Loss of scleraxis leads to distinct reduction of mineralized intermuscular bone in zebra fish
Chunhong Nie, Shiming Wan, Yulong Chen, Dejie Zhu, Xudong Wang,Xiaoru Dong, Ze-Xia Gao,c,*
aCollege of fisheries, Key Laboratory of Freshwater Animal Breeding, Ministry of Agriculture and Rural Affair/Key Laboratory of Agricultural Animal Genetics, Breeding and Reproduction, Ministry of Education, Huazhong Agricultural University, Wuhan, Hubei, 430070, China
bEngineering Research Center of Green Development for Conventional Aquatic Biological Industry in the Yangtze River Economic Belt, Ministry of Education, Wuhan,430070, China
cHubei Engineering Technology Research Center for fish Breeding and Healthy Aquaculture, Wuhan, 430070, China
ABSTRACT
Keywords:
Intermuscular bone
Scleraxis
CRISPR-Cas9
Phenotype
Gene expression
Gene function
1.Introduction
Generally, the fish skeleton system includes the skull, axial skeleton,ribs and fins, and many studies had focused on these components.However, intermuscular bones (IBs) are also a component of fish skeleton, but fewer studies have paid attention to it. Previous studies have demonstrated that IBs are ossified after the main skeleton (Bird &Mabee, 2003; Wan et al., 2014). IBs, which occur only in lower teleosts,are hard-boned spicules located in the myosepta on both sides of the vertebrae (Danos & Ward, 2012; Patterson & Johnson, 1995). There are three series of IBs in lower teleosts: epineurals, epicentrals, and epipleurals (Patterson & Johnson, 1995). Almost half of farmed finfish species worldwide, especially the Cypriniformes, accounting about 70% of aquaculture production and 56% of production value, have a certain number of IBs (epineurals and epipleurals) (Nie, Hilsdorf, Wan & Gao,2019). These small IBs negatively affect the economic value of fish species, restrict processing options, and stress consumers due to the possibility of injury or trauma if lodged in the throat or mouth (Nie,Hilsdorf, Wan & Gao, 2019). Since 1960s, many studies focused on the number, morphology and ossification pattern of IBs (Dong et al., 2006;Jiang, Yang & Bao, 2008; Lv, Bao, Jiang, Yang & Li, 2007; Wan et al.,2014; Yao, Gong, Lv & Bao, 2015). In recent years, some studies also paid attention to the molecular mechanism of IB ossification in blunt snout bream (Megalobrama amblycephala) using high-throughput sequencing technologies (Wan et al., 2015, 2016, 2019; Nie et al.,2017; Nie, Hilsdorf et al., 2019). These reports provided a basic understanding of developmental molecular mechanism of IBs and proved feasibility of genetic improvement of IB traits in farmed fish. However,the key genes that control the occurrence or number of IBs remain unknown, so identification of genes that directly regulate the phenotype of IBs has become the main objective in this field.
Previous studies had reported that IBs are ossified from tendons in myosepta in teleost (Danos & Ward, 2012; Gemballa & Britz, 1998;Patterson & Johnson, 1995). Tendons are dense,fibrous connective tissue that connects muscle to bone, transmitting the forces that allow for body movement, and play an essential role in the muscular control of the body by connecting muscle and skeletal elements (Murchison et al.,2007). As a basic helix-loop-helix (bHLH) transcription factor, scleraxis(scx) is known to be associated with differentiation and maturation of tendon cell lineage, as the earliest marker of tendon progenitors (Cserjesi et al., 1995; Schweitzer et al., 2001). To determine scx function,previous studies have applied gene-editing approach to generate new alleles and evaluate the phenotype of scx mutations in mammals. For instance, Scxmice generated by Cre-mediated excision were viable,but had severely impaired mobility of limbs and back (Yoshimoto et al.,2017). TALEN-mediated scx-deficient mice exhibited hypoplastic tendon formation (Shukunami et al., 2018). In teleosts, species without IBs normally just have one copy of the scx gene; however species(Cypriniformes) with IBs all have two copies of the scx gene (scxa and scxb). Therefore, a functional analysis based on gene knock-out experiment could help us understand the regulatory role of the scx genes on the IB development.
Considering that IBs are ossified from tendons and zebra fish have a certain number of IBs (Nie, Chen, Dai, Wan & Gao, 2018), scxa and scxb mutant lines of zebra fish were generated using the Clustered Regularly Interspaced Short Palindromic Repeats-associated protein 9 (CRISPR--Cas9) in this study. Although Kague et al. (2019) already reported that scxa mutants had severely defected, or decreased number of, ribs, they did not report the impacts on the IBs phenotype. However, our study demonstrated that loss of scxa function had an obvious effect on the number of IBs, with almost 70% reduction compared with the wild type zebra fish. The phenotype of ribs was the same as reported by Kague et al.(2019). The molecular regulation mechanism of scxa impacts on the IB development was further clarified through comparative transcriptome analysis. This is the first study that identified the key gene regulating the number of fish IBs, which may provide a new perspective on the role of scxa in the molecular mechanism of IB development in fish.
2.Materials and methods
2.1.Ethics statement
All experimental protocols in this study were approved by the Animal Experimental Ethical Inspection of Laboratory Animal Center,Huazhong Agricultural University, Wuhan, China (HZAUDO-2016-005,2016-10-26). All efforts were made to minimize the suffering of the animals. All experiments were performed in accordance with relevant guidelines and regulations.
2.2. fish maintenance
The zebra fish were bred and maintained according to standard protocols at the College of fisheries, Huazhong Agricultural University.Zebra fish embryos and larvae were cultured at 28C. The Tg (sp7/eGFP)transgenic line was obtained from Dr. Chung-Der Hsiao, Chung Yuan Christian University, Taiwan, and then bred in the College of fisheries,Huazhong Agricultural University.
2.3.Phylogenetic and genomic structure analysis
For the phylogenetic analysis, we retrieved scx orthologues of seven fish species (Danio rerio, Cyprinus carpio, Carassius auratus, Megalobrama amblycephala, Paralichthys olivaceus, Oryzias latipes and Cynoglossus semilaevis), and sequences from other vertebrates as outgroups (Homo sapiens, Mus musculus, Gallus gallus, Xenopus laevis). All sequences are available in the Supplemental Table 1. To analyze sequence conservation, we performed multiple sequence alignment of scx sequences with Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
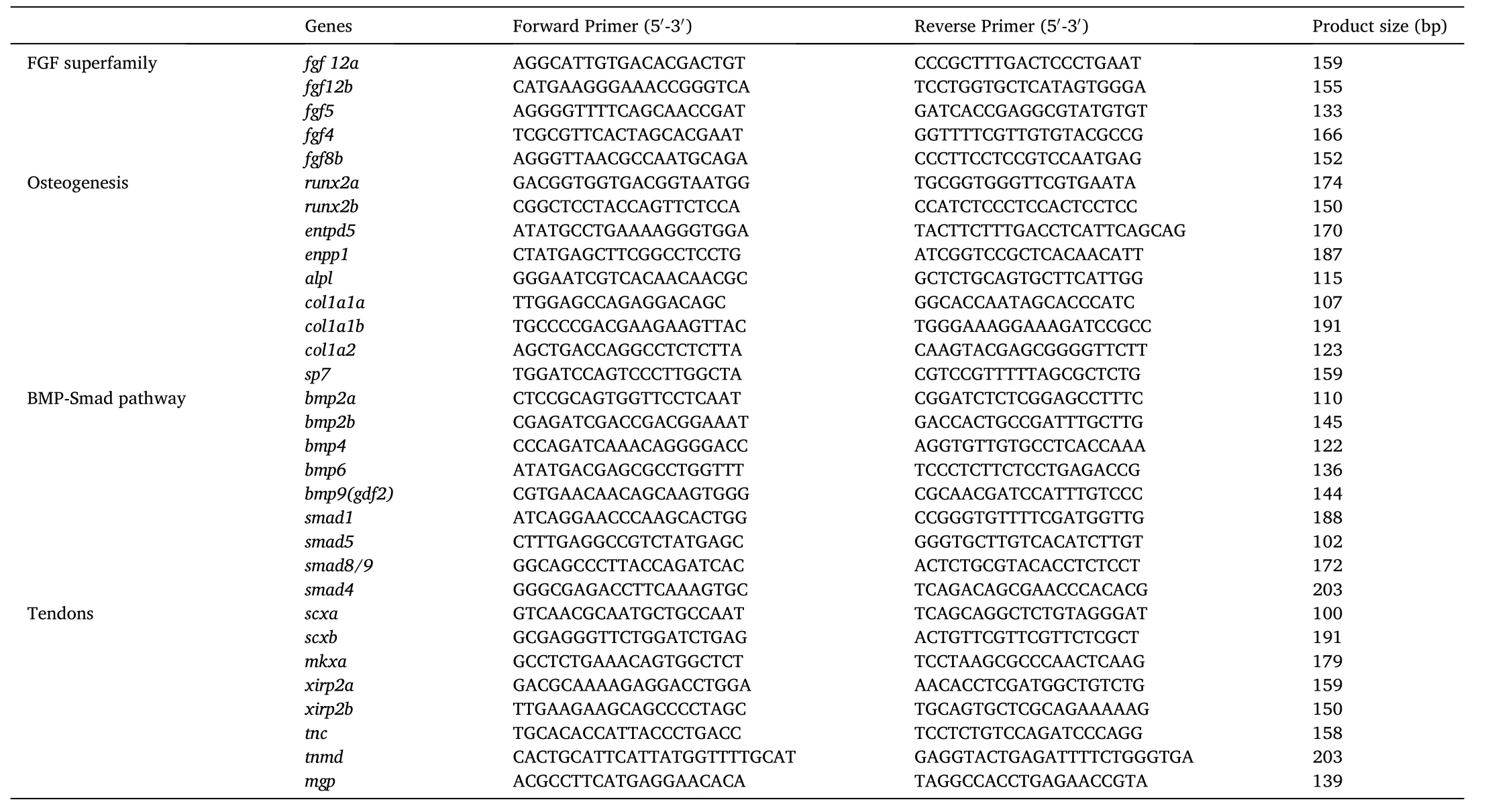
Table 1Nucleotide sequences of the primers used for qPCR.
2.4.Generation of mutant zebra fish lines and genotyping
CRISPR-Cas9 genome editing technology (Fin, Bergamin, Steiner &Hughes, 2017) was used to target scxa (zv11, Chr19: 3001919-3001938,5-GGGGGTGGCGGACGGCTGAT (PAM)-3’ (exon one) and 5-GAGAGAGGAAATCCGCCCTG (PAM)-3’ (exon two)) and scxb (zv11, Chr16:31,396,781-31,396,801, 5-GAGACGGTCAGCCCTGCCTC (PAM)-3’(exon one)). The scxa and scxb mutant lines were developed in a Tg(sp7/eGFP) transgenic line. The scxa and scxb are predicted to cause a translational frame shift. The primer pairs used for PCR genotyping are listed in Supplementary Table 2. The PCR protocol was: step 1 was 94C for 5 min; step 2 was 35 cycles of 94C for 30 s, 63C for 30 s and 72C for 30 s; and step 3 was 72C for 5 min. The PCR products were separated on a 2.5% agarose gel: 183 bp/175 bp for wild/mutant of scxa-1;226 bp/201 bp for wild/mutant of scxa-2; 173 bp/159 bp for wild/-mutant of scxb-1.
2.5.Morphological and developmental observations of IBs at different developmental stages
Bone staining with alizarin red was performed as previously described (Nie, Wan et al., 2019; Nie, Hilsdorf et al., 2019). In order to investigate the phenotypic changes of IBs in mutant and wild types, we used alizarin red staining to observe the phenotype of IBs at different developmental stages, including 20 dpf (days post fertilization), 23 dpf,26 dpf, 29 dpf, 32 dpf, 35 dpf, 38 dpf, 40 dpf, 45 dpf, 50 dpf and 3-month-old adult individuals. After transparency processing, the stained specimens were analyzed and photographed under an Olympus SZX-16 Stereo Microscopes (Japan). The number of IBs distributed across the whole body and different parts of the body (epineurals and epipleurals distributed on both sides of the body) were counted under the microscope. Similarly, the stained ribs on both sides of the body were also photographed and counted. The differences in the number of IBs and ribs between mutant and wild type individuals were compared using the Independent-Samples T Test.
2.6.Comparative transcriptome analysis and validation of DEGs with qPCR
In order to study the molecular regulation mechanism for the IB development, the dorsal muscle tissues from wild type fish (with IBs)and scxa-1 mutants (without IBs) at 60 dpf were collected and the total RNA was isolated using mirVana? miRNA Isolation Kit (Ambion, USA)according to the manufacturer’s protocol, with three biological replicates. The sampling procedure was performed as shown in Fig. 1. The six transcriptome sequencing libraries were generated using TruSeq Stranded mRNA LTSample Prep Kit (Illumina, USA) following manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. After cluster generation, the library preparations were sequenced on an Illumina HiSeq platform. After quality control of raw data, paired-end clean reads were aligned to the zebra fish reference genome (ftp://ftp.ensembl.org/pub/release-92/fasta/dan io_rerio/) using Hisat2 v2.2.1.0 (Kim, Langmead & Salzberg, 2015).HTSeq-count v0.9.1 (Anders, Pyl & Huber, 2015) was subsequently used to count the read numbers mapped to each gene, and FPKM of each gene was calculated using Cufflinks (Trapnell et al., 2010). Differential expression analysis of two conditions/groups (two biological replicates per condition) was performed using the DESeq2 R package (1.18.0)(Anders et al., 2013). Genes with an adjusted P-value <0.05 with fold change >2 or fold change <0.5, adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate, were defined as differentially expressed genes (DEGs). GO function enrichment and KEGG pathway enrichment analysis of differentially expressed genes were then performed using cluster Profiler R package based on hypergeometric distribution (Yu, Wang, Han & He, 2012).
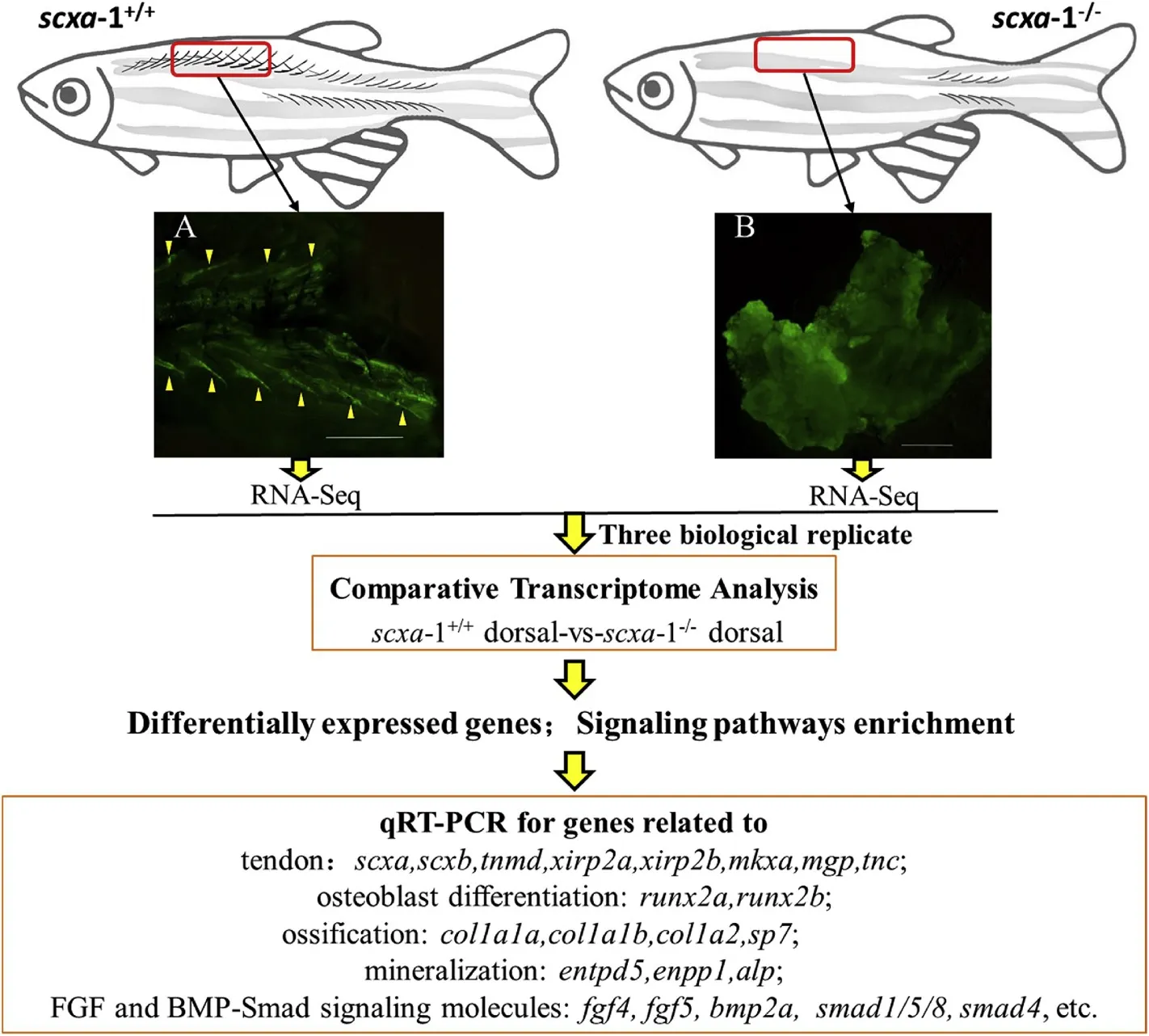
Fig. 1.Flowchart for the comparative transcriptomic analysis. Red rectangular boxes indicate sampling areas for transcriptome analysis. The sampled tissue characteristics from the corresponding area with or without intramuscular bones are shown in panels A and B respectively. The IBs wrapped in the muscle tissue are indicated by the yellow triangle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The expression of bone-related and tendon-related genes was validated using qPCR, which was performed using SYBR Green Premix Ex Taq 177 (TaKaRa) and a QuantStudio 6 Flex real-time PCR System(Applied Biosystems, 178 ABI, USA) according to the manufacturer’s instructions. All of the real-time reactions were performed in triplicate and the relative expression levels of the target genes were normalized to the housekeeping gene β-actin using the equation 2. Levels of significance of gene expression differences were then calculated using T-test. Detailed information of primers used in the study is shown in the Table 1.
3.Results
3.1.Phylogenetic analysis of scx in vertebrates
Mammalian genomes contain a single scx gene, but most teleost genomes have two scx genes called scxa and scxb. To trace the evolutionary origin and phylogenetic distribution of scx, we searched vertebrate genomes for homologues of zebra fish scx (Fig. 2A). The phylogenetic analysis results of scx genes showed that the scxa and scxb of fish with IBs clustered into one clade with two branches, while the scx of fishes without IBs clustered into a different clade (Fig. 2A). Aminoacid (aa) sequence analysis indicated that scxa sequence is highly conserved (identity levels >95%) among the Cyprinidae species with IBs (epineurals and epipleurals), while identity levels of scxb were a bit lower, but still higher than 92%. However, the identity of scxa among Paralichthys olivaceus, Oryzias latipes and Cynoglossus semilaevis, all species without IBs, was less than 73%, while that of scxb was less than 71%.
Scx is a bHLH transcription factor, and the earliest marker of tendon and ligament progenitors. The Fig. 2B shows that the size and number of exons of the scx gene are different in different species: mainly 2 exons,except in frog (Xenopus laevis), which possesses six exons, and medaka,possessing three exons. SMART analysis of aa sequences of different species showed that there is a common region, called bHLH domain(53aa). bHLH domain is coded by exon1 in different species, except in medaka (Fig. 2B), and the bHLH domain-encoding sequences were highly conserved across different species (Fig. 2B).
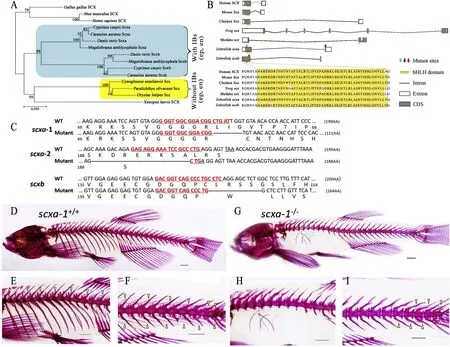
Fig. 2.Genotypic and phenotypic analysis of the wild-type and mutant zebra fish. (A) Relationship of scx protein sequences among the gnathostomes. The blue rectangular box indicates fish species which possess a certain number IBs (en and ep) and the yellow rectangular box indicates the species without IBs (at least en and ep). (B) Structural characteristics and conservation of scx in different species. The black polyline and rectangular box represent introns and exons respectively(equally proportional to gene scale); the gray area in the box represents the CDS sequence. The highly conserved bHLH domains are marked with yellow boxes. The red triangles indicate the mutation sites. Colored arrows indicate the mutation sites. (C) Schematic representation of scxa and scxb DNA and protein sequences as well as the mutant alleles for scxa (scxa-1 and scxa-2) and scxb. CRISPR-Cas9 target sequences are underlined and marked as red letters in the WT and mutant DNA sequences. Deleted nucleotides are shown by (-) in mutants. (D-I) Alizarin red staining for IBs and other mineralized bones of scxa-1+/+and scxa-1-/-zebra fish (3-months-old). (D) Overall mineralized bone phenotypes of scxa-1+/+zebra fish. (E) IBs in the dorsal part of scxa-1+/+zebra fish, indicated by yellow triangle. (F) IBs in the tail part of scxa-1-/-zebra fish, indicated by yellow triangle; (G) Overall mineralized bone phenotypes of scxa-1-/-zebra fish. (H) No IBs visible in the dorsal part of scxa-1-/-zebra fish, and ribs showing defects. (I) Few IBs distributed in the tail segment of scxa-1-/-zebra fish, indicated by yellow triangle. The scale bars shown in the D-I represent 1 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2.scxa-1-/-genotype exhibited a significant reduction of the number of IBs and rib defects
To analyze the effects of a complete loss of function of scxa and scxb in larvae and adult zebra fish, we generated a stable line using Crisper-Cas9 genome editing. We identified 8 bp and 25 bp deletions in scxa-1and scxa-2mutants, respectively, resulting in frameshifts before and after the bHLH domain (Fig. 2C). In scxb, a 14 bp deletion after the bHLH domain caused a frameshift and premature stop codon in exon one(Fig. 2C).
Bone staining results showed that the number of IBs in adult scxa-1zebra fish was significantly reduced compared with the wild type fish(Fig. 2D-I), while scxa-2and scxbmutants had normal IB and skeletal bone phenotype (Fig. 3). scxa-1fish also had rib defects, in agreement with results of Kague et al. (2019); the number of ribs in adult scxa-1(mean 21) was reduced by 57% compared with adult scxa-1(mean 9) (Table 2). However, the adult mutants were viable and fertile. Further, we also counted and analyzed the number of IBs in different body parts (epineurals and epipleurals) and different body sides (left and right) of scxa-1and scxa-1fish (Table 2). In scxa-1mutants, the total number of IBs (mean 24), and the number of epineurals (mean 12) and epipleurals (mean 12) were significantly reduced compared with the wild type (Table 2). The total number of IBs in adult scxa-1was reduced by 70% compared with adult scxa-1,with the significant reduction on both sides of scxa-1fish (Table 2).
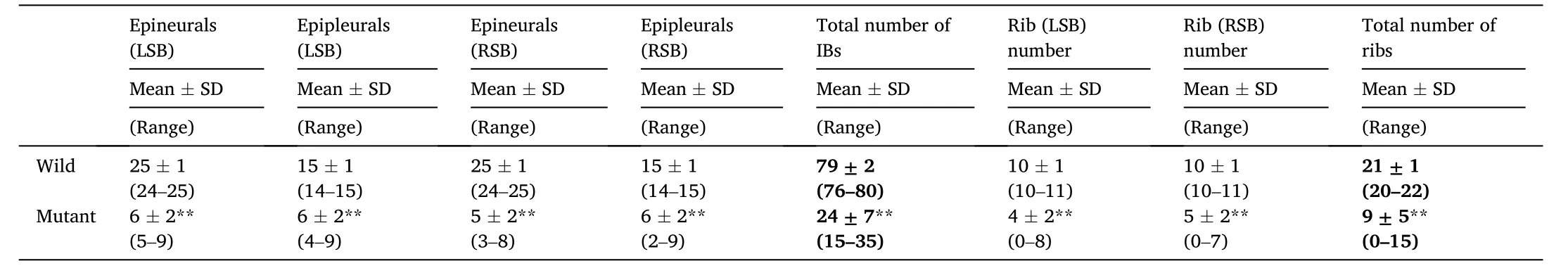
Table 2Statistical analysis of IB numbers in adult wild and scxa-1-/-mutant zebra fish.

Fig. 3.Alizarin red staining for mineralized bone of wild, scxa-2-/-and scxb-/- adult zebra fish (three-months old). (A-C) normal mineralized bone development in wild zebra fish; (D-F) normal mineralized bone development in scxa-2-/-zebra fish; (G-I) normal mineralized bone development in scxb-/- zebra fish.The scale bars in the figure represent 1 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In order to reveal phenotypic changes of IBs after the loss of scxa function, we observed the IB phenotypes of wild and mutant zebra fish at different developmental stages. No IBs appeared in scxa-1and scxa-1zebra fish at 20 dpf. However, scxa-1individuals exhibited rib defects in the early stage of development, manifested as disappearance or reduction of rib structure (Fig. 4). At 26 dpf, more than six IBs had already mineralized in scxa-1, while no mineralized IBs were observed in scxa-1individuals at the same time-point (Fig. 4). At 38 dpf, the IBs of the scxa-1were mineralized and appeared in the dorsal part, while the scxa-1individuals still only had few IBs in the tail part. From the dorsal to ventral, the IBs were distributed and clearly visible from the tail to the head in the scxa-1, while there were only a few IBs in the tail part of scxa-1individuals (Fig. 4). scxa-1and scxa-1fish showed similar IB phenotypes at 50 dpf and adults, with the IBs of scxa-1individuals mineralized from tail to head in adult fish, while scxa-1individuals only had a few IBs in the caudal part.
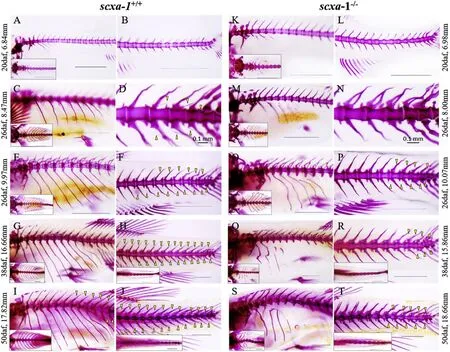
Fig. 4.Phenotype of IBs and ribs at different developmental stages of scxa-1+/+and scxa-1-/-zebra fish. (A-J) phenotypes of IBs and ribs at different developmental stages of scxa-1+/+zebra fish; (K-T) phenotypes of IBs and ribs at corresponding developmental stages of scxa-1-/-zebra fish; the data on the left and right sides of the figure indicate the body length of scxa-1+/+and scxa-1-/-individuals, respectively, at corresponding development stages; rectangular box shows top view of the corresponding part of fish; IBs are indicated by a yellow triangle. The scale bars in the figure represent 1 mm, except in D and N - 0.1 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3.DEGs in response to scxa loss and IB development
In order to further explore the regulatory mechanism of scxa on the mineralization of IBs, we conducted comparative transcriptomic analysis of dorsal tissue between scxa-1(with IBs) and scxa-1(without IBs) (Fig. 1). After raw data quality control, reference genome mapping and comparison of expression levels, a total of 2136 DEGs were identified with a threshold of fold change >2 and p-value <0.05 (Supplementary Table 3). The KEGG pathway analysis revealed a number of important signal pathways with significant enrichment of DEGs,including the TGF-β pathway, calcium pathway, Wnt pathway, Hedgehog pathway, etc. (Fig. 5).
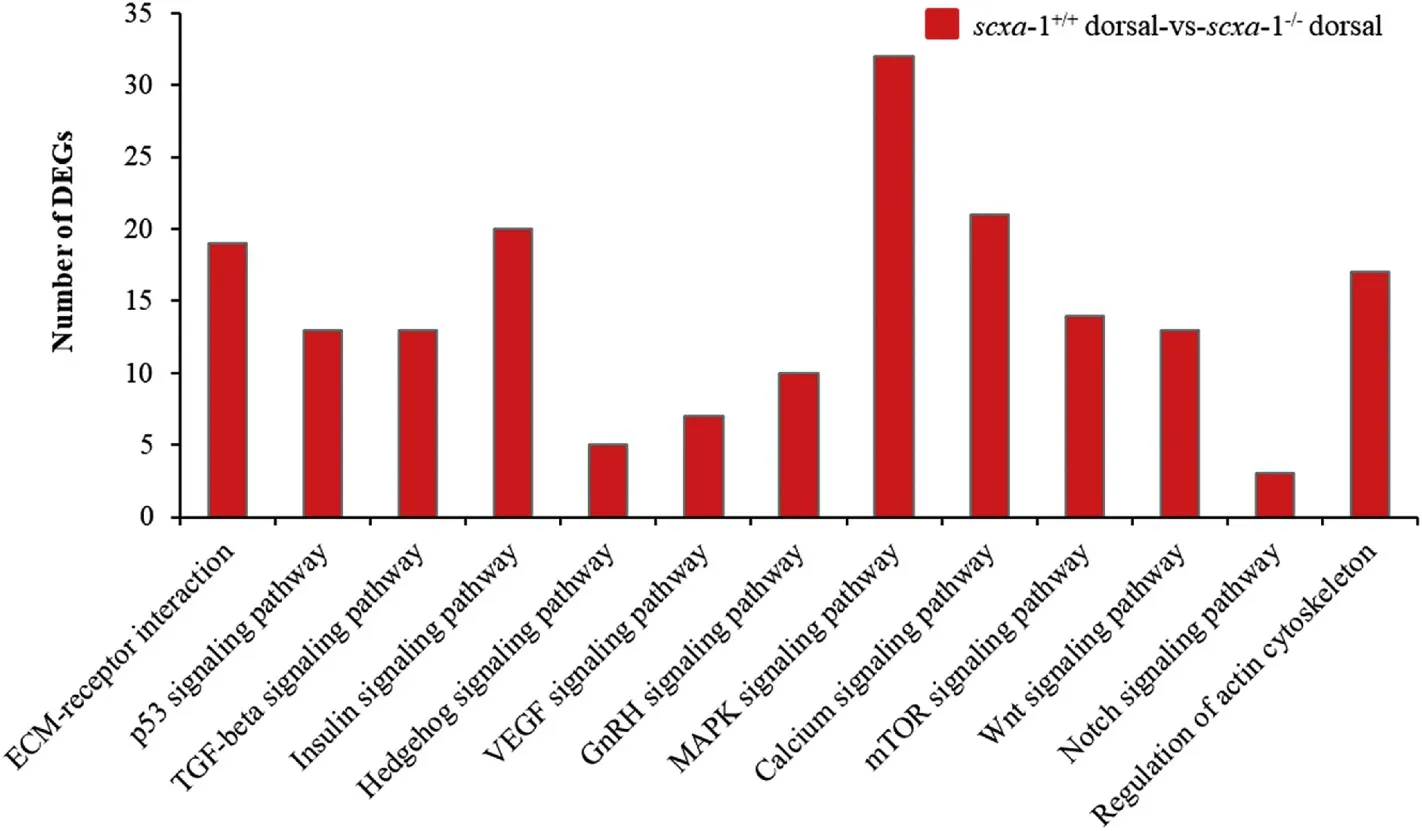
Fig. 5.Signaling pathways related to the bone development enriched in DEGs identified from comparative transcriptome analysis of the dorsal tissue between scxa-1-/-mutant and wild individuals.
The expression of scxa was significantly decreased in the dorsal tissue of scxa-1mutants (Supplementary Table 4). Four types of genes related to tendon differentiation and skeletal development were selected and analyzed for expression. All of these genes showed differences in the expression between scxa-1and scxa-1fish (Supplementary Table 4). For example, mkxa/b, tnmd, xirp2a are associated with tendon development, and they were all down-regulated in scxa-1(Supplementary Table 4). Genes related with osteogenesis and mineralization(col1a2, col1a1a, col1a1b, entpd5, enpp1) were also down-regulated in scxa-1. In the dorsal part of scxa-1, the expression of some genes associated with osteoblasts was up-regulated (bmp4, myog, myf6,smad1), whereas others were down-regulated (bmp2a, runx2b, fgf8a/8b,bmp6, alpl, spp1, smad8) (Supplementary Table 4).
To validate the transcriptomic datas, 31 genes were selected for qPCR, including tendon related genes, osteogenesis related genes, FGF subfamily, and BMP-Smad signaling molecules (Fig. 6). In agreement with the transcriptomic data, the expression of scxa was significantly decreased in scxa-1compared to the scxa-1. Due to the loss of scxa, other tendon marker genes (tnmd, mkxa, tnc) also showed lower expression level in scxa-1(Fig. 6). However, the expression of scxb had no obvious changes between scxa-1and scxa-1. FGF subfamily genes, fgf4, fgf5, fgf8b, fgf12a/b, exhibited different expression levels: fgf4 and fgf8b expression was lower in scxa-1, while fgf12a showed higher expression in scxa-1. Runx2a/2b, related with osteoblast differentiation, did not show significant expression difference between wild and mutant fish. The key genes involved in bone mineralization (col1a1a
,col1a2
,sp7
,entp5
) showed lower expression level inscxa
-1, while the expression ofenpp1
andcol1a1b
was higher inscxa
-1. BMP-Smad signaling molecules (bmp2a
/2b
,bmp4
,bmp9
,smad1
,smad5
/8
andsmad4
) exhibited lower expression inscxa
-1, while expression ofbmp6
was higher inscxa
-1. The expression patterns of genes involved in tendon differentiation and IB development in response to function loss ofscxa
is shown in Fig. 7.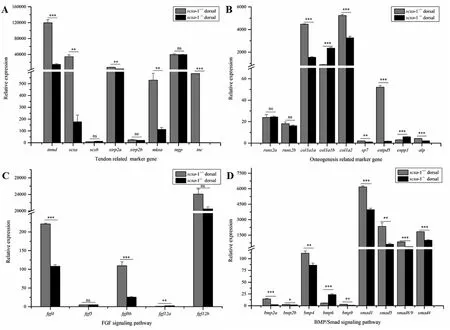
Fig. 6.Quantitative expression analysis of IBs development in the dorsal tissue of zebra fish scxa-1-/-mutant and wild individuals. (A) Relative expression of tendon related genes; (B) Relative expression of osteogenesis related genes; (C) Relative expression of FGF superfamily genes; (D) Relative expression of BMP/Smad signaling molecules. Ns indicates no significant differences; * indicates significant differences at p < 0.05; ** indicates significant differences at p < 0.01; ***indicates significant differences at p <0.001.
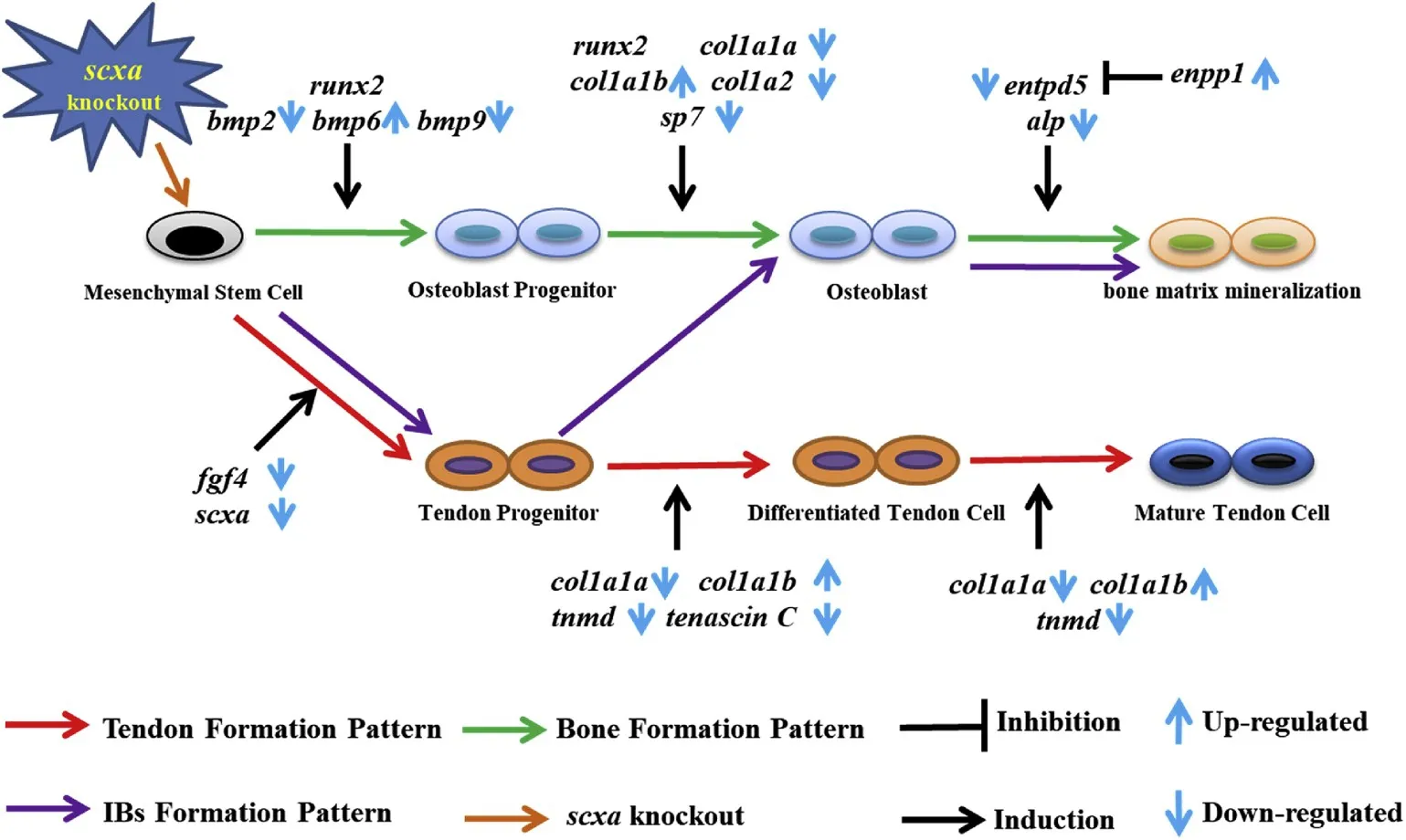
Fig. 7.The expression pattern of related genes involving tendon differentiation and IB development in response to function loss of scxa. Red, green, and purple arrows indicate the process of corresponding physiological functions, and black arrows and symbols indicate the induction or inhibition of specific processes by the corresponding genes. The direction of the blue arrow indicates the expression change of the corresponding gene in IB development after the scxa function loss. (For interpretation of the references to colour in this figure legend,the reader is referred to the Web version of this article.)
4.Discussion
IBs have attracted scientific attention both from the evolutionary aspect, and from the aspect of the improvement of aquaculture and customer preferences. Previous reports identified individuals without IBs in natural populations that predominantly had IBs (Perazza et al.,2017), and showed that a reduction in the number of IBs via genetic improvement is feasible (Xiong et al., 2019). Phylogenetic tree showed thatscx
genes in fishes were divided into two clades, corresponding to fishes with IBs and those without. Moreover,scxa
andscxb
in fishes with IBs were divided into two clades, which showed thatscx
genes may be closely associated with the evolution of the IB phenotype. Positive regulatory impact ofscx
on tendon differentiation by promoting type I collagen expression (L′ejard et al., 2007; Murchison et al., 2007) and the potentially differentiated relationship between IB and tendon (Danos &Ward, 2012) gave us reasons to speculate thatscx
plays a role in the regulation of differentiation and development of IBs. To test this hypothesis, we constructedscx
gene knockout lines and conducted phenotypic analyses in this study.Unlike mammals, cursorial birds, amphibians and some higher teleosts, which have only onescx
gene, zebra fish and some fish species with IBs in phylogenetic tree have two scx orthologous genes, scxa and scxb. This provides an excellent material to explore the evolution and functional differentiation of fish genes. Previous genome replication studies have shown that the main fate of copies of genes may be lost,silenced, sub-functionalized, or even acquire new functions (Lynch &Force, 2000; Zhou, Wang & Gui, 2006). Herein, the expression of scxb was tested in scxa-1and scxa-1and the result showed that the scxb had a lowest expression between scxa-1and scxa-1compared to other genes, which indicated that scxb may lost its function during genome replication. The FSGD ( fish-specific genome duplication) led to the formation of scxa and scxb in zebra fish. Although the proteins encoded by the two genes have nearly the same number of amino acids(scxa, 199aa; scxb, 200aa), their significantly different mRNA sizes(scxa, 1432bp; scxb, 607bp) may indicate their functional differentiation. Subsequently, this was confirmed by the distinct skeletal phenotypes in scxa-1and scxbmutants. scxa mutants with mutation sites in front of and behind the bHLH domain showed significant phenotypic changes (scxa-1) and no phenotypic change (scxa-2), respectively, which indicates that phenotypic difference between scxa-1and scxa-2mutants may be due to structural changes in the bHLH domain.Previous studies mainly focused on the function of scx in tendon development, repair and differentiation in mammals and some fish species. For instance, intermuscular and force-transmitting tendons in the limbs and tail tendons became hypoplastic in scxmice (Murchison et al., 2007). In zebra fish, both scxa and scxb mRNAs were detected in tenocytes at the junctions between skeletal elements and muscles, while scxa is the predominant scx gene expressed at embryonic and juvenile stages (Kague et al., 2019). Our results showed that scxa-1mutants exhibited a significant change in the number of IBs and ribs, with normal survival and reproduction ability. The total number of IBs was reduced by about 70%, which not only confirmed the role of scxa in skeletal development in zebra fish (Kague et al., 2019; Murchison et al., 2007),but also offered clues for genetic improvement of economic fish with IBs.Rib defects were observed at early developmental stages, before IBs formed, and the number of ribs was reduced by about 57% in the scxa-1. Meanwhile, the scxbwithout any observed phenotypic skeletal changes, also verified loss of function of this gene in fish skeletal development observed before (Kague et al., 2019). This indicates that functional studies of scxa in zebra fish may help elucidate the role of scx in the evolution of vertebrates.
In addition to the significance of the scxa-1for the study of gene function evolution and genetic improvement of the IB trait mentioned above, the construction of IB-less mutant lines also provides us with excellent material to reveal the molecular mechanism of IB development in fish. The comparative transcriptome analysis and qPCR results showed that the function loss of scxa lead to a significant change in the expression of tendon-related genes, such as tnmd, mkx, etc., which play an important role in the differentiation and maturation of dense connective tissues, including tendons and ligaments (Dex, Lin, Shukunami& Docheva, 2016; Docheva, Hunziker, F¨assler & Brandau, 2005; Ito et al., 2010; Shukunami et al., 2006, 2018). In addition, lower expression level of scxb in scxa-1mutants indicated the absence of expression compensation effects, which makes us speculate that there is functional divergence between scxa and scxb. In agreement with previous studies, the expression of some key genes related to osteogenesis was also changed significantly as expected, such as collagens (Gistelinck et al., 2016), entpd5a (Huitema et al., 2012), etc. However, there was no significant change in the expression of runx2, the key gene for skeletal development (Yang et al., 2011), so its role in the intramembranous ossification pattern of IBs needs further verification. In addition, we also studied the expression patterns of genes involved in FGF and BMP-Smad signaling pathways. Previous studies have revealed that FGF ligands and signaling molecules play key roles in tendon induction and differentiation (Brent et al., 2003, 2004; Tozer et al., 2005) and BMP signal has widely recognized roles in osteoblast differentiation, bone formation,skeletal development, disease and repair (Chen Deng & Li, 2012; Salazar, Gamer & Rosen, 2016). The qPCR analysis in this study showed that the expression of fgf4 and fgf8b was significantly decreased in scxa-1(Fig. 6C), and the expression levels of BMP ligands and BMP-SMAD signaling molecules also significantly changed (Fig. 6C).Unfortunately, we did not corroborate these data at the protein level, so this needs be conducted in future studies. Taken together, the results reveal not only regulatory roles of these genes in the development of IBs,but also indicate that the formation of IBs is a result of a variety of physiological processes, including differentiation and mineralization of tendons (Fig. 7).
In conclusion, this study for the first time revealed the important role of scxa in the development of fish IBs. The potential regulatory mechanisms of related genes and signaling pathways during the IB development were also discussed. The significant reduction of IB count in zebra fish caused by the loss of scxa function provides a new window of opportunity for genetic improvement of IB in cultured fish species.
CRediT authorship contribution statement
Chunhong Nie: Methodology, Data curation, Formal analysis,Writing - review & editing. Shiming Wan: Methodology, Data curation,Formal analysis, Writing - review & editing. Yulong Chen: Methodology, Data curation, Formal analysis, Validation. Dejie Zhu: Methodology, Validation. Xudong Wang: Methodology. Xiaoru Dong:Methodology. Ze-Xia Gao: Funding acquisition, Project administration,
Writing - review & editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31872559), National Key Research and Development Program (Grant No. 2018YFD0900102), Modern Agriculture Industry Technology System Construction Projects of China titled as-Staple Freshwater fishes Industry Technology System (Grant No. CARS-46-08), Fundamental Research Funds for the Central Universities (Grant No. 2662018PY035) and State Key Laboratory of Developmental Biology of Freshwater fish (2018KF003).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aaf.2020.04.006.
 Aquaculture and Fisheries2021年2期
Aquaculture and Fisheries2021年2期
- Aquaculture and Fisheries的其它文章
- Editorial: Global fish passage issues
- Analysis of the impacts of socioeconomic factors on hiring an external labor force in tilapia farming in Southern Togo
- Using Bayesian Bio-economic model to evaluate the management strategies of Ommastrephes bartramii in the Northwest Pacific Ocean
- Endosymbiotic pathogen-inhibitory gut bacteria in three Indian Major Carps under polyculture system: A step toward making a probiotics consortium
- Expression of multi-domain type III antifreeze proteins from the Antarctic eelpout (Lycodichths dearborni) in transgenic tobacco plants improves cold resistance
- A chromosome-level genome assembly of the red drum, Sciaenops ocellatus
