Chemical renal artery denervation with appropriate phenol in spontaneously hypertensive rats
Ming WANG, Wen-Zheng HAN, Min ZHANG, Wei-Yi FANG, Xin-Rong ZHAI,
Shao-Feng GUAN1,2, Xin-Kai QU1,2
1Department of Cardiology, Huadong Hospital Affiliated to Fudan University, Shanghai, China
2Shanghai Key Laboratory of Clinical Geriatric Medicine, Shanghai, China
3Department of Cardiology, Shanghai Chest Hospital, Shanghai Jiaotong University, Shanghai, China
Abstract Objective To explore the effectiveness of renal denervation (RDN) on blood pressure with the appropriate dosage of phenol/ethanol solution in spontaneously hypertensive rats (SHRs). Methods RDN was performed on the bilateral renal artery. Forty SHRs were divided into four groups according on the dosage of phenol (10% phenol in absolute ethanol): sham group, 0.5 mL phenol group, 1 mL phenol group and 1.5 mL phenol group (n = 10 in each group). Blood pressure was measured by tail-cuff plethysmography. Plasma creatinine was determined four weeks after the treatment. The kidneys and renal arteries were collected and processed for histological examination. Results A sustained decrease in systolic blood pressure (SBP) was only observed after the application of 1 mL phenol for four weeks, while SBP was lowered during the first week after RDN and increased in the following three weeks in the 0.5 mL and 1.5 mL phenol groups compared with the sham group. Renal norepinephrine (NE) was significantly decreased four weeks after RDN in the 1 mL and 1.5 mL phenol group compared with the sham group, but not in the 0.5 ml group. RDN with 1 mL phenol obviously reduced glomerular fibrosis. Histopathological analysis showed that tyrosine hydroxylase immunoreactivity was lower in the 1 mL and 1.5 mL phenol groups compared with the sham group. Moderate renal artery damage occurred in the 1.5 mL phenol group. Conclusion Chemical denervation with 1 ml phenol (10%phenol in absolute ethanol) effectively and safely damaged peripheral renal sympathetic nerves and contributed to the sustained reduction of blood pressure in SHRs.
Keywords: Hypertension; Norepinephrine; Phenol; Renal denervation; Spontaneously hypertensive rats
1 Introduction
Hypertension is a highly prevalent chronic condition across the world and contributes to heart failure, stroke, chronic kidney disease and even death.[1,2]The total number of adults with hypertension in 2000 was estimated at 972 million,which is predicted to increase to 1.56 billion in 2025.[3]By using three or more antihypertensive agents, one of which is a diuretic, 8.9% of the population in the United States achieve a goal of blood pressure (BP) < 140/90 mmHg,[4]but these people may have drug resistant hypertension.
Recently, renal sympathetic denervation (RDN) has become a promising strategy for the treatment of resistant hypertension.[5–9]The SYMPLICITY HTN-1 trial and the HTN-2 trial have found that percutaneous renal artery ablation with catheter-based radiofrequency energy can effectively reduce arterial blood pressure in humans.[5,6]RDN not only lowers BP, but also plays a pivotal role in disorders with sympathetic hyperactivity, including tachyarrhythmia,heart failure, insulin resistance and chronic kidney disease.[10–13]Nonetheless, the negative result of the first prospective, randomized, SYMPLICITY HTN-3 challenged the feasibility of RDN.[14]There are several possible reasons for this negative result. On one hand, catheter-based RDN and chemical-based RDN are blind procedures because the accurate interprocedural makers of success have not been identified yet. On the other hand, incomplete interruption of renal nerves with RDN may have led to the negative result.[15]RDN has not been abandoned yet.[16]Therefore, it is necessary to establish a stable RDN model to exploit the effect of RDN in the treatment of hypertension.
There are several RDN approaches, such as cryoablation,[17]intravascular or extracorporeal ultrasound[18,19]and local application of a chemical or neurotoxin.[20–26]
In 1997, Muhlbauer, et al.[27]applied a 10% phenol/ethanol solution for 2 min to make an RDN model. In 2004,Luippold, et al.[28]used the same method to establish the RDN rat model again. However, a commonality of these two studies and previous research was the lack of a set dose of phenol.[29,30]More importantly, the details of the experimental procedure was unclear. Thus, it is necessary to explore the details of the RDN rat model and determine the best volume of phenol.
The details of the method of chemical corrosion of renal sympathetic nerve with a mixture of phenol and ethanol are unclear, including the ablation criteria and ablation time, in several previous studies,[25,26,28,31–33]contributing to different results. Our study aimed to develop a model with spontaneously hypertensive rats (SHRs) that permitted the quantification of RDN by delivering different dosages of phenol-ethanol to local renal artery.
2 Methods
2.1 Ethics statement
All procedures were performed following the relevant ethical standards of the National Institute of Health (NIH Publications No. 8023, revised 1978) and were approved by the committees on animal research of Huadong Hospital affiliated to Fudan University.
2.2 Animals and protocol
Forty male SHRs were used at the age of 12 weeks(240–260 g) from Vital River Laboratory Animal Technology Company (Beijing, China). All animals were randomly divided into four groups: sham group, 0.5 mL phenol group(denervation with 0.5 mL 10% phenol in absolute ethanol),1 mL phenol group and 1.5 mL phenol group (n = 10 in each group). BP was measured while the rats were conscious by tail-cuff plethysmography (BP-2000 Blood Pressure Analysis System II, America) at baseline and every week after surgery. Body weight was measured every week.Four weeks later, plasma was collected after anesthetization with sodium pentobarbital (100 mg/kg, i.p.). Then the animals were killed. After perfusion with 0.9% saline solution intracardially, the right renal artery and the kidney were harvested for histological analysis.
2.3 Renal artery denervation
Animals received general anesthesia with sodium pentobarbital (100 mg/kg, i.p.). After the abdomen was opened with an incision in the dorsal midline, renal arteries and veins were identified after isolation of the surrounding connective tissue and periadventitial fat. Phenol (10% phenol in ethanol) was applied to the surface of the renal artery (from renal hilus to the abdominal aorta) with a cotton swab and a small paint brush over 2 min. Then, vessels were washed with saline before closing the skin of the abdomen (Figure 1).In the sham group, saline was used to proceed to renal artery denervation instead of phenol. After surgery, rats were placed on warm pads until fully recovered.
2.4 Evaluation renal norepinephrine (NE)
Kidney samples were quantified using a commercial ELISA kit (Beijing FuRuiZe Biological and Technology Company, China).

Figure 1. Images of RDN surgery. (A): The right renal artery and kidney are exposed; (B): the right renal artery is isolated, and the periadventitial fat and connective tissues are removed; (C): phenol was applied to the surface of the renal artery with a small paint brush from the renal hilus to the abdominal aorta over 2 min. RDN: renal sympathetic denervation.
2.5 Histological examination and immunohistochemistry
Renal arteries and kidneys were fixed with 4% paraformaldehyde, dehydrated, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin (HE) and periodic acid-Schiff (PAS). Renal nerves received HE staining and immunohistochemical staining for tyrosine hydroxylase(TH, Abcam, Cambridge, UK).
Renal fibrosis was evaluated as described.[34]The percentage of the fibrous area was calculated by Image-Pro Plus 6.0. The intensity and distribution of TH staining was assessed by a semiquantitative scoring system: 0 = no reaction, 1 = patch/very weak reaction, 2 = weak to moderate reaction, and 3 = strong reaction. The renovascular damage was also scored by an ordinal semiquantitative grading system: 0 = no injury (no endothelial loss or medial change), 1= minimal injury (< 25% vessel circumference in endothelial loss and < 25% of media thickness in depth in medial change), 2 = mild injury (25 to <50% vessel change), 3 =moderate injury (50 to < 75% vessel circumference in endothelial loss and 50 to < 75% of media thickness in depth in medial change), and 4 = severe injury (≥ 75% vessel circumference in endothelial loss and ≥ 75% of media thickness in depth in medial change or injured media thickness <50% of unaffected media).[35]
2.6 Statistical analyses
All data are presented as the means ± SE. Comparisons between groups were determined by one-way ANOVA with the Bonferroni posthoc test using SPSS 22.0. Differences were considered statistically significant at a value of P < 0.05.
3 Result
3.1 Survival characteristics of rat model
One rat died five days after surgery in the 1.5 mL phenol group because of severe synechia of abdominal tissues and intestinal obstruction.
3.2 BP and body weight
The one-week follow-up showed systolic blood pressure(SBP) was significantly lower in the 0.5 mL phenol, 1 mL phenol and 1.5 mL phenol groups. In the following three weeks, SBP was comparable between the 0.5 mL phenol and sham group (P > 0.05), while the 1.5 mL phenol group had higher SBP than the sham group (P < 0.05 at 16 weeks).In contrast, SBP in the 1 mL phenol group was significantly reduced compared to the control over the whole four weeks after the surgery (P < 0.01 each week, Table 1).
Compared with the sham group, RDN did not affect body weight in the 0.5 mL, 1 mL or 1.5 mL group throughout the following four weeks (Table 2).
3.3 Renal NE and plasma creatinine
Renal NE in the 1 mL phenol and 1.5 mL phenol groups was markedly decreased four weeks after the surgery compared to the sham group and 0.5 mL phenol group (P <0.01). There no significant differences in plasma creatinine between any groups (Table 3).
3.4 Histological examination
Obvious injury to nerves was caused by RDN in the 1 mL phenol and 1.5 mL phenol groups, while the sham group and 0.5 mL phenol group showed almost intact renal nerves (Figure 2). The TH semiquantitative score was significantly decreased in the 1 mL phenol and 1.5 mL phenol groups compared to the other two groups (P < 0.01, Table 3).Compared with the sham group, renal fibrosis was attenuated by RDN in the 1 mL group, but there were no differences among the sham group, 0.5 mL group and 1.5 mL group (Figure 3). These results demonstrate that 0.5 mLphenol was not enough to destroy the sympathetic nerve and that chemical ablation had little influence on renal function.Significant vascular intimal hyperplasia was observed in the renal artery in the 1.5 mL phenol group. In contrast, the sham group, 0.5 mL phenol group and 1 mL phenol group had a normal renal artery (Figure 4). Renal artery damage score was obviously increased in the 1.5 mL phenol group compared to the other three groups (P < 0.01, Table 1),which means it might be excessive to ablate the renal nerve chemically with 1.5 mL phenol.
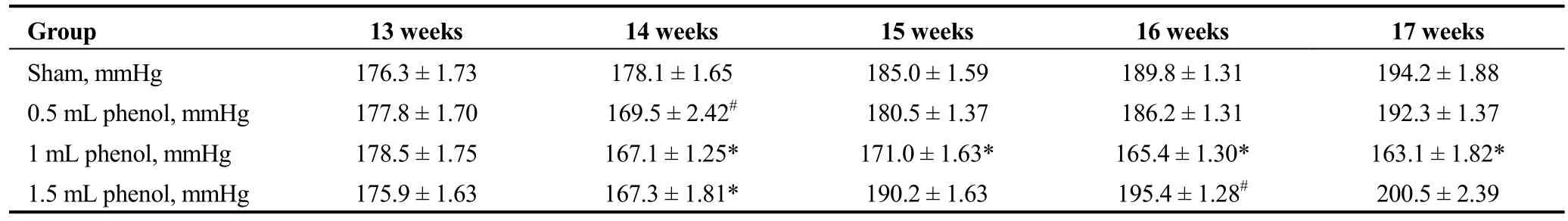
Table 1. Effect of RDN on systolic blood pressure.
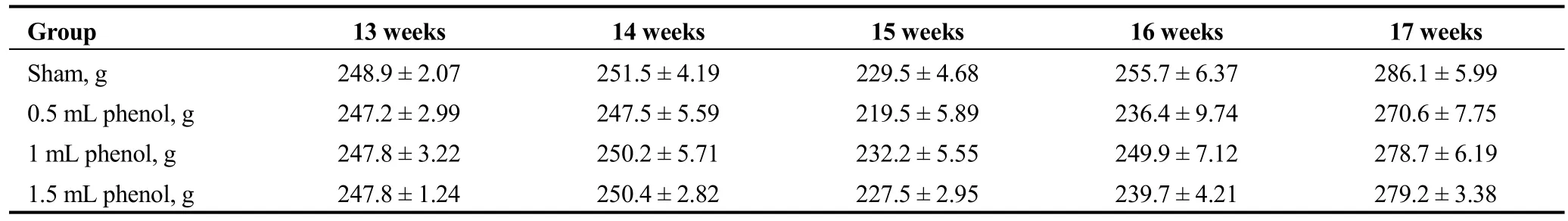
Table 2. Effect of RDN on body weight.
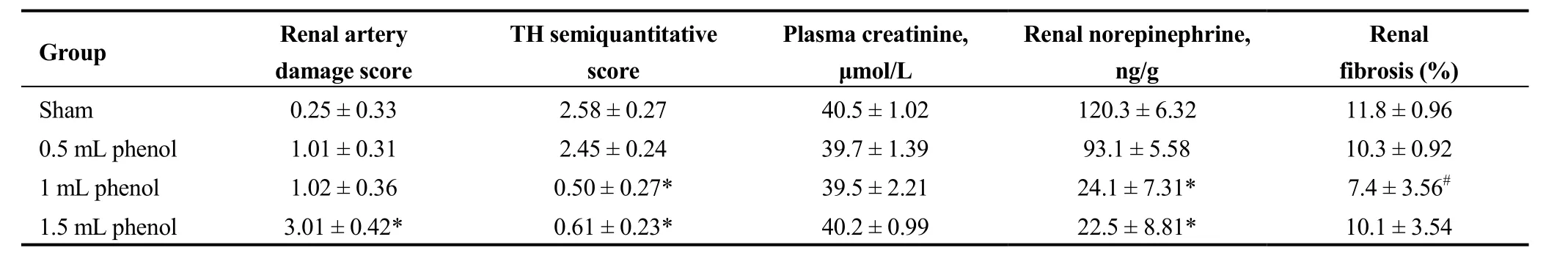
Table 3. Effect of RDN on renal artery damage score, TH score, creatinine, renal norepinephrine and renal fibrosis.
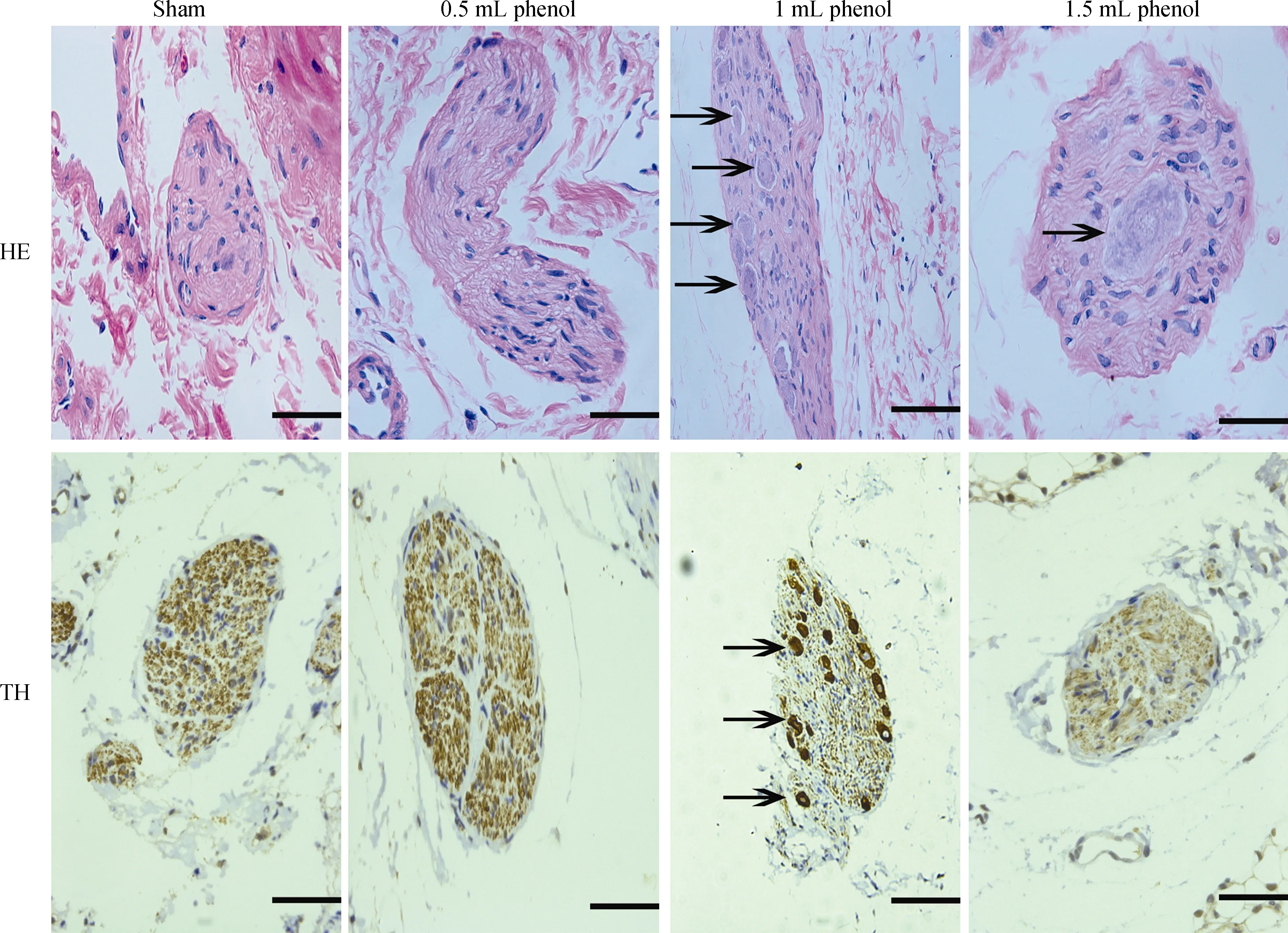
Figure 2. Representative images of renal nerves with HE and TH staining four weeks after RDN or sham operation. Fragmented and unclear nuclei (arrow), × 400. Scale bar = 50 μm. RDN: renal denervation.
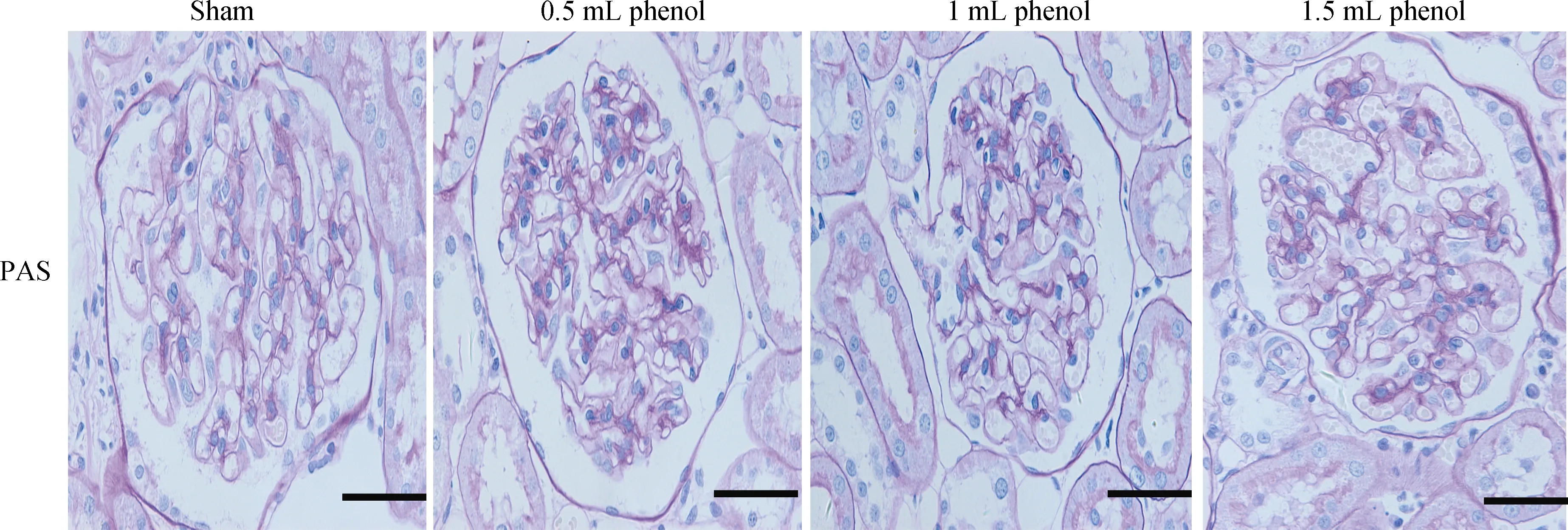
Figure 3. Representative images of sections of kidney in PAS staining (× 400). Scale bar = 50 μm. PAS: periodic acid-Schiff.
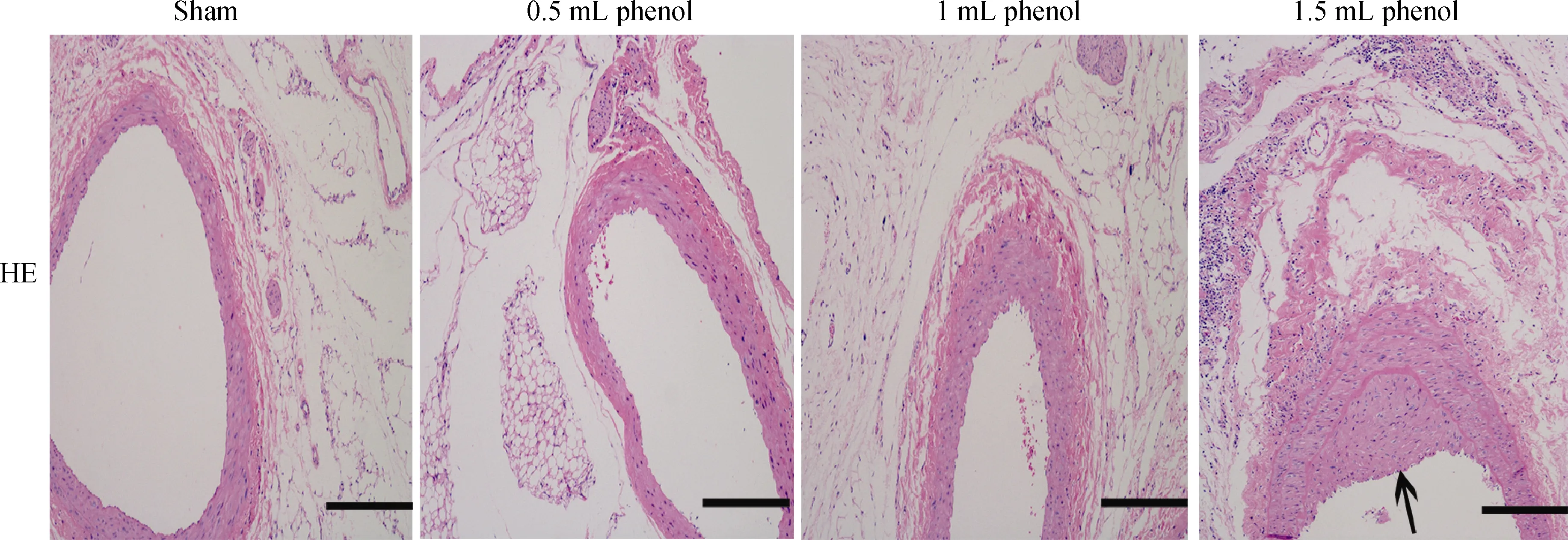
Figure 4. Representative images of renal artery in HE staining (× 100). Scale bar = 200 μm. A thickened intima-media layer was observed in the 1.5 mL phenol group (arrow).
4 Discussion
The major findings of our study are: (1) the RDN SHR model was successfully established with 1 ml phenol/ethanol solution; and (2) we found a precise dose of phenol that could attenuate renal fibrosis and effectively and safely lower SBP, while excess phenol could cause renal artery damage and intestinal obstruction.
As a method for chemical corrosion of the renal sympathetic nerve with a mixture of phenol and ethanol, the details of ablation were unclear, including the ablation criteria and ablation time in a large number of previous studies,[25,26,28,31–33]contributing to different results. In pilot experiments, we observed a phenomenon of severe intestinal obstruction when applying a 10% phenol/ethanol solution for 2–5 min and a 20% phenol/ethanol solution for 2 min. Therefore,consistent with previous research,[27–29]we adopted a 10%phenol/ethanol solution for 2 min in our experiment.
In this study, we developed the RDN rat model with 1 mL phenol/ethanol solution and validated the RDN model in several ways. First, as one of the major models of hypertension, SHRs normally have high blood pressure during the first 10 weeks after birth. The development of hypertension in SHRs is similar to essential hypertension in humans.[36]In previous research, renal denervation has been effective in the treatment of refractory and drug-resistant hypertension in human.[6,37]It can lead to a decrease in blood pressure in SHRs.[38–41]In line with these studies, we found reduced in blood pressure in all treatment group within one week after the surgery. However, SBP kept decreasing only in the 1 mL phenol group and not in the 0.5 mL and 1.5 mL phenol groups in the following three weeks.
Second, as an accurate index of renal sympathetic nerve activity, increased renal NE spillover occurs in hypertensive subjects.[42]Biochemical and pharmacological studies have shown that the neurotransmitter released by the renal sympathetic nerve is NE. Thus, there is a reduction of over 90%in renal tissue NE content after surgical section or destruction of the renal sympathetic nerve.[38,41,43]In this study,renal NE was significantly decreased after application of 1 ml phenol/ethanol to the isolated renal artery than sham group within four weeks, which was similar to the findings of Trostel and Osborn[21]and Katayama, et al.[38]and was consistent with the change in SBP.
Third, as the rate-limiting enzyme, tyrosine hydroxylase is involved in catecholamine synthesis within the postganglionic nerve terminals. It has been used as a marker of sympathetic innervation.[44,45]In 2010, Burgi, et al.[46]found that tyrosine hydroxylase immunoreactivity could be an indicator of sympathetic activity. Sakakura, et al.[35]adopted immunohistochemical staining of tyrosine hydroxylase to assess the ablation of the periarterial sympathetic nerve,because tyrosine hydroxylase converts tyrosine to L-DOPA.In this study, the result showed weaker reaction of TH staining in the renal nerve in the 1 mL and 1.5 mL phenol groups than the sham group and 0.5 mL phenol group.These results indicated that 1 mL and 1.5 mL phenol, but not 0.5 mL, can effectively destroy the renal nerve and decrease sympathetic activity.
There was a slight reduction of renal NE in the 0.5 mL phenol group compared to the control group. The response to TH staining of renal nerves in 0.5 mL phenol was the same as the sham group. Therefore, 0.5 mL phenol was not enough to destroy the renal nerve. In contrast, there was a marked reduction of renal NE and a weaker reaction to TH staining in the 1.5 mL phenol group than the sham group,which means 1.5 mL phenol indeed does work. There was obvious injury of the renal artery on HE staining in the 1.5 mL phenol group, which may have been related to the increase in SBP over the following three weeks. Additionally,no obvious glomerulosclerosis was found in any group.More importantly, compared with the control group, application of 1 mL phenol/ethanol relieved glomerulosclerosis.Although phenol is a toxic compound, it has little influence on renal function.
Renal nerve regeneration can occur after RDN. Sakakura,et al.[47]found that focal nerve regeneration at the sites of radiofrequency ablation occurred approximately 60-180 days after the surgery in a swine model. Moreover, evidence from Rousselle, et al.[48]demonstrated that poorly organized neuromatous regeneration occurred as early as seven days after RDN with the same model. These results were different from ours, where no renal nerve regeneration occurred four weeks after the RDN. This might be associated with the method of RDN. Generally, RDN is done by radiofrequency ablation to large arteries in swine, sheep and dogs,[17,23,49]while it done chemically to small renal arteries in rats. RDN was performed on the intima of the renal artery in the former, while it was in the adventitia in the latter. Bai, et al.[50]found that the effect of RDN was more effective in adventitia than intima. Moreover, more phenol does not necessarily lead to better results. Similarly, it is crucial to choose the appropriate radiofrequency ablation energy or endovascular ultrasound nerve ablation energy to confirm the effect of RDN in clinical trials.
4.1 Limitation
The postoperative follow-up time was only four weeks,and the sample sizes were relatively small. The effect of long-term RDN on BP and the potential mechanism will be investigated in future studies.
4.2 Conclusion
RDN with 1 mL phenol/ethanol could effectively and safely lower BP. The efficacy of nerve ablation was determined by measuring renal NE depletion and the reduction in blood pressure, especially in histological examination of the renal nerve.
Acknowledgement
This work was partly supported by the National Natural Science Foundation of China (81370361), Science and Technology Commission of Shanghai Municipality (12140902800),Scientific and Technical Project of Shanghai Chest Hospital(2014YZDH20300).
 Journal of Geriatric Cardiology2018年11期
Journal of Geriatric Cardiology2018年11期
- Journal of Geriatric Cardiology的其它文章
- Atypical electrocardiographic manifestations of ischemia: a case of dynamic Wellens patterns
- Inoperable severe aortic valve stenosis in geriatric patients: treatment options and mortality rates
- Increased index of microcirculatory resistance in older patients with heart failure with preserved ejection fraction
- Prevalence of iron deficiency in patients aged 75 years or older with heart failure
- Frailty significantly impairs the short term prognosis in elderly patients with heart failure
- Association of invasive treatment and lower mortality of patients ≥ 80 years with acute myocardial infarction: a propensity-matched analysis
