Expression of multi-domain type III antifreeze proteins from the Antarctic eelpout (Lycodichths dearborni) in transgenic tobacco plants improves cold resistance
Qiao Huang, Ruiqin Hu, Hui zhu, Changlian Peng, Liangbiao Chen
aKey Laboratory of Exploration and Utilization of Aquatic Genetic Resources (Ministry of Education) and International Research Center for Marine Biosciences, College of fisheries and Life Science, Shanghai Ocean University, Shanghai, 200120, China
bInternal Joint Research Center for Marine Biosciences (Ministry of Science and Technology), College of fisheries and Life Science, Shanghai Ocean University, Shanghai,200120, China
cGuangdong Provincial Key Laboratory of Biotechnology for Plant Development, College of Life Science, South China Normal University, Guangzhou, 510631, China
dLaboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Shandong, Qingdao, 266000, China
eSchool of Food Engineering and Biotechnology, Hanshan Normal University, Guangzhou, 521041, China
ABSTRACT
Keywords:
Type III antifreeze proteins
Multidomain proteins
Cold tolerance
Electrolyte leakage
MDA
Proline
1.Introduction
In the polar Oceans, seawater temperature can seasonally or perennially reach (around -1.9C) and is the existence of antifreeze proteins(AFPs) in polar fish species that enable them to survive in such hostile environments (Kelley, Aagaard, MacCoss, & Swanson, 2010). Four different types of antifreeze proteins (AFP I to IV) and one antifreeze glycoprotein (AFGP) have been characterized in polar fishes (A. L.Devries & Lin, 1977; Pickett et al., 1984; Yang, Sax, Chakrabartty, &Hew, 1988). Recently, Zona pellucida proteins of Antarctic notothenioid fishes are also shown to have freeze prevention activities (Cao et al.,2016). Despite antifreeze proteins have different structural characteristics, their common function is to protect organisms tissues from freezing. These proteins impede the formation and growth of nucleating ice crystals in tissues by depressing the freezing point of the fish below the ocean temperature by a non-colligative process (Raymond & DeV-ries, 1977). Type III AFPs evolved from the sialic acid synthase gene(Deng, Cheng, Ye, He, & Chen, 2010) and they are a small group of globular proteins homologues to the C-terminal domain of sialic acid synthase with a flat, amphipathic ice-binding face that include several conserved hydrophilic residues (Gln9, Asn14, Thr15, Thr18, Gln44).These residues establish hydrogen bonds with water molecules on the ice surface and inhibit ice crystals to growth (Z. C. Jia, DeLuca, Chao, &Davies, 1996; Sonnichsen, DeLuca, Davies, & Sykes, 1996).
Three major and at least five minor variants of AFPIII have been isolated from the Antarctic eelpout (Lycodichthys dearborni) (Wang,Devries, & Cheng, 1995a; 1995b). The two major AFPs (RD1, RD2) are of 7 kDa in size, contain a unique 64-aa AFP domain and are similar to AFPIII characterized from other fishes. In 1995, a third (RD3) isoform was identified containing two 7-kDa AFP domains connected in tandem through a 9-residue linker sequence (-Asp-Gly-Thr-Thr-Ser-Pro-Gly--Leu-Lys-) (Wang et al., 1995a). Wang et al. also suggested a widespread presence of multi-domain AFP gene structure in L. dearborni and these multi-domain AFP genes were expressed, as well as the 2.7 kb mRNA product detected by Northern blot analysis (Wang, Devries, & Cheng,1995b). Previously, we have cloned an AFP transcript, ld12, encoding 12 tandemly linked AFP domains (Yu, Cheng, DeVries, & Chen, 2005).Analysis of the AFPIII loci of L. dearborni using bacterial artificial clones indicated the existence of AFPIII genes encoding various number of AFPIII domains ranging from 1, 2, 3, 4 and 12 (Deng et al., 2010).However to date the proteins that contain more than three domains have not yet been purified from the fish plasma and the function of the multiple domain in respect to survival in cold environments have not been established.
Low temperature is one of the major limiting environmental factors that in fluence growth, development, productivity and geographical distribution of plants and transgenic expression of fish AFP has been previously suggested to increase plant cold tolerance and freeze resistance (K. D. Kenward, Altschuler, Hildebrand, & Davies, 1993). Preliminary tests have shown that vacuum-infiltration of AFPI from the winter flounder into the extracellular spaces of canola, potato and Arabidopsis thaliana leaves resulted in an average of 1.8C decrease in the spontaneous freezing temperature (Cutler et al., 1989). However,Bernard (Duncker, Chen, Davies, & Walker, 1995; Duncker, Hermans,Davies, & Walker, 1996) and Rancourt (Rancourt, Davies, & Walker,1992; 1987) suggested that AFPII and AFPIII might be better options for antifreeze activity in other organisms when compared to the linear AFPI,because this protein has no tertiary structure and is less stable. Identification of hyperactive AFPIII can improve cold resistance in other organisms including plants. Functional studies on AFPIII size variants demonstrated that artificially synthesized multi-domain AFP III proteins exhibited significantly higher activity in freezing-point depression when compared to single-domain AFPs at same concentrations (Miura et al.,2001; Nishimiya, Ohgiya, & Tsuda, 2003). In this study, we have cloned cDNAs that encode the 7-kDa small globular (ld1), its dimer (ld2), trimer(ld3), and tetramer (ld4) respectively from the multimer AFPIII gene ld12, and studied their performance in improving stress cold resistance on transgenic tobacco plants. We have found that the multiple-domain fish AFPIIIs protect tobacco cells from non-freezing hypothermic stress, apart from their known function as ice-binding and ice-inhibition proteins at freezing temperatures.
2.Materials and methods
2.1.Vector construction and Agrobacterium-mediated transformation of tobacco plants
The full length cDNA coding for ld1, ld2, ld3 and ld4 were amplified from the ld12 cDNA of the Antarctic eelpouts (Lycodichthys dearborni)using forward primer 5-CGACGCGTATGAAGTCAGTTGTTTTAAC-3and reverse primer 5-CGGAATTCCTACTTCTCATAGTTTTTCACC-3,(underlined sequences of the primers correspond to the MuLI and EcoRI restriction sites), respectively. The PCR product of ld1, ld2, ld3, ld4 were cloned into the pCAPE2-gfp vector of the pEBV-VIGS vector system by replacing the gfp region (Fig. 1) and generate new recombinant vector pCAPE2- ld1, pCAPE2-ld2, pCAPE2-ld3 and pCAPE2-ld4. DNA sequencing was performed to ensure that the conjunction and the gene sequences were correctly inserted.
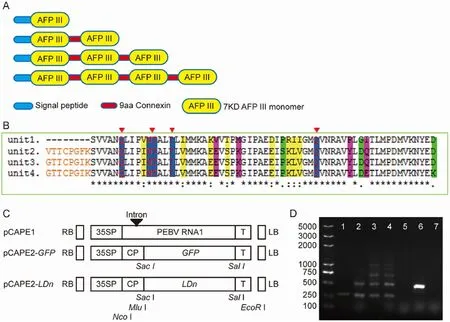
Fig. 1.Structural analysis and cloning of ld AFPIII size isoforms. (A) Simple structure model of the deduced amino acid sequences of the ld1, ld2, ld3 and ld4. (B)Amino acids alignment of the AFPIII domains. Ice-binding residues (blue) and linker sequence (orange) are indicated, different residues with highly conserved(yellow), similar (green) or dissimilar (pink) properties are colored. (C) Diagram illustrating the PEBV clones used in this study. 35SP, CaMV 35S promoter; CP, the coat protein coding region of PEBV; T, the NOS terminator; LB and RB, the left and right borders of the T-DNA region, respectively. (D) RT-PCR analysis of AFPIIIs expression in transgenic lines. Transgenic lines (1: ld1; 2: ld2; 3: ld3; 4: ld4), vector control (5: gfp) of tobacco, tetracycline gene fragment (6: positive control) and H2O (7: negative control) were used as PCR templates. (For interpretation of the references to color in thisfigure legend, the reader is referred to the Web version of this article).
2.2.Reverse transcription-PCR (RT-PCR) analysis
Total RNA from the leaves of 2 weeks old tobacco grow in the greenhouse (25C) environment was extracted using TRIzol Reagent according to the manufacturer’s protocol (Invitrogen). Reverse transcription-PCR reaction was carried out according to the standard protocol (Takara) and RT-PCR products were checked on agarose gel electrophoresis.
2.3.Cold resistance analysis
To evaluate the effect of ld1, ld2, ld3 and ld4 over-expression on plant cold tolerance,five independent pCAPE2-gfp pCAPE2-ld1, pCAPE2-ld2,pCAPE2-ld3 and pCAPE2-ld4 transgenic lines were cultured under normal conditions for 2 weeks, and the plants were placed in a 4C temperature chamber for 30 days, followed by returning them to growth chamber (25 ± 2C) for 10 days to recover. Phenotypes were observed at three different time points.
2.4.Electrolyte leakage measurements
The percentage of electrolyte leakage showed the injury degree of tobacco seedlings under cold stress and was determined by relative conductivity as describe (Nunes, Eug′enia, & Smith, 2003). Brie fly, leaf segments (1 cm) obtained from fully unfolded leaves of tobacco were immersed in 15 mL distilled water and vacuum infiltration, the electrical conductivity of the supernatant (S1) was detected using a DDS-307 Conductivity Meter (LEICI Company, China). The samples were subsequently then boiled for 15 min to determine the ultimate conductivity(S2: maximum conductivity of tissues). Electrolyte leakage data (EL)were calculated using the formula: electrolyte leakage (%) =S1/S2 ×100. Experiments were independently replicated 3 times under identical conditions.
2.5.MDA measurements
The level of lipid peroxidation was measured for MDA content by thiobarbituric acid (TBA)-reactive substances. Tissue samples (0.3 g)were homogenized in 5 mL of 10% TCA and quartz sand. After centrifugation at 12,000 × g for 10 min at 4C, a 2-ml supernatant was mixed with 2 mL of 0.6% TBA. The mixture was heated at 100C for 15 min,immediately cooled, and centrifuged at 12,000 ×g for 5 min and supernatant was measure at 532, 600 and 450 nm. The MDA concentration was calculated from: MDA (nmol/g FW) =[6.45(A- A)-0.56A] Ve×Vt×Vs×FW[Ve: Total volume of reaction (mL); Vt:Total volume of extract (mL); Vs: Extract volume was determined with(mL); FW: Sample fresh weight (g)]. Experiments were independently replicated 3 times under identical conditions.
2.6.Proline content measurements
Free proline content was measured using a method described as before (1991). Leaves (0.3 g) were homogenized with 5 mL 3% sulfosalicylic acid and quartz sand and the homogenates were centrifuged at 3000 g for 20 min, then 2 mL of sample supernatant was mixed with 2 mL of acetic acid and 2 mLof 2.5% acid ninhydrin and were boiled for 30 min, and the reaction terminated on an ice bath. The reaction mixture was extracted with 4 mL toluene, mixed vigorously 15-20 s and centrifuged at 3000 g for 10 min. The supernatant was used to measure the absorbance at 520 nm using toluene as blank. Proline concentration was determined based on a standard curve calculated on a fresh weight basis as follows: pg proline/mg of fresh weight material =[(pg proline/mL × mL toluene)/(g sample)]×1000.
2.7.Chlorophyll fluorescence measurements
During cold treatment, chlorophyll fluorescence was determined using a portable pulse-modulated fluorimeter PAM-2100 (Walz, Efeltrich, Gemany). Seedlings were dark-adapted for 30 min before measurement of maximum photochemical efficiency of PSII (Fv/Fm).Minimal fluorescence (Fo) was measured under a weak pulse of modulating light and maximal fluorescence (Fm) was measured under a strong flash of light (4,000 μmol ms) by a 0.8 s pulse. The maximal photochemical efficiency of PSII (Fv/Fm) was calculated as Fv/Fm =(Fm - Fo)/Fm.
2.8.Statistical analyses
Each treatment was repeated six times in separate petri dishes. Data were expressed as means ±standard deviation (SD) and analyzed using one-way ANOVA by SPSS 11.5. Experiments were repeated at least three times. Significant differences were compared between groups at P <0.05.
3.Results
3.1.Generating cDNA clones
We amplified AFPIII coding sequences containing one to four of the 7 kD AFPIII domains from the LD12 transcript (Fig. 1A). ld1, ld2, ld3 and ld4 encode a 87, 157, 227 and 297 amino acid polypeptide respectively.All clones contained a putative signal peptide, and the domains are linked by slightly different 9-amino acid linkers. The individual AFPIII domains are very similar in amino acid sequences. The first domain is 77% amino acid identical to the other three domains. The variable sites between the four domains are non ice-interacting residues (Fig. 1B).
ZHAO Jia-yi, HAN Yi-ping, YANG Li-xin, JIN Hai, CHEN Wei, SHENG Jing, ZUO Chang-jing, ZHENG Jian-ming
3.2.Generation of transgenic tobacco plants
In order to verify the cold resistant function of the ld1, ld2, ld3 and ld4 genes, a virus-induced expression construct was developed (Fig. 1C)and they were transformed into tobacco. We successfully generated transiently expressed transgenic plants for all four constructs (Fig. 1D).
3.3.Cold resistance analysis
The ld1, ld2, ld3 and ld4-expressing plants exhibited no obvious phenotypic difference from the gfp-expressing plants under a long-day photoperiod (16 h of light/8 h of dark) and optimum growth temperatures (25 ± 2C). To determine whether the plants expressing the AFPs improved cold resistance, the control and the transgenic plants were treated with 4C (16 h of light/8 h of dark) for 30 days. Leaves of the transgenic plants showed different degrees of dehydration among the plants (Fig. 2). Plants with the gfp and ld1 transgenes showed the most serious phenotypes in which almost all leaves were dehydrated. The plants with ld2 transgene showed a less server phenotype in which most of the leaves were dehydrated except the ones on the top. In contrast, the ld3 and ld4 transgenic plants showed visible signs of dehydration in the bottom leaves and but the rest leaves appeared healthy. The results were further manifested after recovery at 25C and the ld3 and ld4 expressing plants overcame dehydration and achieved complete recovery (Fig. 2),while the ld2 expressing plants only half of the leaves recovered. The gfp control plants displayed severe chlorosis and sufferred irreversible damage.

Fig. 2.Phenotype of transgenic tobacco plants with different AFPIII genes under cold stress and during recovery.
These results showed that transgenic plants expressing the ld2, ld3 and ld4 genes maintained greater stamina than gfp and ld1 during cold treatment and were able to recover and continue to grow under the normal temperature. The ld3 and ld4 are the most potent in conferring the cold resistant effects to the plants.
3.4.Electrolyte leakage
The effect of low temperature on the electrolyte leakage in the leaves of the transgenic plants was examined. As shown in Fig. 3A, gfp and four transgenic plants have a similar level of electrolyte leakage at normal growth temperature. Cold stress greatly increased electrolyte leakage in both control and transgenic plants, but it is more dramatically in the gfp and ld1 plants (4.5 and 4.4 folds) when compared to ld2, ld3, ld4 plants(3.8, 2.6 and 2.4 folds). After 10 days recovery, electrolyte leakage in the ld3 and ld4 plants decreased about 71.15% and 47.33% while no significantly decrease was detected in the gfp, ld1, ld2 plants.
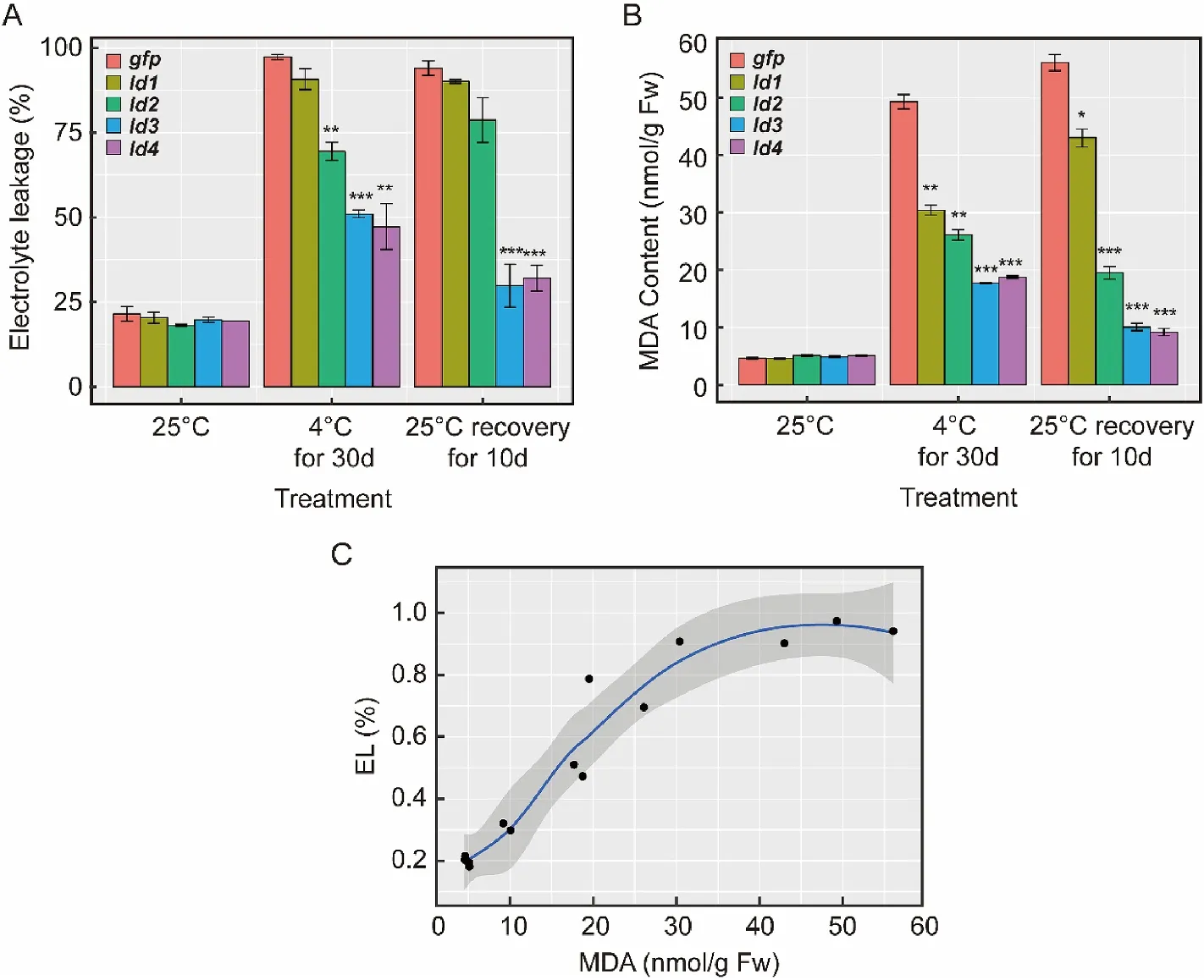
Fig. 3.Effects of cold treatment on the electrolyte leakage (A) and MDA content (B) in the leaves of control and transgenic tobacco plants. (C) Correlation analysis between MDA content and electrolyte leakage. Error bars, mean±s.d., n =3 (biological replicates).
3.5.MDA content
Low temperature stress induces the accumulation of free radicals,which damage cells by initiating or accelerating the membrane lipid peroxidation (Wise & Naylor, 1987) and yields elevated level of MDA(malondialdehyde). We examined the MDA content by measuring the relative conductivity which is parallel with the content of MDA. No significant differences between the control and transgenic plants under normal growth condition, but after cold stress, MDA content of the gfp expressing plants were 1.6, 1.9, 2.8, 2.6 folds higher, respectively, than those of ld1, ld2, ld3, ld4 plants. After recovery, no MDA reduction was observed in the gfp and ld1 plants, but was reduced in the ld2 plants and drastically reduced in the ld3 and ld4 plants. It is noteworthy that in all plants, there is an increase in the MDA levels even after recovery when compared to the plants without cold treatment (Fig. 3B). Overall, the results indicated that over expression of ld1, ld2, ld3 and ld4 could alleviate the lipid peroxidation in plant cells under low temperature stress but ld3 and ld4 are more potent.
When level of ion leakage with the MDA content were compared, a significant correlation (R=0.8631) was observed between MDA content and permeability of cell membranes to ions (Fig. 3C), indicating membrane leakage is associated with peroxidation of fatty acids. Protection of the antifreeze proteins probably involved with protection of membrane integrity of the cold exposed cells.
3.6.Proline content
Proline (Pro) is an osmolyte that copes with cellular dehydration and, in Arabidopsis and other plants, the increase of Pro levels during cold acclimation correlates to cold tolerance (Koster & Lynch, 1992;McKown, Kuroki, & Warren, 1996; Wanner & Junttila, 1999). To verify whether low temperature stress modified the cytoplasmic amino acid content, the Pro content from the gfp and the ld1, ld2, ld3, ld4 transgenic plants were measured at cold exposure and after recovery (Fig. 4A).During normal growth conditions, there were not significant differences between gfp and the four transgenic tobaccos. However, after 30 days of cold temperature stress, Pro content in all plants increased with the fish ld transgenic plants showing a higher increase, especially for the ld3 and ld4 plants. After recovery, the Pro content in ld2, ld3 and ld4 plants declined mostly in ld3 and ld4 plants, suggesting a better recovery. Pro levels were not recovered in the gfp and ld1 plants.
3.7.Chlorophyll fluorescence
The chlorophyll fluorescence technique is an effective way to investigate the efficiency and kinetics of the photosynthetic apparatus in plants. In our study, Fv/Fm was investigated every 10 days under 4C exposure (Fig. 4B). The chlorophyll fluorescence parameters (Fv/Fm) of ld2, ld3 and ld4 decreased during cold exposure and increased to normal levels during recovery. Fv/Fm of gfp and ld1 plants constantly decreased even after the 10 days of recovery.
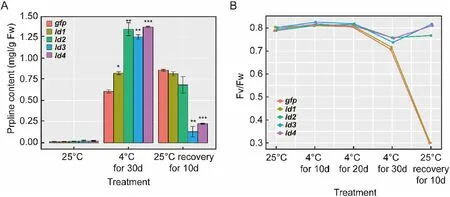
Fig. 4.Changes of proline content in seedlings (A) and maximal photosystem II (PSII) photochemical efficiency (Fv/Fm) in leaves (B) of control and transgenic plants under cold treatment or recovery. Error bars, mean±s.d., n =3 (biological replicates).
4.Discussion
Cold stresses imposed severe negative impacts on agriculture. It has been conjectured that a 1C decrease in the World average temperature would result in a 40% reduction of the rice production. AFPs are well known for their thermal hysteresis and ice recrystallization inhibition activities in both fishes and insects ( DeVries, 1988; Duman, 2001). In the past forty years, considerable efforts have been devoted to utilizing the antifreeze activity of the AFPs in plants as seen on the considerable advances achieved with freezing resistance in the tomato (Hightower,Baden, Penzes, Lund, & Dunsmuir, 1991), tobacco(K. Kenward, Brandle,McPherson, & Davies, 1999; 1993), potato (Wallis, Wang, & Guerra,1997) and spring wheat (Khanna & Daggard, 2006). Under most circumstances, freezing condition is an extreme end of the cold stress, and hypothermic stress above the freezing temperature is a major challenge for crops and also for aquaculture species.
It has been shown that vacuum-infiltration of winter flounder AFPI into the extracellular spaces of canola, potato and Arabidopsis thaliana leaves resulted in an average of 1.8C decrease in the spontaneous freezing temperature (Cutler et al., 1989). While Bernard (Duncker,Hermans, Davies, & Walker, 1996) and Rancourt (Rancourt et al., 1992;1987) suggested that the globular fish AFPII and AFPIII AFPs are better choices for antifreeze transfer to other organisms functional studies on the AFPIII size variants demonstrated that artificially synthesized multi-domain proteins exhibited significantly higher activity in freezing-point depression when compared to single-domain proteins concentrations (Miura et al., 2001; Nishimiya, Ohgiya, & Tsuda, 2003).
Our results revealed that overexpression of Lycodichths dearbornd AFPIII gene confers increased cold-tolerance in tobacco. We found that a positive correlation between the numbers of AFPIII domains and the capability of cold tolerance exists. On the other hand, increase of repeating domains is also a mean to improve the ice-binding and iceinhibition activity of many AFP proteins, such as for the antifreeze glycoproteins (Devries, 1982), and AFPI (Chao, Hodges, Kay, Gauthier,& Davies, 1996). In our study, the ld4 and ld3 transgenic tobacco plants acquired similar levels of cold resistance activity. Yoshiyuki et al.(Yoshiyuki Nishimiya, Satoru Ohgiya, & Sakae Tsuda, 2003) reported that no significant difference in the antifreeze activity was detected between the trimers (RD3NNC, RD3NCC, and RD3NCN) and a tetramer(RD3NCNC). The mechanisms underlying the correlation between the antifreeze activity and non-freezing cold resistance and the different protein isoforms requires further investigation. Nevertheless, the ld3 and ld4 isoforms show both the non-freezing cold protection and ice-inhibition functions. The versatility of these proteins render their usefulness on improving cold and freezing tolerance.
Declaration of competing interest
The authors declare that there is no conflicts of interest.
Acknowledgements
The work is supported by Natural Science Foundation of China[31572611], the National Key Research and Development Program of China [2018YFD0900601] and the Major Science Innovation Grant[2017-01-07-00-10-E00060] from the Shanghai Education Committee to L. Chen.
 Aquaculture and Fisheries2021年2期
Aquaculture and Fisheries2021年2期
- Aquaculture and Fisheries的其它文章
- Editorial: Global fish passage issues
- Analysis of the impacts of socioeconomic factors on hiring an external labor force in tilapia farming in Southern Togo
- Using Bayesian Bio-economic model to evaluate the management strategies of Ommastrephes bartramii in the Northwest Pacific Ocean
- Endosymbiotic pathogen-inhibitory gut bacteria in three Indian Major Carps under polyculture system: A step toward making a probiotics consortium
- A chromosome-level genome assembly of the red drum, Sciaenops ocellatus
- Loss of scleraxis leads to distinct reduction of mineralized intermuscular bone in zebra fish
