Increased index of microcirculatory resistance in older patients with heart failure with preserved ejection fraction
Zhuo XU, Hui-Ping GU, Yang GU, Wei SUN, Kun YU, Xi-Wen ZHANG, Xiang-Qing KONG
1Department of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China
2Department of Cardiology, the Affiliated Huai'an No.1 People’s Hospital of Nanjing Medical University, Huai’an, China
Abstract Objective To study the coronary microvascular function in older patients with heart failure with preserved ejection fraction (HFpEF)using an invasive pressure–temperature sensor guidewire. Methods Patients undergoing echocardiography and cardiac catheterization examinations for exertional dyspnea and a positive stress test were retrospectively enrolled from January 2014 to November 2017, and were allocated into the control group or HFpEF group. The HFpEF group was secondary divided into two groups according to the age of 65.Comparing the clinical features and values obtained in examinations between the three groups, multivariate regression analysis was used to analyze the predictors of left ventricle end diastolic pressure (LVEDP). Results There were 87 patients enrolled in this study. The older HFpEF patients (n = 32) were more likely to be female; and had the most comorbidities, such as diabetes mellitus, atrial fibrillation, and chronic kidney dysfunction (CKD) with a low estimated glomerular filtration rate (eGFR), and had a similar hypertensive prevalence as the adult HFpEF group (n = 24), whose mean LVEDP and index of microcirculatory resistance (IMR) were highest in comparison to the adult HFpEF patients and controls (n = 31). The coronary flow reserve (CFR) in the older HFpEF and adult HFpEF groups was similarly reduced.In the regression analysis, the IMR linearly correlated to LVEDP, and was the only independent predictor of LVEDP. Conclusions An increased IMR and reduced CFR were characteristics of microvascular dysfunction in older HFpEF patients. The IMR independently had a positive linear correlation with LVEDP. Microvascular rarefaction might be a subsequent pathological progression in the development of HFpEF.
Keywords: Heart failure; Ejection fractions; Microcirculatory resistance; Microvascular dysfunction
1 Introduction
Heart failure with preserved ejection fraction (HFpEF)has been acknowledged as an entirely different type of heart failure (HF) in the past decade.[1]Exertional dyspnea is the typical symptom of HEpEF. Elevated diastolic left ventricle(LV) stiffness, higher filling pressure, impaired diastolic dysfunction and preserved ejection fraction (EF) are observed in cardiac imaging as the features of HEpEF.[2]In regard to comorbidities accumulating with age, the prevalence of HFpEF has surpassed that of heart failure with reduced ejection fraction (HFrEF) in an older population past age 65.[3,4]A favorable strategy for HFpEF treatment is one of the largest unmet needs. The achievement of neurohumoral inhibition treatment in HFrEF has not yielded beneficial results in the documented literature on HFpEF. Trials of pharmacologic agents based on the postulated mechanisms have had largely neutral results.[5]The pathology involved in HFpEF has not exactly been elucidated. Coronary microvascular dysfunction is probably a key driver in HFpEF development.[6]Microvascular rarefaction, interstitial fibrosis and myocardial hypertrophy were validated in older patients in reference to HFpEF in an autopsy study.[7]However, the in vivo microvascular function of HFpEF in older patients has not been quantitatively identified in the available literature.
The index of microcirculatory resistance (IMR) is calculated from values obtained using a coronary temperatureand pressure-sensing guidewire. Fearon WF, et al.[8]demonstrated that the IMR has a strong positive correlation with microvascular rarefaction induced by embolized microspheres. This parameter was proven to be quantitative, reproducible, specific, and independent from the epicardial stenosis severity, and was proposed for evaluating coronary microvascular function.[9]We hypothesized that coronary microvascular rarefaction in older patients with HFpEF increases coronary microcirculatory resistance. This study sought to quantitatively investigate the coronary microvascular function in older patients with HFpEF using an invasive coronary physiological examination in a cardiac catheterization laboratory.
2 Methods
2.1 Study population
This was a retrospective study. Patients complaining of exertional dyspnea and with a positive stress test were enrolled. Clinical features such as demographics, laboratory data and medications were documented. Transthoracic echocardiography and cardiac catheterization were performed during hospitalization in our division of cardiology from January 2014 to November 2017. Inclusion criteria were as follows: symptoms of HF, EF > 50% on echocardiography, stenosis of epicardial coronary arteries ≤ 50% on quantitative coronary angiography (QCA) and coronary flow fraction reverse (FFR) > 0.8. Exclusion criteria were prior myocardial infarction or angioplasty, severe valvular disease, cardiac transplantation, infiltrative disorders and constrictive pericarditis. All patients were allocated into the control group or HFpEF group according to a left ventricle end diastolic pressure (LVEDP) of 16 mmHg, and the patients in the HFpEF group were divided into two groups according to the age of 65 years.
2.2 Diagnosis of HFpEF
Our diagnosis of HFpEF was according to the criteria of the European Society of Cardiology,[10]as follows: clinical signs and symptoms of HF, preserved systolic function with EF > 50%, normal LV size (LV end-diastolic volume index(LVEDI) < 97 mL/m2), and LVEDP > 16 mmHg.
2.3 Echocardiographic examination
Doppler and tissue Doppler measurements were performed by a Siemens ACUSON SC2000 (Siemens AG,Germany) echocardiography machine with a full-volume transducer (1.5–3.5 MHz). Ventricular volumes and LVEF were calculated using the modified Simpson method.[11]Early transmitral velocity (E wave) was obtained by pulse wave Doppler at the tip of the mitral leaflet. Peak LV velocity (e’) was measured from the lateral and septal mitral annulus and was averaged. The E/e’ ratio was calculated as the E wave divided by the e’ velocity. LV mass was calculated using a linear method formula.[12]
2.4 Coronary angiographic, hemodynamic examination
Selective coronary angiography was performed using a standard procedure via radial access. Intracoronary nitrate(100 or 200 mg) was administered before the angiographic views. Quantitative coronary angiography was performed under optimal projections with a computer-assisted coronary angiographic analysis system (CAAS II/QUANTCOR;Siemens, Berlin and Munich, Germany)LVEDP was obtained during LV angiography using either a Judkins right or a pigtail catheter.
2.5 Coronary physiological examination
The FFR, coronary flow reserve (CFR), and IMR were measured as described previously.[13]After a 6F guide catheter engaged the coronary artery, a pressure-temperature sensor-tipped guidewire (Pressure Wire; Abbott Vascular,St. Paul, MN, USA) was introduced. Equilibration was fulfilled when the sensor was at the tip of the catheter. The pressure sensor was consistently positioned at the distal segment of a target vessel, intracoronary nitrate (100 or 200 mg) was administered before each measurement., and 4 mL of room temperature saline was briskly injected into the vessel three times to derive the resting mean transit time(Tmn) using a thermodilution curve. Hyperemia was induced by intravenous infusion of adenosine (140 mg/kg per minute) via a peripheral vein. Hyperemic proximal aortic pressure (Pa), distal arterial pressure (Pd), and hyperemic Tmn were measured during sustained hyperemia. FFR was calculated as the lowest average of three consecutive beats during stable hyperemia. CFR was calculated as the ratio of resting Tmn/hyperemic Tmn. The IMR was calculated using the equation Pd × Tmn during hyperemia.
2.6 Statistics
Normally distributed variables are expressed as the mean± SD, whereas non-normally distributed variables are expressed as the median [25th, 75thpercentile]. Categorical data are presented as numbers (percent). One-way ANOVA followed by Tukey’s post-hoc test was used to compare continuous variables between older HFpEF subjects, adult HFpEF subjects, and the control subjects, whereas categorical variables were compared using χ2test. Univariate linear regression was used to screen the variables related to LVEDP, and significant variables in the univariate linear regression and those affecting factors of LVEDP reported in the literature were included in the multivariable linear regression model to test the predictive power. The analyses were performed using SPSS version 20.0 (International Business Machines, Armonk, NY, USA). A 2-sided P <0.05 was considered to indicate statistical significance.
3 Results
kidney dysfunction (CKD) with a low estimated glomerular filtration rate (eGFR), was highest in the older HFpEF group, and the prevalence of hypertension was increased in the HFpEF cohort compared to the control group. The albumin to creatinine ratio (ACR) and B-type natriuretic peptide (BNP) levels were higher in the HFpEF cohort compared to the control group. The mean body mass index(BMI) of the adult HFpEF group was higher than that of the older HFpEF and control groups. 22/32 patients had poorer HF symptoms than NHYA Class III. The prevalence of chronic obstructive pulmonary disease and hyperlipidemia were not different between the groups. Hemoglobin was not significantly different between groups, and the finding was similar for medication use.
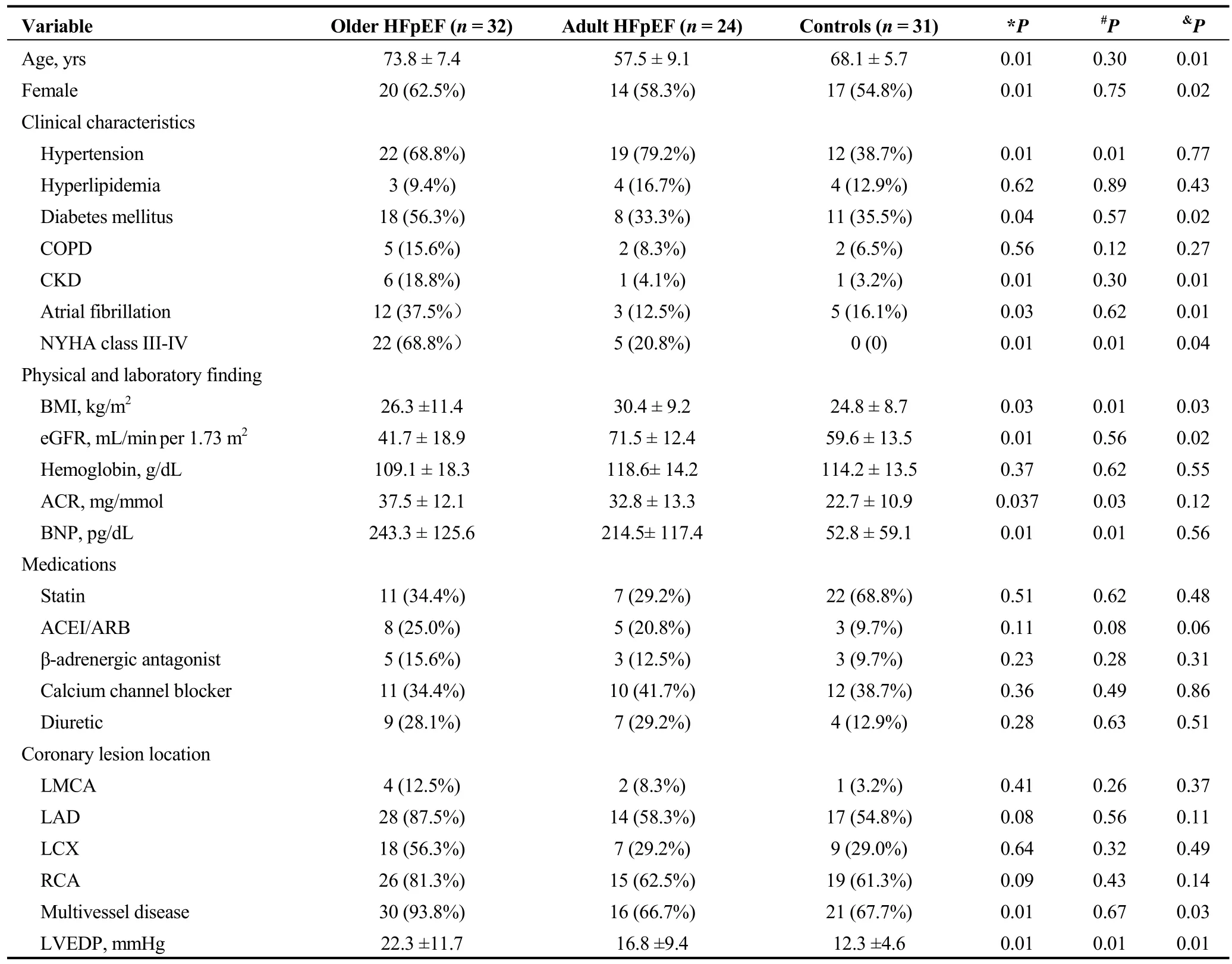
Table 1. Clinical features and angiographic findings.
3.1 Clinical characteristics and angiographic findings
There were 87 patients enrolled in this study; 31 patients with LVEDP ≤ 16 mmHg were allocated to the control group; and 56 subjects met the HFpEF diagnosis criteria, of which 24 patients were stratified into the adult HFpEF group according to age ≤ 65, and 32 patients were stratified into the older HFpEF group. The clinical features of all three groups are shown in Table 1. The mean age of the older HFpEF subjects was 73.8 ± 7.4 years, and 20/32 patients were female. The prevalence of comorbidities, including diabetes mellitus, atrial fibrillation, and chronic
Thirty of 32 older HFpEF patients presented with comorbid multivessel coronary atherosclerosis. Atherosclerosis affected nearly the same ratio of adult HFpEF and control subjects. The LVEDP obtained during LV angiography significantly increased in older HFpEF patients compared to that in adult HFpEF and control subjects.
4.2 Echocardiographic and coronary physiologic parameters
Parameters from the echocardiographic study are summarized in Table 2. No differences were found in LV EDVI,LV ESVI, LVEF or HR between the groups. The highest LVMI was 122.4 ± 26.7 g/m2in the adult HFpEF group,compared to 101.3 ± 28.9 g/m2in the older HFpEF group, P< 0.05, and 87.4 ± 17.6 in the control group, P < 0.05. The values of the LAD and E/e' ratio in the older HFpEF group were the highest of the three groups.
No difference was found in FFR between the groups.Resting mean transit time was significantly different by comparison between any two means, and hyperemic mean transit time was longer in the older HFpEF group than in the other two groups. The calculated CFR in both HFpEF cohorts was lower than in the control group, and no difference was found between the older and adult HFpEF groups. The mean IMR was significantly increased in HFpEF patients,and the highest average IMR was calculated in the older HFpEF group.
4.3 Uni- and multivariate linear regression analyses to examine independent factors of LVEDP
Resultsof the regression analyses are showed in Table 3.In the entire study population, the univariate linear regression analysis showed that age, BNP and IMR had significant relationship with LVEDP. A scatter plot of IMR and LVEDP is shown in Figure 1, depicting a linear relationship between IMR and LVEDP (Y = 1.24.X + 5.11, P < 0.01).The multivariate linear regression analysis showed that the IMR was the only independent predictor of LVEDP.
4 Discussion
To our knowledge, this is the first study to quantitatively evaluate coronary microcirculation of older patients with HFpEF using an invasive pressure–temperature sensor guidewire. There were two main findings in this observational retrospective study: (1) compared to the control subjects without HF, microcirculatory resistance (measured by the IMR), was increased in patients diagnosed with HFpEF,especially in those over 65 years of age. Comorbidities,including diabetes mellitus, atrial fibrillation, albuminuria,CKD and hypertension were more frequently present in the older HFpEF population; (2) an increased IMR was significantly associated with elevated LVEDP, which is the parameter of diastolic dysfunction. The IMR was a significant and independent predictor related to LVEDP. These results indicate that an increased IMR might play an important role in the pathophysiology of HFpEF and might reflect the disease progress.
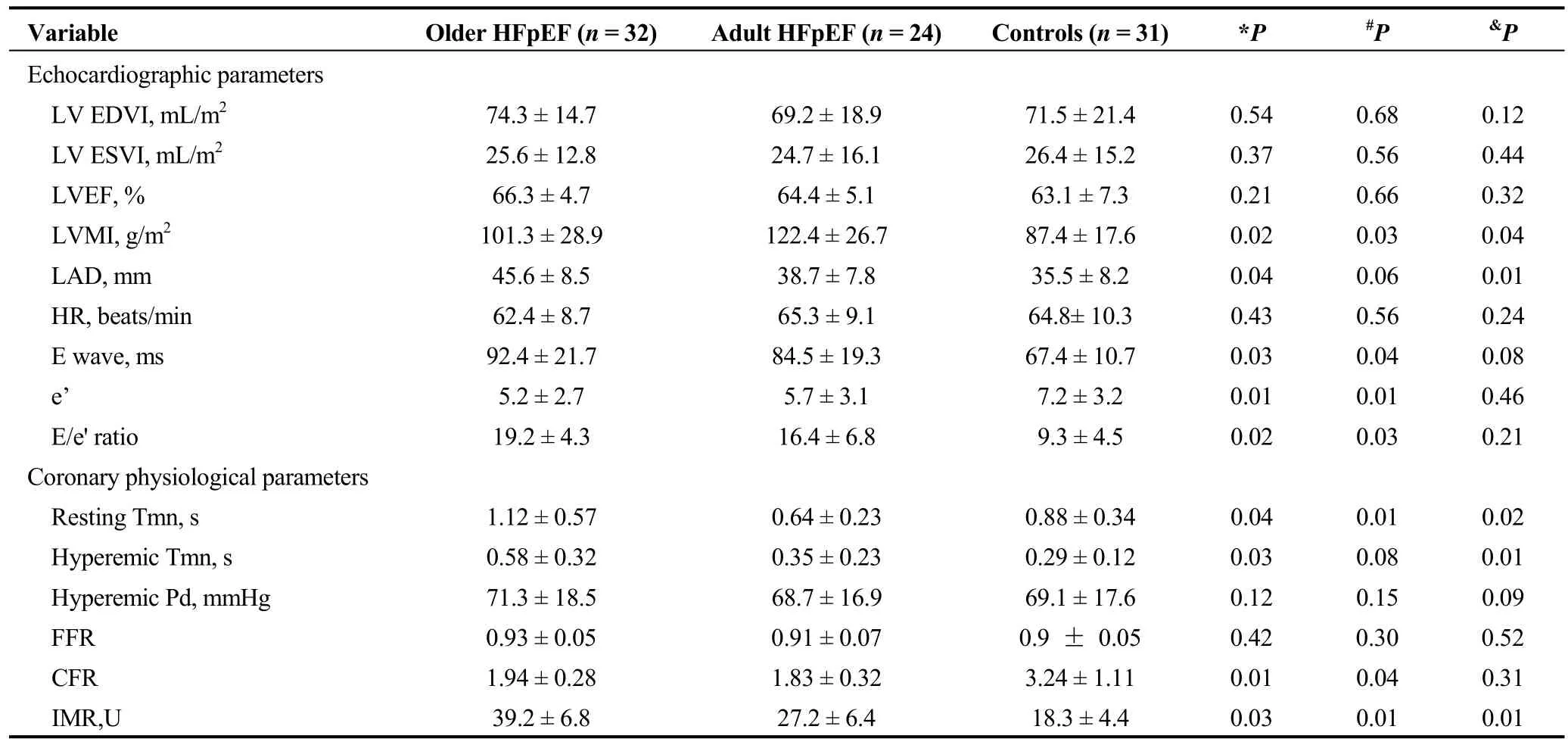
Table 2. Echocardiographic and coronary physiological parameters.

Table 3. Uni- and multi-variate linear regression analyses to examine independent factors for LVEDP.
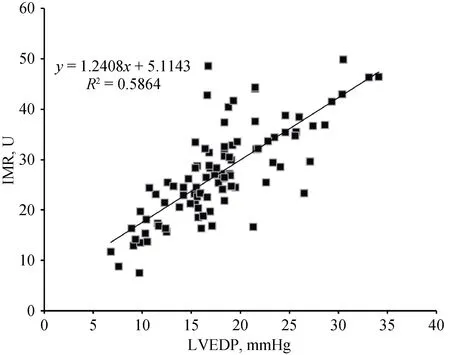
Figure 1 Scatter plot of IMR and LVEDP. IMR: index of microcirculatory resistance; LVEDP: left ventricle end diastolic pressure.
Increased microcirculatory resistance in HFpEF results from a sequence of events consisting of the following: (1)microvascular rarefaction increases microcirculation resistance and reduces coronary flow in states of rest and hyperemia.[8,14,15]In our study, both the rest and hyperemic Tmn in the older HFpEF group were longer than in the control group. Additionally, the IMR in HFpEF patients was significantly higher than in non-HF subjects, especially in the population older than 65. (2) Endothelial cells with deficient nitric oxide (NO) bioavailability fail to initiate a sufficient coronary microvascular vasodilator response induced by ATP,[12,14]thus microcirculatory resistance does not decline as briskly as in non-HF subjects. In our study, CFR,the ratio of resting and hyperemic Tmn, was decreased in HFpEF patients compared to non-HF patients.
The IMR has been proposed and validated as a simple and specific invasive method of assessing the coronary microcirculation,[8,9]which has been shown to have prognostic value for predicting the outcome of acute coronary syndrome interfered by angioplasty.[16–19]In a porcine model study, after microvascular obstruction induced by microsphere infusion into the coronary microvascular bed, the IMR and true microcirculatory resistance were both increased and significantly correlated in the percentage changes,[8]which is a fundamental proof of the effectiveness of IMR in the evaluation of microvascular density reduction.When compared to other noninvasive measures, the flow reserve of the coronary microvasculature was assessed by magnetic resonance imaging and positron emission tomography, and the IMR was equivalent for predicting the outcome of left ventricular function after primary angioplasty for acute coronary syndrome.[20-22]In spite of the similar decreased CFR, a higher IMR indicted poorer HF syndrome in older HFpEF patients, which was consistent with a previous finding of reduced CFR and an elevated IMR in an HFpEF population.[23]More information about microvascular function was gained by analyzing the components used to calculate CFR. Reduced resting Tmn in the adult HFpEF group can be explained by the fact that arterioles are regulated to increase the resting coronary flow to meet additional myocardial demands. Prolonged resting Tmn in the older HFpEF group may be explained by microvascular rarefaction in older patient accountings for the predominant reason for microvascular dysfunction and increasing microvascular resistance beyond coronary flow regulation,which may indicate an advanced stage of microvascular dysfunction.
A new paradigm for HFpEF development has been gradually accepted, which is that HFpEF originates from the comorbidities, which drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.[14]Acknowledged comorbidities, such as diabetes mellitus, atrial fibrillation, CKD, hypertension, overweight, and albuminuria were prevalent in the HFpEF cohort in our study, which was consistent with the available documents.[16,24,25]The Maastricht Study showed that microvascular rarefaction in skin was positively related to albuminuria in patients with type 2 diabetes, which implied that systematic capillary rarefaction involving the kidney increased intraglomerular pressure to promote albuminuria.Urine ACR was positively related to the diastolic dysfunction measured by the E/e’ ratio and caused adverse cardiovascular outcomes.[26]An increase in the E/e’ ratio and ACR was observed in the older HFpEF group in our study. Diabetes mellitus, atrial fibrillation, and CKD were more frequently present in the older HFpEF population, and significant multiple coronary atherosclerosis existed in the older HFpEF subjects; advanced age is probably the same independent predicting factor of HFpEF and these comorbidities.[27–29]Endothelial inflammation and myocardial fibrosis are the same mechanisms in atrial fibrillation and HFpEF development.[30]Atrial fibrillation also decreases exercise capacity and right ventricle function, finally increasing the risk of death. Obesity is an independent predictor for HFpEF. The mean BMI of the HFpEF cohort was higher than that of the control group, and the mean BMI of the adult HFpEF group was higher than that of the older HFpEF group. Central obesity might accelerate age-associated ventricular stiffening, especially in women.[27,28]Visceral adipose tissue is infiltrated by macrophages, which produces a systemic inflammatory state, increases nitrosative/oxidative stress, and therefore; limits NO bioavailability in both the heart and the vasculature.[31,32]Obesity also contributes to mortality, as evidenced from the U-shaped relationship in HFpEF between BMI and mortality.[33]
The downstream consequence of coronary microcirculation inflammation and dysfunction, cardiac hypertrophy and myocardial fibrosis, reduces compliance and increases LVEDP. The microvascular function was identified to be inversely related to the diastolic function. In a phase contrast cine-magnetic resonance imaging study, CFR was significantly decreased in HFpEF patients compared to hypertensive LVH patients and control subjects.[34]Even in a rest state, the TIMI frame count (TFC) and myocardial blush grade (MBG) of major coronary arteries free from stenosis in HFpEF patients was still longer than in non-HFpEF patients.[35]An observational study revealed that the index of LV mass was increased by 35% in older HFpEF patients versus age/sex matched healthy controls.[36]In our study, the IMR had a positive linear correlation with LVEDP, and the highest IMR was seen in older HFpEF patients with severe HF and elevated LVEDP and E/e’ ratio. To figure out the relationship between LVEDP and the parameters of coronary hemodynamics and structural echocardiography, a multivariate regression analysis was conducted, which revealed that the IMR was the only independent factor for LVEDP. The result was probably explained by the finding that microvascular density was inversely associated with myocardial fibrosis.[7]
The gender of HFpEF patients in an older population was likely to be predominantly female, due to their longer life expectancy[37]and distinctive myocardial remodeling response to afterload.[38]Comorbidities including advanced age were accumulated in this cohort, which stir up lowgrade systemic inflammation, the downstream response of microvascular dysfunction and myocardial stiffness that results in diastolic dysfunction.[14]Microvascular rarefaction indicated an advanced stage of microvascular dysfunction in older HFpEF patients, which was quantified by increased IMR and decreased CFR during an invasive coronary physiological examination. The LVEDP and E/e’ ratio reflected the ventricle stress and were significantly elevated in an older population with HFpEF. In the regression analysis,the IMR had an independent linear relationship with LVEDP. The IMR increased in older HFpEF patients, suggesting that microvascular rarefaction triggered by inflammation is likely to be a subsequent systemic histopathological progression toward promoting HFpEF.
4.1 Limitations
The single-center, cross-sectional, retrospective design was the main limitation of this study. A large-scale, multi-center study may provide stronger proof to validate our findings. In consideration of the impact of angioplasty on the IMR, subjects with severe coronary stenosis and an FFR< 0.8 were ruled out in our study; while older patients with HFpEF referred for coronary revascularization are very common,[39]the exclusion of them may bring selection bias.The idea of famale susceptibility to HFpEF is controversial;an age-matched control group is needed to confirm the gender difference in an older HFpEF population. The role of the IMR in predicting the outcome of HFpEF patients needs to be elucidated in a follow-up study.
4.2 Conclusion
In conclusion, microvascular dysfunction in older HFpEF patients was characterized as an increased IMR and reduced CFR. The IMR had an independent positive linear correlation with LVEDP. These results indicate that microvascular rarefaction might be a subsequent pathological progression in the development of HFpEF.
 Journal of Geriatric Cardiology2018年11期
Journal of Geriatric Cardiology2018年11期
- Journal of Geriatric Cardiology的其它文章
- Atypical electrocardiographic manifestations of ischemia: a case of dynamic Wellens patterns
- Inoperable severe aortic valve stenosis in geriatric patients: treatment options and mortality rates
- Chemical renal artery denervation with appropriate phenol in spontaneously hypertensive rats
- Prevalence of iron deficiency in patients aged 75 years or older with heart failure
- Frailty significantly impairs the short term prognosis in elderly patients with heart failure
- Association of invasive treatment and lower mortality of patients ≥ 80 years with acute myocardial infarction: a propensity-matched analysis
