Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
Zichao Hu,Chao Li,Dengfeng Zhang*
Faculty of Chemical Engineering,Kunming University of Science and Technology,Kunming 650500,China
State Key Laboratory of Shale Oil and Gas Enrichment Mechanisms and Effective Development,Beijing 100083,China
Keywords:Coal Sequestration Moisture Supercritical carbon dioxide Methane
ABSTRACT The interactions between CO2 and coals during CO2-ECBM (CO2 sequestration in deep coal seams with enhanced coal-bed methane recovery) could change pore morphology and chemistry property of coals,thereby affecting adsorption,diffusion and flow capability of CO2 and CH4 within coal reservoirs.To simulate CO2-ECBM process more practically,the dynamic interactions of supercritical CO2 (scCO2) and moisture-equilibrated coals were performed at temperature of 318.15 K,pressure of 12.00 MPa,and duration of 12.00 h.The impacts of the interactions on physicochemical properties of coals were investigated.Results indicate that scCO2/H2O exposure shows minor effect on micropores of coals.However,the exposure significantly decreases the mesopore surface area of bituminous coals,while increases that of anthracites.The mesopore volume and the average mesopore diameter of all the coals after scCO2/H2O exposure decrease.The multi-fractal analysis verifies that the scCO2 exposure can enhance the pore connectivity of various rank coals.Apart from the pore morphology,the exposure of scCO2/H2O also affects the oxygenic functional groups on coal surface.Particularly,the exposure of scCO2/H2O reduces the content of C-O and C=O of coals.The content of COOH of low rank coals including Hehua-M2# coal,Zhongqiang-4# coal,Buliangou-9# coal and Tashan-5# coal decreases,while the high rank Laochang-11#coal and Kaiyuan-9#coal witness a growth in COOH.The content of total oxygenic functional groups of all coals after interaction with scCO2/H2O decreases;on the contrary,that of C-C/C-H of all coals after scCO2/H2O exposure increases.In summary,the interaction with scCO2/H2O significantly changes the pore system and oxygenic functional groups of various rank coals,which needs further attention regarding CO2-ECBM.
1.Introduction
Human activities are producing ever-increasing amounts of carbon dioxide (CO2),a typical kind of greenhouse gas (GHG),which causes climate change issues.The statistical data from International Energy Agency (IEA) show that approximately 33.14 billion tons of CO2is released into atmosphere in 2018.Thus,reducing CO2emission is of great importance and urgency.Among a wide variety of CO2mitigation options,onshore geologic settings including depleted oil and gas reservoirs,oil and gas reservoirs,deep saline formations and unmineable coal reservoirs are suitable for safe and long-term CO2storage[1].Regarding sequestration of CO2into coal reservoirs,numerous investigations confirm that the adsorption ability of coal matrix is considered as the primary CO2trapping mechanism [2].Furthermore,the adsorption affinity between CO2and coals is greater than that betweenin-situcoalbed methane(CH4)and coals[3].Once CO2is compressed and injected into the target coal seams,and subsequently adsorbed on coal matrix,CH4is anticipated to be recovered.It is estimated that injecting the concentrated CO2into coal seams can store approximately 2.48 × 1014m3of CO2;meanwhile,it can recover approximately 5.00×1013m3of CH4around the world[4].Moreover,the main coal-production country,China in particular,is abundant in coal reservoirs with extremely lower permeability (9.87 × 10-8–9.87× 10-7μm2),thereby bringing huge difficulty to CH4production[5].It is reported that CO2-ECBM is capable of achieving higher CH4recovery,thereby gaining broad interest.
The practical CO2-ECBM process often involves complex interactions between multiple fluids,such as CO2,CH4and H2O,and coals[6].First of all,the coal matrix contains plenty of micropores and oxygenic functional groups,which accommodate the main adsorption sites for fluids[7,8].Besides the adsorption on pore surface of coal matrix,CO2can even penetrate and dissolve in coal matrix,thereby inducing coal matrix swelling [9].Next,the injected CO2could react with H2O to form carbonic acid (H2CO3),which probably leads to the dissolution and precipitation of minerals of coals [10,11].Finally,the injected CO2in the coal reservoirs with the recommended depth for CO2sequestration,i.e.800–1000 m[1],exists as supercritical fluid,which is of excellent diffusivity and dissolving capacity.Therefore,the supercritical CO2fluid(scCO2) is capable of extracting and mobilizing some smallmolecule organic compounds dispersing in coal matrix [12,13].The aforementioned coal matrix swelling,mineral dissolution and precipitation,and supercritical fluid extraction are able to alter pore morphology[14,15];permeability[15–17],surface chemistry property[18],strength of coals[19–21].Consequently,the adsorption,diffusion and flow of CO2and CH4within the coal seams could be affected,which will further impact CO2-ECBM.Therefore,the interactions between scCO2fluid and coals,and their effects on physicochemical properties of dry coals gain broad attention.Particularly,scCO2exposure changes the pore structure parameters of coals.It is found that the micropore structure of dry coals with middle and high rank degrades due to scCO2exposure (temperature (T) of 318.15 K,pressure (P) of 10.00 MPa,time duration (t)of 240.00 h),whereas the adverse trend is found for low rank coals;in addition,scCO2could also enhance the accessibility of macropore space within all rank coals [22].In addition,scCO2exposure(T=308.15–338.15 K,P=10.00 MPa,t=24.00 h) could decrease the mesopore surface area of two dry coals with lower rank,but increase that of one dry coal with higher rank.Instead,scCO2exposure(T=318.15 K,P=12.00 MPa,t=12.00 h)has no obvious influence on micropore structure of dry coals[23].Interestingly,scCO2-induced coal matrix swelling is capable of inducing fracturing of the mineral phase within the coal seams [15].On the other hand,scCO2exposure (T=318.15 K,P=12.00 MPa,t=12.00 h) could decrease the oxygenic functional groups of coals [23].Moreover,scCO2exposure (T=313.15 K,P=10.00 MPa andt=120.00 h)decreases the weak polar functional groups of coals,including lipid,epoxy and hydrocarbons compounds [18].
Given the practical coal seams containing a certain amount of moisture (H2O),researchers have begun to focus on the scCO2-H2O-coal system in recent years.Zhanget al.employed novel Xray micro-computed tomograph to study the H2O adsorption induced coal matrix swelling on the coal micro-structure.They conclude that approximately 80% of the cleats within coal matrix close due to H2O adsorption,while the change in cleats existed in the mineral phase is negligible.Similar to CO2-induced coal matrix swelling,the cleat closure due to H2O adsorption dramatically reduces porosity and permeability of coals[24].Duet al.indicate that scCO2/H2O binary exposure (T=318.15 K,P=10.00 MPa andt=240.00 h,andT=353.15 K,P=20.00 MPa andt=240.00 h,and deionized water volume of 600 ml)improves the pore fracture system and the permeability of coals;the increasing magnitude positively correlates with interaction temperature and pressure[10].They further confirm that the change of pore fracture system of coals mainly arises from dissolution of carbonate minerals of coals in H2CO3formed by scCO2and H2O.Liuet al.indicate that scCO2/H2O binary exposure (T≤ 373.15 K,P=40.00 MPa,t=240.00 h,and deionized water volume of 800 ml) increases gas adsorption volume,specific surface area and pore connectivity of coals [25].Liuet al.conclude that scCO2/H2O binary exposure(T=353.15 K,P=20.00 MPa,t=240.00 h,and deionized water volume of 300 ml) increases the volume of micropores with pore diameter less than 0.46 nm of high-volatile bitumite,but decreases that of both semi-anthracite and anthracite [23].However,the changing rule regarding the volume of micropores with pore diameter larger than 0.46 nm is reverse for high-volatile bitumite,and semi-anthracite and anthracite.They also point out that scCO2/H2O binary exposure increases the volume of macropores with pore diameter larger than 30 μm of bitumite and anthracite[26,27].Zhanget al.conclude that the interaction duration of scCO2/H2O binary exposure has a significant impact on permeability of high-volatile bituminous coal containing 2.5% (mass) moisture.Specifically,after 7.00 h scCO2exposure (T=313.15 K,P=8.00 MPa and confining pressure of 10.00 MPa),the permeability of coal reduces owing to coal matrix swelling phenomenon induced by CO2;instead,the permeability increases dramatically when the exposure duration is extended to 53.00 h.They attribute the increase in permeability to mineral dissolution and supercritical fluid extraction [28].Apart from the aforementioned negative effect of scCO2/H2O binary exposure on the permeability of coal,Zhanget al.point out that scCO2mixed with formation H2O can dissolve the calcite-fusinite mix phase within calcite-rich coals,thereby leading to an remarkable increasing absolute porosity and connectivity of coal [29].The aforementioned studies well address the influences of scCO2/H2O binary exposure on pore structure of coals.Apart from pore structure,the surface chemistry property of coals,functional groups in particular,still affects coal adsorption capability for CO2and CH4.However,the change of main functional groups on coal surface as a result of scCO2/H2O binary exposure is still ambiguous.Furthermore,CO2-ECBM is a dynamic process involving continuous CO2injection as well as CH4production.However,the existing investigations are often performed under static process,i.e.the interaction between scCO2,H2O and coals occurring in a closed high-pressure space.The dynamic interaction of CO2,H2O and coals and its effects on physicochemical properties of coals still need in-depth elucidation.Finally,previous studies often use scCO2blended with liquid water to simulate scCO2-H2O-coal system,which slightly differs from the practical moisture-containing coal reservoirs [25–27].To gain further insight into scCO2-H2O-coals system,the interactions of scCO2with six moisture-equilibrated coal samples are performed on Model SFE-500 extraction system.It is a dynamic CO2extraction apparatus,bridging the gap between laboratory study and practical CO2-ECBM.The pore morphology including micropores with pore diameter less than 2 nm,and mesopores with pore diameter ranging between 2 nm and 50 nm of coals before and after scCO2/H2O exposure is studied.Moreover,the changing rule regarding the main oxygenic functional groups on coal surface is analyzed.Finally,the effects of change regarding pore parameters and oxygenic functional groups of coals after scCO2/H2O exposure on the implementation of CO2-ECBM are addressed in this study.
2.Materials and Methods
2.1.Materials
In this study,six coal samples,i.e.Zhaotong coal,Tianji coal,Buliangou coal,Datong coal,Fumin coal and Yangquan coal,were collected from the typical Chinese coal reservoirs.The abovementioned coals are abbreviated with Hehua-M2# coal,Zhongqiang-4# coal,Buliangou-9#,Tashan-5# coal,Laochang-11#coal and Kaiyuan-9#coal,respectively.To avoid air oxidation,the coal samples were kept using plastic containers with inert helium inside [30].
The proximate analysis of coals was analyzed based on Chinese Standard GB/T 212-2008 [31].The equilibrium moisture content was analyzed based on Standard ASTM D1412/D1412M-19 [32].The maximum vitrinite reflectance of coals,designated asRo,max,was determined following Standard ASTM D2798-20[33].The analytic results are summarized in Table 1.The data of Zhongqiang-4#coal,Tashan-5#coal and Kaiyuan-9#coal were cited from our previous study[34].Hehua-M2#coal,Zhongqiang-4#coal,Buliangou-9#coal and Tashan-5#coal have higher volatile matter yield(VM)and lower fixed carbon(FC)content.Ro,maxof the above-mentioned coals ranges between 0.66% and 0.83%,which are classified into bitumite.Both Laochang-11#coal and Kaiyuan-9#coal have lower VM and higher FC.TheRo,maxof Laochang-11# coal and Kaiyuan-9# coal are 2.59%and 2.62%,respectively,and they are anthracite.
Table 1Property of studied coals
Given that the coal powder instead of coal plug helps enhance the interaction between scCO2and coal matrix,and thus all the coals were ground carefully and sieved to generate particles with diameter range of 125.00–150.00 μm.The image of the coal particle samples is displayed in Fig.1.Subsequently,the coal particles dried in a vacuum oven at 333.15 K for 72.00 h were used to prepare moisture-equilibrated coal samples.The dry coal samples were moved into a desiccator placed in an oven with temperature of 303.15 K.Given that coals saturated under relative humidity of 96.00% is extremely close to the occurrence state of coals in practical reservoirs [35],the desiccator was preloaded with saturated potassium sulfate (K2SO4) solution with the relative humidity of 97%.All the coals were completely saturated with moisture in the desiccator until reaching the moisture-equilibrated state.
2.2.Exposure of dynamic scCO2 to moisture-equilibrated coals
The interaction process of dynamic supercritical CO2fluid with different rank moisture-equilibrated coal samples was carried out on Model SFE-500 extraction apparatus manufactured by Thar Instruments Inc.,Pittsburgh,Pennsylvania,USA.The entire extraction system typically comprises a pressurization unit,an extraction unit and an extract collection unit (Fig.2).CO2with purity of 99.99% in volume fraction from gas cylinder was cooled down in a low-constant temperature bath.Subsequently,it was pressurized by P-series booster pump,which is able to generate continuous high-pressure CO2fluid with mass flow rate of 0–30.00 g·min-1and pressures up to 60.00 MPa.In order to prevent moisture loss from coal samples during the entire interaction process,a series III liquid injection pump with accuracy of 0.01 ml·min-1was used in this study to provide water with flow rate of 1 ml·min-1.The high-pressure CO2fluid and H2O were mixed in a T-connector and heated in a heat exchanger,and finally moved into the extraction chamber previously loaded with moisture-equilibrated coal samples.
An automated back pressure regulator valve connected with the outlet of extraction chamber maintains the pressure in the extraction chamber unchanged only with fluctuation range within±0.10 MPa.CO2after interacting with moisture-equilibrated coals as well as the dissolved coal-extracts was vented out from the extraction apparatus.The external surface of extraction chamber and collection chamber were wrapped with electrical heater,regulating temperature of the chambers within 298.15–423.15 K.It is worth noting that the performed extraction process is a dynamicprocess,where the high-pressure CO2is continuously generated and introduced into the extraction chamber to further interact with moisture-equilibrated coals inside.Therefore,the interaction process above is compatible with the practical ECBM process.
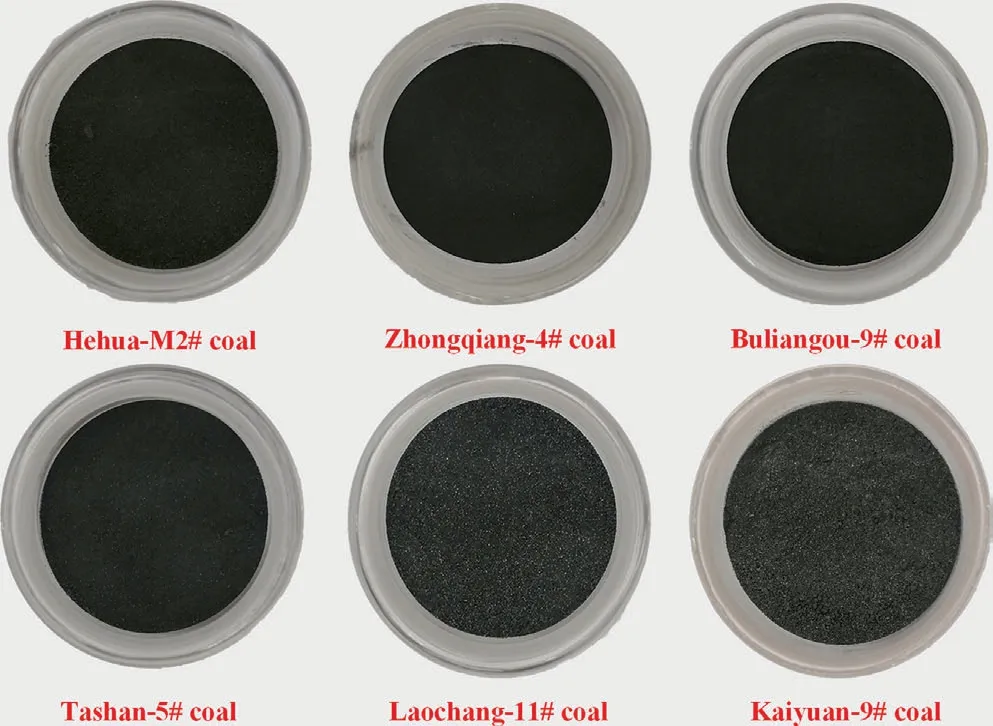
Fig.1.Images of coal samples.
With regard to CO2-ECBM,the recommended reservoir depth is between 800 and 1000 m,where the density of stored CO2is as high as approximately 500–700 kg·m-3[1].Combining the optimum depth and the geothermal gradient of the target coal seams,318.15 K was fixed as the interaction temperature between CO2and moisture-equilibrated coals.The pressure of the extraction chamber was maintained at 12.00 MPa.The mass flow rate of scCO2was set as 10.00 g·min-1.Previous work has revealed that 0.50–12.00 h is sufficient for the adsorption between CO2and coals to reach equilibrium state [36].Thus,the interaction duration regarding dynamic scCO2and moisture-equilibrated coals was determined as 12.00 h.(100 ± 0.0005) g of moisture-equilibrated coals were weighted for each scCO2/H2O exposure.
2.3.Pore structure analysis
The pore classification standard,i.e.micropores with pore diameter less than 2 nm,and mesopores with pore diameter range of 2–50 nm,raised by International Union of Pure and Applied Chemistry (IUPAC) [37],is adopted in present study.The ASAP 2020 Physisorption Analyzer,provided by Micromeritics Instrument Corporation,U.S.A.,was adopted to determine micropore morphology of coals.Given that CO2molecules have stronger accessibility to micro-or ultramicro pores[38],CO2was selected as the molecular probe to measure micropore structure parameters of coals.The operating temperature of CO2adsorption is 273.15 K.Both the specific surface area and volume of micropores can be determined from Dubinin-Radushkevich (D-R) model [39].The average pore size was determined by Dubinin-Astakhov (D-A) model [39].The micropore size distribution(PSD)profile was obtained by Non-Local Density Functional Theory (NLDFT) model [40].
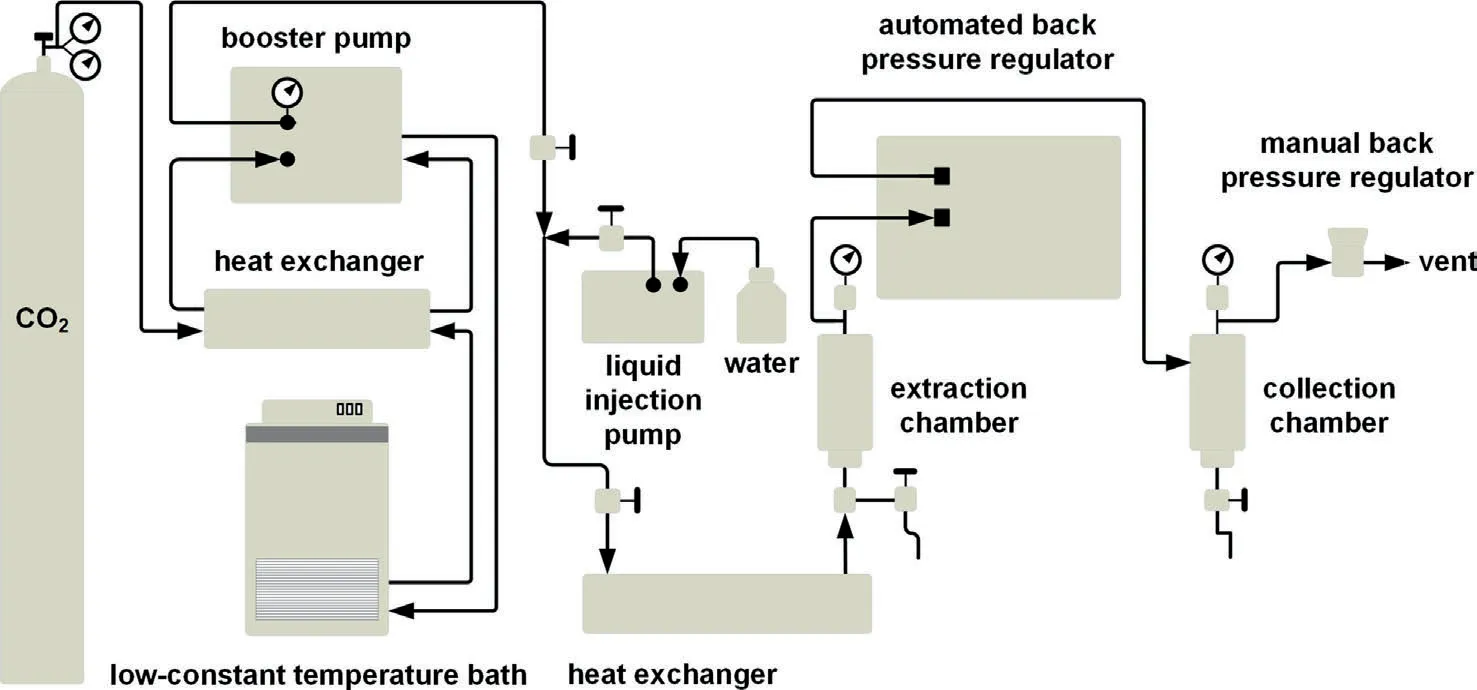
Fig.2.Schematic diagram of Model SFE-500 extraction system selected for interaction of dynamic scCO2 exposure with moisture-equilibrated coals.
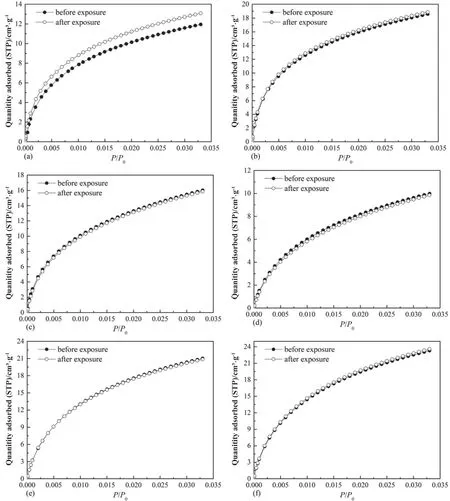
Fig.3.CO2 adsorption isotherms of coals, T=273.15 K,(a) Hehua-M2# coal,(b) Zhongqiang-4# coal,(c) Buliangou-9#,(d) Tashan-5# coal,(e) Laochang-11# coal and (f)Kaiyuan-9# coal.
The estimation of mesopore structure parameters of coal samples was also carried out on ASAP 2020 Physisorption Analyzer.Nitrogen (N2) was selected as molecular probe.The adsorption and desorption isotherms of N2corresponding to 77.00 K were collected at relative equilibrium pressure(P/P0)range of 0.005–0.996.The mesopore surface area was generated by Brunauer-Emmet-Teller (BET) model in terms of the adsorption data withinP/P0range between 0.05 and 0.30 [41].The mesopore volume was determined from Barrett-Joyner-Halenda (BJH) model using the entire desorption data [42].The average mesopore size was calculated by the total pore volume and surface area.The PSD profile of mesopores of coals was generated by DFT model and the adsorption data [43].
To enhance the reliability of pore structure analysis,all the coals were degassed at temperature of 353.15 K,and extremely lower vacuum pressure of 0.1333 Pa to completely remove residual gas in coals prior to pore morphology analysis.
2.4.Mineral analysis
The mineral characteristics of coals prior to and after scCO2/H2O exposure were estimated by X-Ray diffraction (XRD) characterization.The detail operation parameters and procedures could be found in Ref.[44].
2.5.Oxygenic functional group analysis
The main oxygenic functional groups on moisture-equilibrated coal samples prior to and after scCO2exposure were determined by X-ray photoelectron spectroscopy (XPS) analysis.It was operated on Model PHI 5000 Versaprobe II (VP-II) XPS analyzer(ULVAC-PHI,Inc.,Japan) equipped with Al Ka X-ray source.The parameter setting and operating procedure could be found in our previous study [34].
3.Results and Discussion
3.1.Pore structure
The raw CO2adsorption isotherms are presented in Fig.3.The adsorbed quantity of CO2shows a concave trend with elevatedP/P0,and a limiting value at higherP/P0.Thus,in terms of the classification method proposed by Brunauer,Deming,Deming,and Teller (BDDT) [41],these isotherms fall into the category of Type I isotherm [37,41].In general,scCO2/H2O exposure shows little effect on CO2adsorption isotherms of coals except Hehua-M2#coal.
The raw adsorption and desorption isotherms of N2are displayed in Fig.4.It is found that N2adsorption isotherms of coals prior to and after interacting with scCO2/H2O belong to Type IV isotherm,suggesting that the exposure process cannot alter the adsorption mechanism between N2and coals.Moreover,the adsorption/desorption hysteresis,i.e.hysteresis loop assembled by adsorption branch and desorption branch,exists for all the coal samples.The N2adsorption and desorption hysteresis derives from the widely-reported capillary condensation[45].The shape of hysteresis loop can be employed to estimate pore shape of porous materials.Combing previous classification method of hysteresis loops [37],and the shape of hysteresis loops as displayed in Fig.4,the hysteresis loops of Hehua-M2# coal and Zhongqiang-4# coal with lower metamorphic degree are Type H1,indicating that these two coals are abundant in open cylindrical pores at both ends.However,the hysteresis loops of Buliangou-9#coal,Tashan-5# coal,Laochang-11# coal and Kaiyuan-9# coal belong to Type H3,indicating the plentiful slit-shaped pores.Compared with the raw coals,no distinct changes in the shape of hysteresis loops are detected for the coals after scCO2/H2O exposure,which suggests that scCO2/H2O hardly change the pore shape of coals.Unlike adsorption isotherm and pore shape,scCO2/H2O exposure significantly changes N2adsorption and desorption capability of all coals.
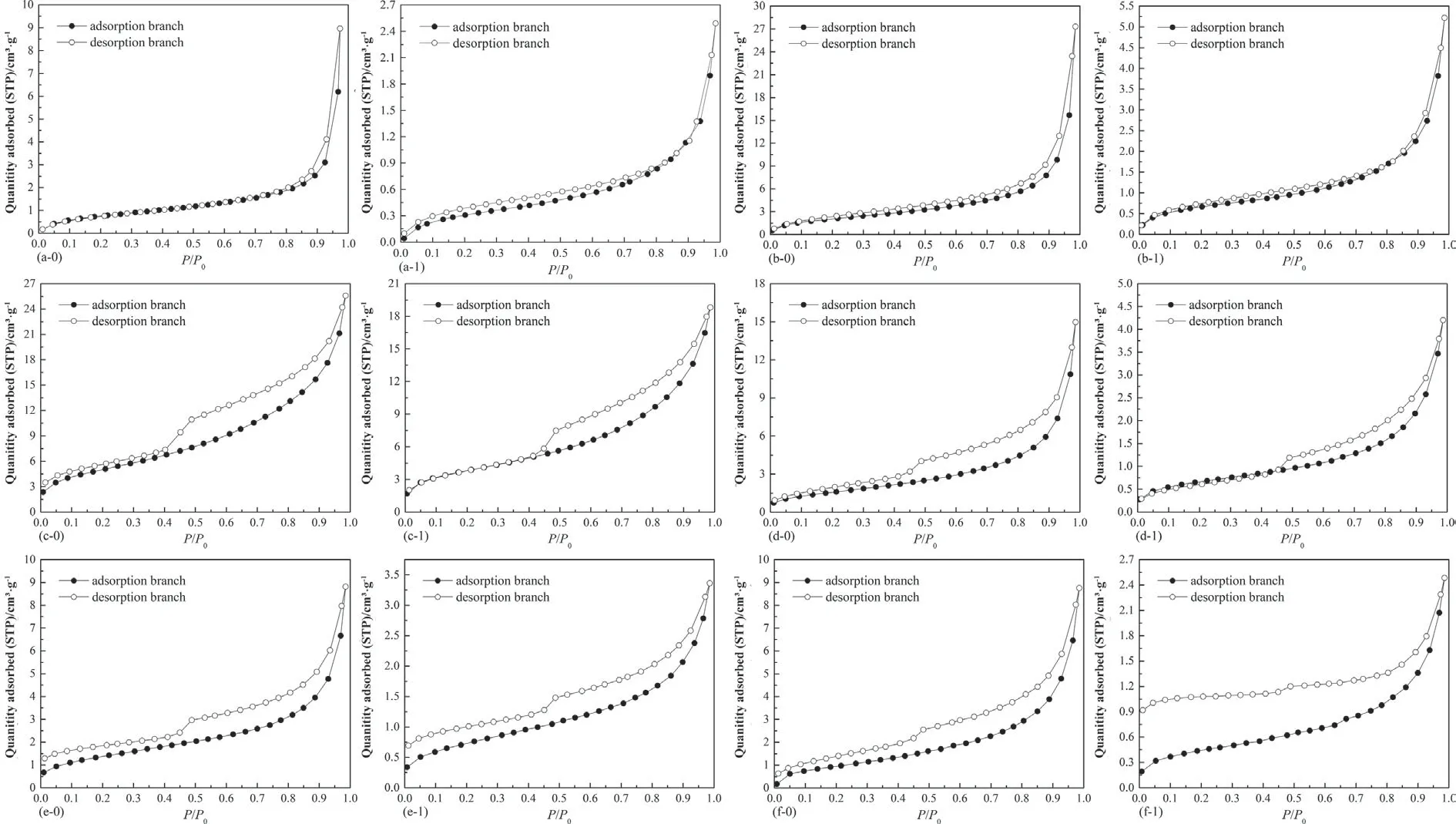
Fig.4.Adsorption and desorption isotherms of N2 on coals,T=77.00 K,(a-0)Hehua-M2#coal before exposure,(a-1)Hehua-M2#coal after exposure,(b-0)Zhongqiang-4#coal before exposure,(b-1)Zhongqiang-4#coal after exposure,(c-0)Buliangou-9#coal before exposure,(c-1)Buliangou-9#coal after exposure,(d-0)Tashan-5#coal before exposure,(d-1)Tashan-5#coal after exposure,(e-0)Laochang-11#coal before exposure,(e-1)Laochang-11#coal after exposure,(f-0)Kaiyuan-9#coal before exposure and(f-1) Kaiyuan-9# coal after exposure.
Based on the raw data of CO2adsorption and N2adsorption and desorption,and pore parameter analytical methods presented in Section 2.3,the main pore structure parameters can be generated.Fig.5 displays pore structure parameters of micro-and mesopores of coal samples.It is noticed that from Fig.5(a),(c) and (e) that scCO2/H2O exposure almost has no remarkable effect on micropore surface area(Smic),micropore volume(Vmic)and average micropore diameter(Dmic)of all the coals.The variation range regardingSmic,Vmic,andDmicis calculated as 0.21%-4.65%,0.29%-4.67%,and 0–3.7%,respectively.On the contrary,scCO2/H2O exposure significantly affects mesopore structure parameters of coals.Specifically,the mesopore surface area (Smeso) of bituminous coals after scCO2/H2O exposure,i.e.Hehua-M2# coal,Zhongqiang-4# coal,Buliangou-9# coal and Tashan-5# coal,decreases significantly.The decreasing magnitude is between 5.00%and 29.83%.However,Smesoof two anthracites,i.e.Laochang-11# coal and Kaiyuan-9#coal,after scCO2/H2O exposure increases by 23.67% and 17.47%,respectively.Moreover,both the mesopore volume (Vmeso) and the average mesopore diameter(Dmeso)of all the coals after scCO2/H2O exposure decrease by 20%–66.67%,and 0.93%–56.59%,respectively.In general,the gas transport capability increases with the increasing average pore diameter [46].With regard to CO2-ECBM,the decreasingDmesoof all the coals implies that scCO2/H2O exposure could probably lower the transport capability of CO2and CH4within coal seams,thereby showing negative influence to the implementing efficiency of CO2-ECBM.

Fig.5.Pore structure parameters of micro-and mesopores of coal samples,(a) Smic,(b) Smeso,(c) Vmic,(d) Vmeso,(e) Dmic and (f) Dmeso.
Fig.6 displays the PSD profiles of micropores of coals before and after scCO2exposure.In general,the PSD profiles of micropores of coals before and after scCO2exposure exhibit bimodal characteristic,mainly distributing in 0.40–0.70 nm and 0.70–0.90 nm.Moreover,scCO2/H2O exposure cannot affect PSD profiles of micropores of coals.Fig.7 displays PSD profiles of mesopores of coals before and after scCO2exposure.The PSD profiles of mesopores of six raw coal samples present two different patterns.For Hehua-M2#coal and Zhongqiang-4# coal with lower metamorphic grade,the distribution of their PSD profiles is broad,typically from 2 nm to 36 nm.By contrast,the PSD profiles of the other four coal samples with metamorphic grade higher than that of Hehua-M2# coal and Zhongqiang-4# coal are simple,which mainly distributes around 2–15 nm.The aforementioned patterns of the mesopore PSD profiles of different rank coals are probably related to coal compressibility.The enhanced coalification induces coal compressibility and further leads to the collapse of meso-and macropore system of coals[47].Thus,the PSD profiles of mesopores of coals with higher metamorphic grade seem rather simple than that of low rank Hehua-M2# coal and Zhongqiang-4# coal.Moreover,scCO2/H2O exposure has a significant impact on PSD profiles of mesopores.Particularly,scCO2/H2O exposure remarkably decreases the amount of pores with pore diameter ranging between 2 nm and 36 nm for low rank Hehua-M2# coal and Zhongqiang-4# coal;while the decreasing trend of mesopores of the other four coals is merely noticed between 2 nm and 5 nm.
The pore morphology of different rank coals is of higher heterogeneity and complexity[48–50],thereby requiring an effective tool to describe pore characteristics of various coals.The fractal theory based on fractal geometry is acknowledged as a viable option to study pore morphology of porous media.The fractal theoretic system consists of single fractal theory and multi-fractal theory.The single fractal model is known to only generate apparent information of the pore structure of coals [6].However,the multi-fractal theory can be adopted to divide a fractal material into many subregions with different degrees of singularity.Generally,the multi-fractal theory comprises singularity spectrum and generalized dimension [51].In this study,the generalized dimension is used to reveal pore characteristics of different coals due to its convenience and validity regarding fractal analysis.
The key point of multi-fractal theory is adopting mass probability to quantitatively describe local feature of porous media in different scales.With respect to multi-fractal analysis based on N2adsorption method,the relative equilibrium pressure (P/P0) is selected as intervalJ=[a,b].Accordingly,a series of boxes ofJwith an equal length (ε) are required.The dyadic scaling down algorithm is frequently employed to partitionJinto a number of boxesN(ε)=2kof box size,ε=L×2-k,wherek=0,1,2,3...,andLis the length ofJ.Aiming to ensure that the minimum box contains N2adsorption capacity,the maximumkis determined as 3.The mass probability (pi(ε)) of each box can be given through estimating N2adsorption capacity.whereNi(ε)is N2adsorption capacity of theith box(i=0,1,2,3...);Ntis the total N2adsorption capacity.
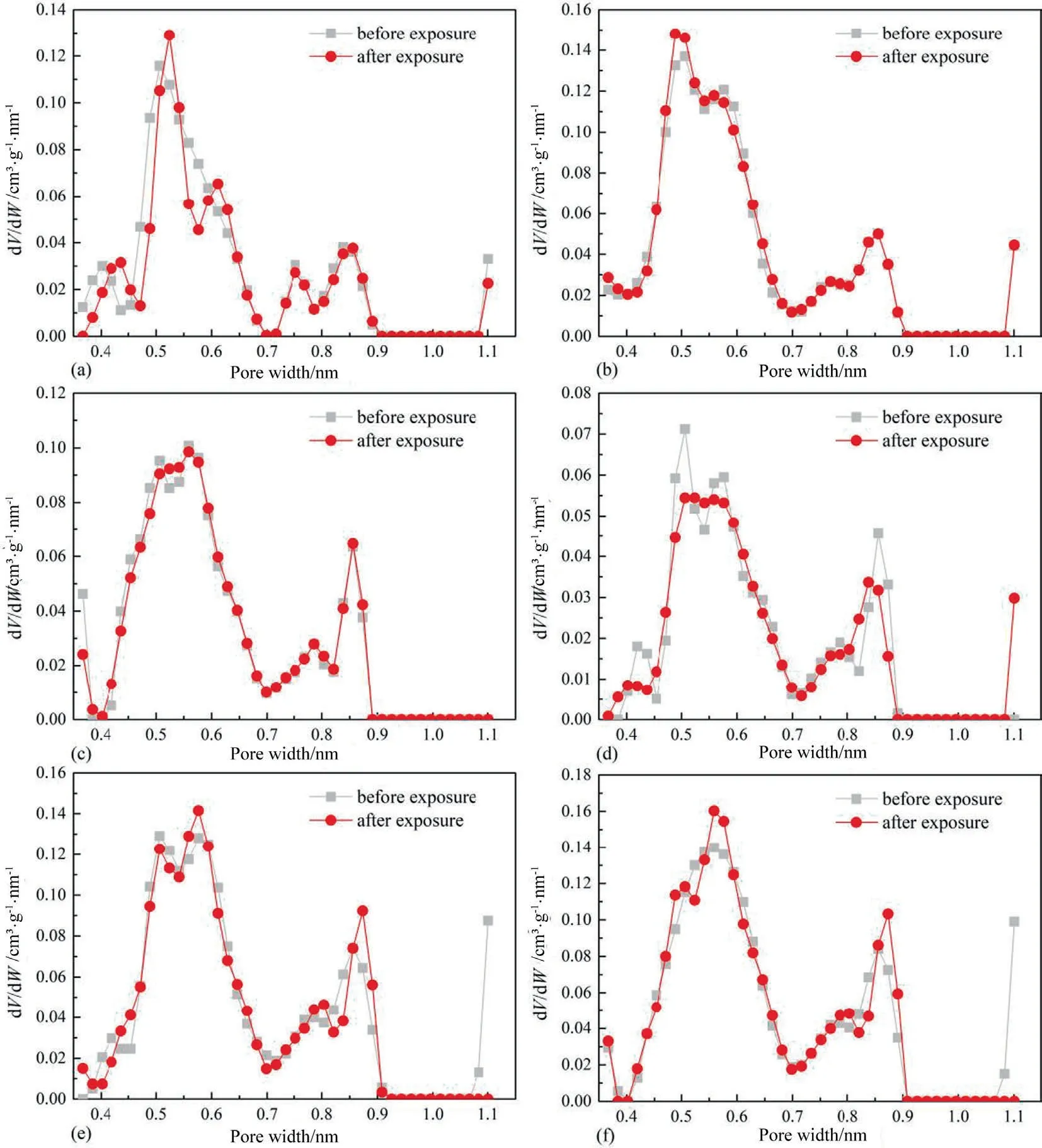
Fig.6.PSD profiles of micropores of coal samples,(a) Hehua-M2# coal,(b) Zhongqiang-4# coal,(c) Buliangou-9# coal,(d) Tashan-5# coal,(e) Laochang-11# coal and (f)Kaiyuan-9# coal.

The probability density distribution ofPiis analyzed by using the partition function χ(q,ε) calculated from Eq.(2).

in which the moment orderqis a real number ranging from-∞to+∞.In this study,the range ofqis between-5 and 5.For the case ofq?1,the value of χ(q,ε)is mainly dominated by small data ofPi(ε).Otherwise,the value of χ(q,ε) is greatly dominated by the large data ofPi(ε) [51,52].Thus,the multi-fractal theory is able to partition pore structure data into locally high or low concentration of porosity.

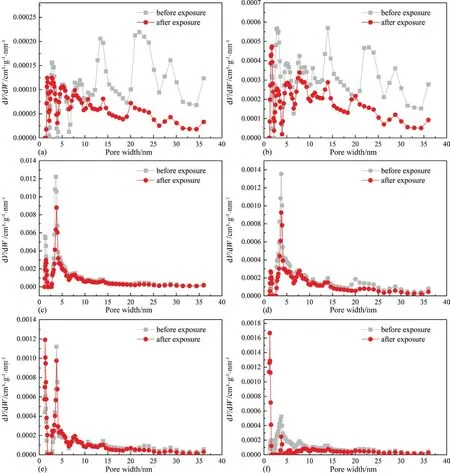
Fig.7.PSD profiles of mesoropores of coal samples,(a) Hehua-M2# coal,(b) Zhongqiang-4# coal,(c) Buliangou-9# coal,(d) Tashan-5# coal,(e) Laochang-11# coal and (f)Kaiyuan-9# coal.
The value ofDqatq=0,q=1 andq=2 represents the capacity dimension (D0),information dimension (D1),and correlation dimension (D2) of porous media,respectively.With regard to the single fractal theory,D0=D1=D2.By contrast,the order ofD0>D1>D2exists within the scope of multi-fractal theory.Particularly,theDqspectrum is a decreasing sigmoidal function ofq.
Fig.8 is the curve of lgχ(q,ε)versuslg(ε).The multiple correlation coefficients(R2)of all the curves are above 0.93,thereby indicating that the PSDs of coals possess multi-fractal characteristic[52].
Furthermore,as shown in Fig.9,theDqspectrum presents a decreasing sigmoidal shape with increasingq.Table 2 summarizes the results of generalized fractal dimension.It is found that the three types of fractal dimension follow the sequence ofD0>D1>D2,which is consistent with the existing knowledge regarding multi-fractal theory as previously mentioned.Among all the coals,the minimum value ofD-5–D5is found for Buliangou-9# coal,indicating that it has the lowest heterogeneity degree within different intervals [53];by contrast,the maximum value ofD-5–D5is found for Zhongqiang-4# coal,indicating its highest pore heterogeneity [53].With regard to coals after scCO2exposure,the value ofD-5–D5of coals except Buliangou-9# coal presents a decreasing pattern.This result confirms that the scCO2exposure enhances the pore complexity of Buliangou-9# coal,while reduces the pore heterogeneity of the remaining four coals.It is worth nothing that the value ofD-5–D5merely describes the heterogeneity of pore size of coals in general,which cannot deeply reveal the minor distinction in PSDs of coals with different pore sizes[50].Instead,theDqspectrum can be employed[54].Particularly,forq>0,theDqspectrum reflects the pore structure with large pore size.By contrast,forq<0,theDqspectrum reflects the pore structure with small pore size [51,54].Fig.9 shows that the values ofD0–D5andD-5–D0of all coals after scCO2exposure decrease.Thus,the scCO2exposure decreases the variability and heterogeneity in the inner pore size distribution of coals.
Table 2 indicates thatD0equals to 1 for all coals,which agrees with previous studies[54,55].Such result is due to the fact that the partition function χ(0,ε) equals to the number of boxesN(ε) [50].Moreover,scCO2exposure increases theD1of coals,and further implies that the pore homogeneous over the entire pore size distribution of coals is enhanced[51].Finally,theH,i.e.the autocorrelation of pores in different pore sizes [55],increases for all the coals after scCO2exposure.To sum up,the aforementioned multi-fractal analysis verifies that the scCO2exposure can enhance the pore connectivity of various rank coals.
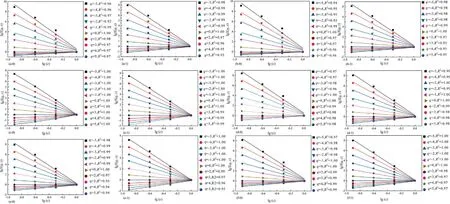
Fig.8.Plots of lgχ(q,ε) versus lg(ε)for PSDs of coals,(a) Hehua-M2# coal,(b) Zhongqiang-4# coal,(c) Buliangou-9# coal,(d) Tashan-5# coal,(e) Laochang-11# coal and (f)Kaiyuan-9# coal.
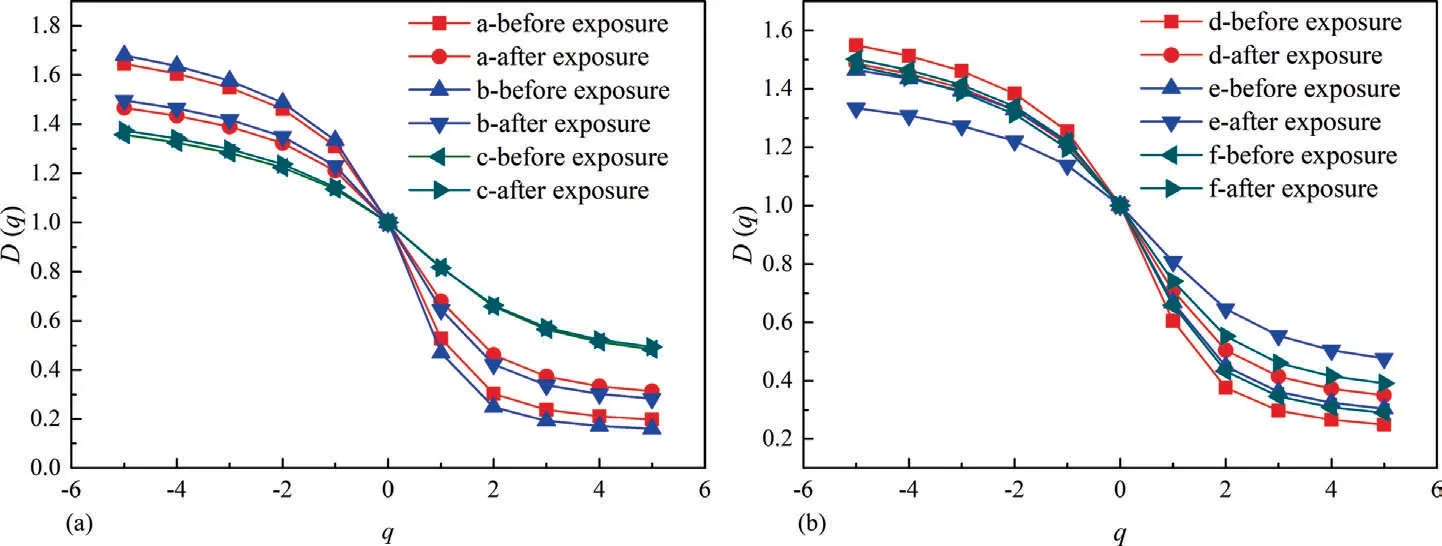
Fig.9.Relationship between D(q)and q,(a)Hehua-M2# coal,(b) Zhongqiang-4# coal,(c)Buliangou-9#coal,(d)Tashan-5# coal,(e) Laochang-11# coal and (f)Kaiyuan-9#coal.
scCO2/H2O induces coal matrix swelling,reducing surface area and volume of pores of coal matrix [56].Furthermore,interaction with H2O could induce clay mineral swelling.The clay minerals in coal typically comprise smectite (Mx[Si8]Al3.2Fe0.2Mg0.6O20(-OH)4),illite (Mx[Si6.8Al1.2]Al3Fe0.25Mg0.75O20(OH)4),and kaolinite(Al2Si2O5(OH)4),exist within coal.[57].The clay minerals have large pore surface area and exchangeable cations,such as Na+,K+,Mg2+and Ca2+,within interlamellar space of clay layers [58].Therefore,clay mineral in coal could combine H2O moleculesviaadsorption and exchangeable cationic hydration,thereby enlarging interlamellar gap between clay layers and finally inducing clay mineral swelling [59].Next,the extraction capability of scCO2/H2O can mobilize small-molecule organic molecules dispersing in coal matrix,which can alter pore structure of coals [12].Finally,the mineral dissolution and precipitation effects due to the reaction between inorganic minerals in coals and carbonic acid,formed by scCO2/H2O,change pore morphology of coals [60].It is noticed from Fig.10 that almost no clear change in ash content is found between coals prior to and after interacting with scCO2/H2O.Next,as shown in Fig.11,no obvious difference in X-Ray diffraction patterns is detected between coals prior to and after scCO2/H2O exposure.It is worth noting the reaction rate constants between various minerals and scCO2/H2O is extremely lower.For instance,the rate constant regarding quartz dissolved in H2O at 308.15 K is 1.1367 × 10-13mol/m2/sec [61].Thus,the mineral dissolution and precipitation effects due to scCO2/H2O exposure are always found at long interaction time,such as 72.00 h [62],or 240.00 h[63].Here,the interaction time between scCO2/H2O and coals is limited to 12 h,which is extremely shorter than previous literature[62,63].The mineral dissolution and precipitation effects are not significant in this study.Thus,the coal matrix swelling and the extraction capability of scCO2/H2O could probably account for pore morphology change of coals after interaction with scCO2/H2O.Specifically,the coal matrix swelling effect induced by CO2is pronounced for low rank coals [64].Although the migration of smallmolecule organic molecules due to the extraction capability of scCO2/H2O is helpful to increase the porosity of coals [11,65],the influence of CO2-induced coal matrix swelling on pore structure of coals is probably superior to small-molecule organic compound extraction effect.Thus,Smesoof low rank Hehua-M2# coal,Zhongqiang-4# coal,Buliangou-9# coal and Tashan-5# coal after scCO2/H2O exposure decreases.With regard to high rank Laochang-11# coal and Kaiyuan-9# coal,scCO2-induced coal matrix swelling is unremarkable compared with the extraction effect,which could account for the increase inSmeso.
3.2.Functional groups
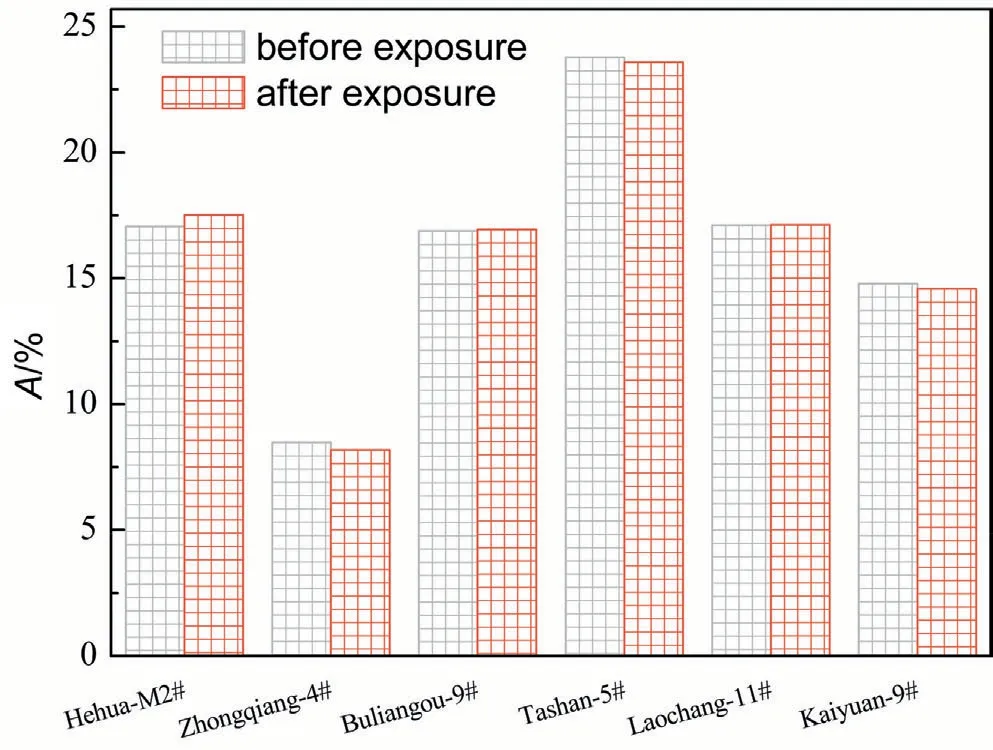
Fig.10.Ash (A) content of coal samples.
Apart from the pore structure parameters,the oxygenic functional groups of coals still affect fluid adsorption capability of coals[66].Given that scCO2/H2O can extract small-molecule organic compounds dispersing within coal matrix,and the chemical adsorption still exists between CO2and coals [67],scCO2/H2O exposure is capable of changing oxygenic functional groups on coal surface.Hence,XPS analysis was employed to estimate the change of oxygenic functional groups of coals after scCO2exposure.Fig.12 presents the observed C (1s) spectrum of each coal sample.The spectra of Zhongqiang-4#coal,Tashan-5#coal,Buliangou-9#coal and Kaiyuan-9#coal prior to scCO2/H2O exposure were cited from our previous study [68].The C (1s) spectra of coals are resolved into peaks with binding energy (BE) of 284.80 eV,286.30 eV,287.50 eV and 289.00 eV.These observed peaks indicate C-C,C-O,C=O and COOH,respectively [69].The observed C (1s) spectrum of each coal sample is resolved using Gaussian (80%)-Lorentzian (20%) function after deducting a Shirley background applying XPS Peak 4.1 software[68,70].The composition of different species of each coal sample generated from C (1s) spectral is tabulated in Table 3.The total content of the oxygenic functional groups including C-O,C=O and COOH decreases with the elevated coal rank.Such changing rule is consistent with the viewpoints of Xinet al.[71] and Liet al.[72].For high rank coals,they were always exposed to the higher reservoir temperature,hereby leading to a decomposition and conversion of the oxygenic functional groups.Moreover,the enhanced metamorphism increases the condensation degree of aromatic rings,while decreases alkyl chains and some small-molecule compounds [73].Therefore,COOH is not detected in the curve-resolved spectra of high rank Laochang-11# coal and Kaiyuan-9# coal before scCO2/H2O exposure,while graphite does exist.
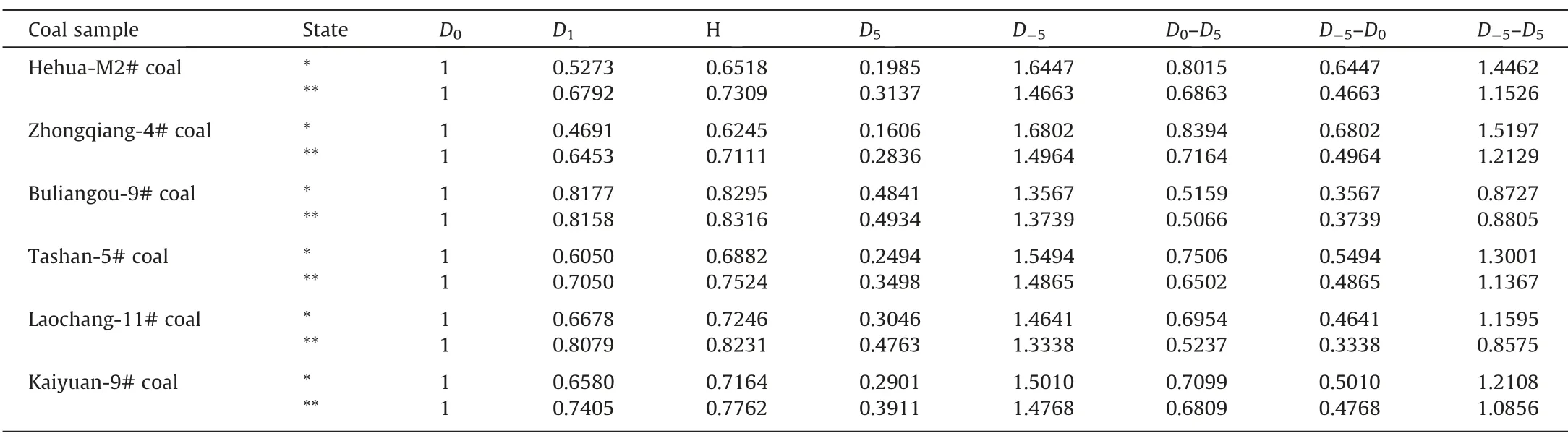
Table 2Results of generalized fractal dimension
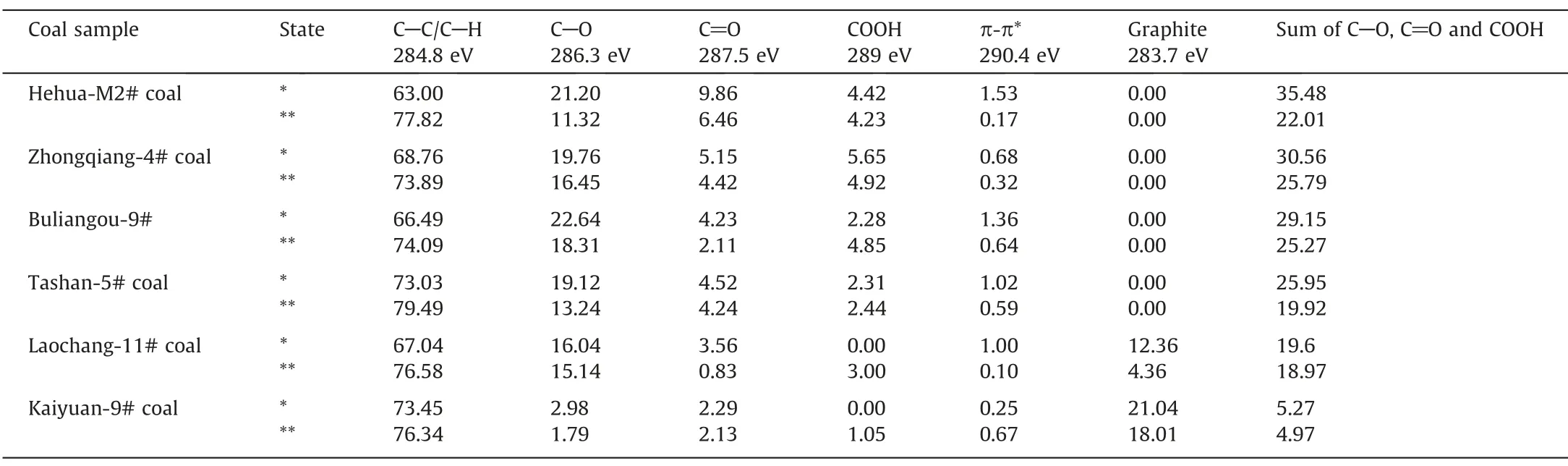
Table 3Main chemical composition of coals(%)
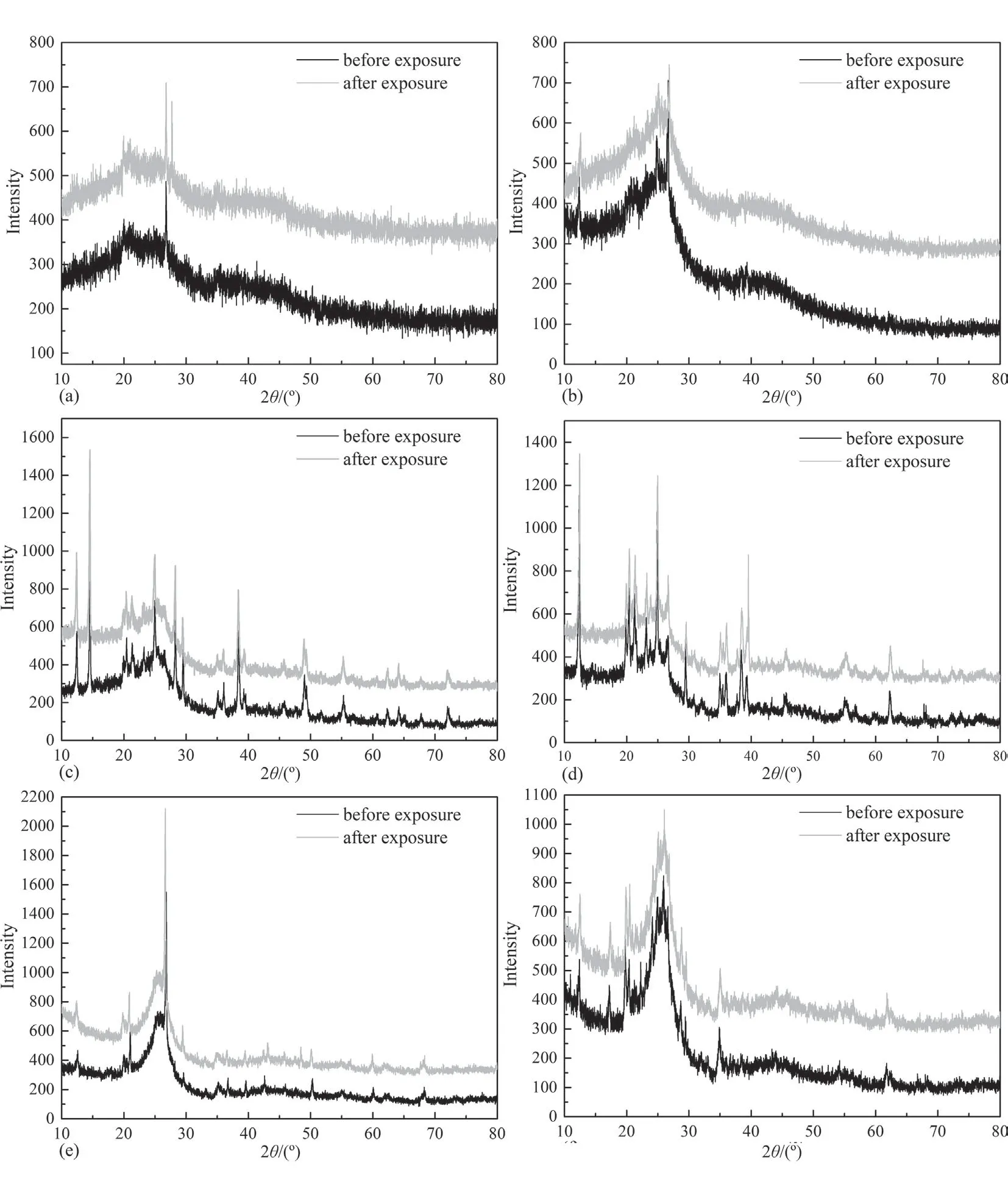
Fig.11.X-ray diffraction patterns of coal samples.(a) Hehua-M2# coal,(b) Zhongqiang-4# coal,(c) Buliangou-9# coal,(d) Tashan-5# coal,(e) Laochang-11# coal and (f)Kaiyuan-9# coal.
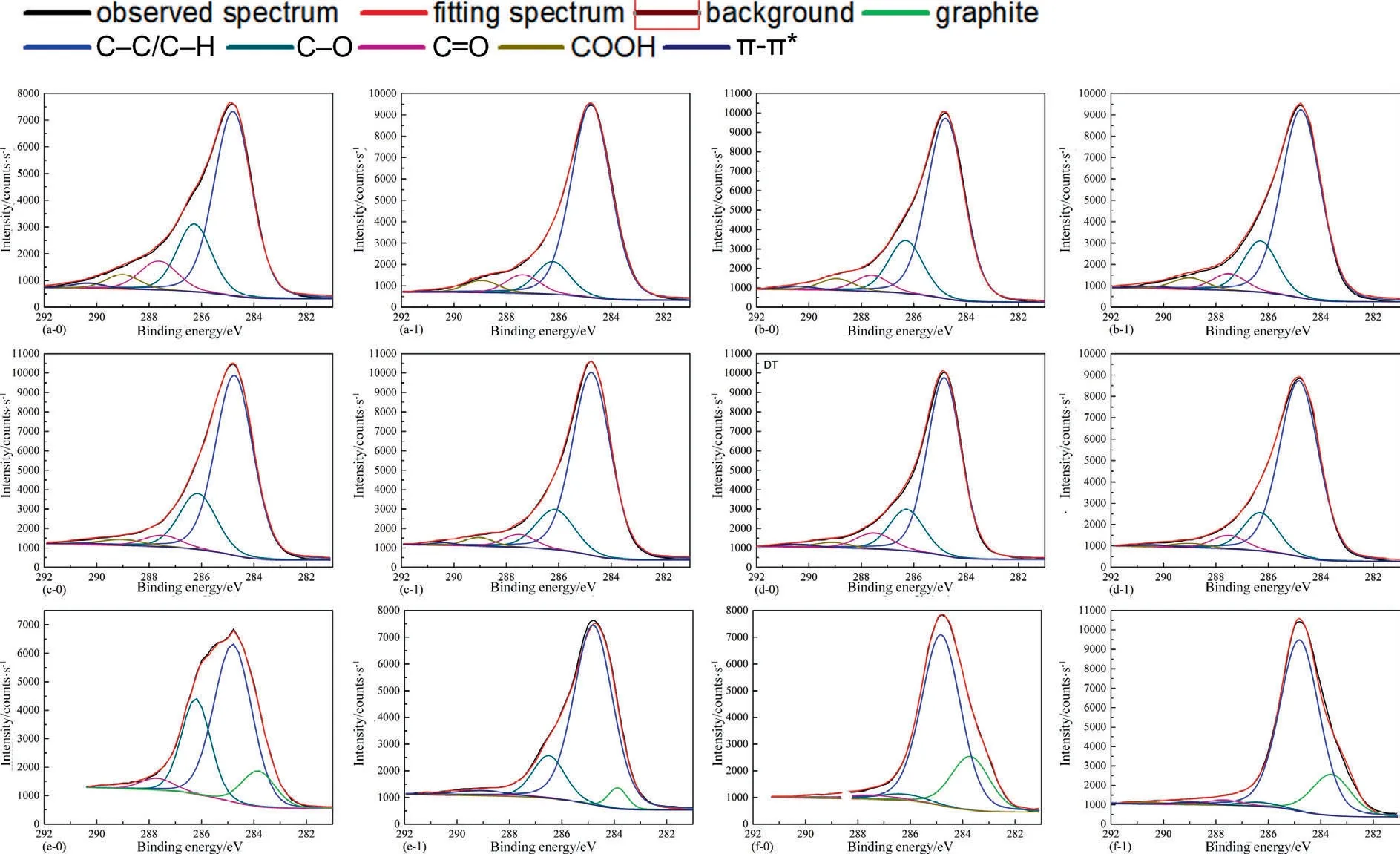
Fig.12.Calibrated C(1s)spectra and their curve-resolved spectra of coals.(a-0)Hehua-M2#coal before exposure,(a-1)Hehua-M2#coal after exposure,(b-0)Zhongqiang-4#coal before exposure,(b-1) Zhongqiang-4# coal after exposure,(c-0) Buliangou-9# before exposure,(c-1) Buliangou-9# coal after exposure,(d-0) Tashan-5# coal before exposure,(d-1)Tashan-5#coal after exposure,(e-0)Laochang-11#coal before exposure,(e-1)Laochang-11#coal after exposure,(f-0)Kaiyuan-9#coal before exposure and(f-1) Kaiyuan-9# coal after exposure.
Table 3 clearly shows that scCO2/H2O exposure reduces the content of C-O and C=O of coal samples after scCO2/H2O exposure.The data of Zhongqiang-4# coal,Tashan-5# coal,Buliangou-9#coal and Kaiyuan-9# coal prior to scCO2/H2O exposure were cited from our previous study [68].The variation ranges are 5.61%–46.60% and 6.19%–76.69%,respectively.The scCO2/H2O exposure dependence of the oxygenic functional groups is mainly associated with two aspects.On the one hand,scCO2/H2O exposure extracts small-molecule organic compounds in coal matrix,thereby leading to decreasing oxygenic functional groups [23].On the other hand,CO2molecules are of quadrupole moment promoting the chemical adsorption between CO2,and defects and functional groups on coal surface [74–76].The chemical adsorption is of higher adsorption strength,and it is a nonreversible process.Given that the extraction effect of CO2is probably predominant over the chemical adsorption effect,the oxygenic functional groups including C-O and C=O of all the coals after interacting with scCO2/H2O decrease.The effect of scCO2/H2O exposure on COOH of coals is complex.Particularly,the content of COOH of low rank coals including Hehua-M2# coal,Zhongqiang-4# coal,Buliangou-9# coal and Tashan-5# coal decreases,probably associated with the dominant extraction effect of scCO2/H2O.On the contrary,high rank Laochang-11# coal and Kaiyuan-9# coal witness a growth in COOH.Particularly,as shown in Eq.(7),COOH could be formed between CO2and aromatic compounds within coals under coal reservoir condition [77].The aforementioned mechanism will be performed in our future work.In addition,the total oxygenic functional groups of coal samples after exposure to scCO2/H2O present a decreasing trend.The decreasing amplitude of the four low rank coals,i.e.Hehua-M2#coal,Zhongqiang-4#coal,Buliangou-9#coal and Tashan-5# coal,is 15.61%-37.97%,higher than that of high rank Laochang-11#coal and Kaiyuan-9#coal (3.21%-5.69%).Thus,scCO2/H2O exposure dependence of the total oxygenic functional groups of low rank coals is stronger than that of high rank coals.Apart from pore structure parameters,the oxygenic functional groups are also a leading factor influencing fluid adsorption capability of coals.The oxygenic functional groups with basic adsorption sites have a strong capability to donate their electrons to the acidic C atom in CO2[8].Hence,the C atom in CO2is strongly attracted to the oxygenic functional groups,promoting CO2adsorption on coal surface[74].On the contrary,the oxygenic functional groups donate the electrons to the acidic H atom in CH4.The C atom in CH4is highly electronegative,leading to a strong repulsion between C atom and the oxygenic functional groups.Thus,the oxygenic functional groups on coal surface show negative effect to CH4adsorption[8].The CO2-ECBM process involves CO2adsorption and CH4desorption within coal reservoirs.The aforementioned oxygenic functional groups change is not beneficial to CO2adsorption and CH4desorption.Thus,scCO2/H2O exposure dependence of the oxygenic functional groups needs attention for CO2-ECBM.Moreover,the increase in the content of C-C/C-H is found for all the coals after scCO2/H2O exposure.On the one hand,CO2is prone to combine with coal surfaceviathe aforementioned nonreversible chemical adsorption effect.On the other hand,the coal matrix swelling occurs owing to CO2dissolution within coal matrix.Numerous studies have indicated that the swelling is a nonreversible process [78,79].In summary,both the chemical adsorption effect and the swelling effect could probably account for the growing content of C-C/C-H.Interestingly,Table 3 shows that the scCO2/H2O exposure clearly decreases the graphitization of Laochang-11#coal and Kaiyuan-9#coal.Numerous studies have demonstrated that scCO2could peel off graphite to grapheme[80,81],which well accounts for the decreasing graphitization of the aforementioned high-rank coals.

Aiming to gain further insight into the interaction mechanism between CO2and coals during CO2sequestration in coal reservoirs,the following future research work is of great significance.The current investigations mainly employed the experimental methodology to study CO2fluid dependence of pore and chemical structure of coals;however,the theoretical elaboration of which seems unsatisfactory.In order to tackle this issue,the experimental characterization and theoretical simulation by adopting the existing physical and chemical structure models regarding coals are recommended to further account for the mechanism of CO2fluid altering pore and chemical structure of various rank coals.Combination of physical experiments and numerical simulations is recommended to predict the changes in porosity and permeability of coals due to long-term CO2sequestration.Moreover,previous studies mainly focused on the interaction of CO2with coals performed at short duration,i.e.12.00–720.00 h [82].Given that CO2-ECBM is aiming to store CO2in geologic time scale,the investigation on long-term CO2exposure to coals is of more significance in the near future.Finally,the coal core plug instead of coal particle is more practical to gain further insight into complex interactions existed between scCO2and moisture-equilibrated coal,including dynamic supercritical CO2fluid exposure dependence of coal matrix and cleat system.
4.Conclusions
In order to further address the supercritical CO2fluid dependence of pore morphology and surface chemical property of coals,the dynamic interaction between supercritical CO2and moistureequilibrated coals was performed.The following conclusions derived from this study are summarized.The scCO2/H2O exposure shows minor effect on micropores of coals.However,the exposure significantly decreases the mesopore surface area of bituminous coals,while increases that of anthracites.Both the mesopore volume and the average mesopore diameter of all the coals after scCO2/H2O exposure decrease.In addition,the scCO2exposure can enhance the pore connectivity of various rank coals.Apart from the pore morphology,the scCO2/H2O exposure also affects the oxygenic functional groups of coals.Particularly,scCO2/H2O exposure reduces the content of C-O and C=O of all coals after scCO2/H2O exposure.The content of COOH of the low rank coals including Hehua-M2# coal,Zhongqiang-4# coal,Buliangou-9# and Tashan-5# coal decreases,while the high rank Laochang-11# coal and Kaiyuan-9# coal witness a growth in COOH.The total oxygenic functional groups of all coals after interaction with scCO2/H2O present a decreasing trend.The content of C-C/C-H of all coals after scCO2/H2O exposure increases.In summary,the interaction with scCO2/H2O changes pore structure and oxygenic functional groups of coals,requiring further attention with respect to practical CO2-ECBM.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research is sponsored by the National Natural Science Foundation of China (41762013),the Open Foundation of Key Laboratory of Shale Oil and Gas Exploration&Production,SINOPEC(G5800-19-ZS-KFZY001),and the National Major Project during the 13th Five-Year Plan Period (2016ZX05061).
 Chinese Journal of Chemical Engineering2021年7期
Chinese Journal of Chemical Engineering2021年7期
- Chinese Journal of Chemical Engineering的其它文章
- Selective preparation of light aromatic hydrocarbons from catalytic fast pyrolysis vapors of coal tar asphaltene over transition metal ion modified zeolites
- The effect of hydrothermal pretreatment on the structure and fast pyrolysis behaviors of ShengLi lignite
- Formation and emission characteristics of VOCs from a coal-fired power plant
- Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers
- Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition
- Kinetics of steam regeneration of SAPO-34 zeolite catalyst in methanol-to-olefins (MTO) process
