Selective preparation of light aromatic hydrocarbons from catalytic fast pyrolysis vapors of coal tar asphaltene over transition metal ion modified zeolites
Yongqi Liu,Qiuxiang Yao,Ming Sun*,Xiaoxun Ma*
School of Chemical Engineering,Northwest University,International Science &Technology Cooperation Base of MOST for Clean Utilization of Hydrocarbon Resources,Chemical Engineering Research Center of the Ministry of Education for Advanced Use Technology of Shanbei Energy,Shaanxi Research Center of Engineering Technology for Clean Coal Conversion,Collaborative Innovation Center for Development of Energy and Chemical Industry in Northern Shaanxi,Xi’an 710069,China
Keywords:Coal tar asphaltene Transition metal ion modification Deoxygenation Lightweight Light aromatic compounds
ABSTRACT The catalytic cracking of coal tar asphaltene (CTA) pyrolysis vapors was carried out over transition metalion modified zeolites to promote the generation of light aromatic hydrocarbons(L-ArHs)in a pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS) micro-reactor system.The effects of ultra stable Y(USY),Co/USY and Mo/USY on the selectivity and yield of L-ArHs products and the extent of deoxygenation (Edeoxygenation),lightweight (Elightweight) from CTA pyrolysis volatiles were investigated.Results showed that the yields of L-ArHs are mainly controlled by the acid sites and specific surface area of the catalysts,while the deoxygenation effect is determined by theirs pore size.The Elightweight of CTA pyrolysis volatiles over USY is 9.65%,while the Edeoxygenation of CTA pyrolysis volatiles over Mo/USY reaches 20.85%.Additionally,the modified zeolites (Mo/USY and Co/USY) exhibit better performance than USY on L-ArHs production,owing to the synergistic effect of metal ions (Mo,Co) and acid sites of USY.Compared with the non-catalytic fast pyrolysis of CTA,the total yield of L-ArHs obtained over USY (4032 mg?kg-1),Co/USY (4363 mg?kg-1) and Mo/USY (4953 mg?kg-1) were increased by 27.03%,38.19%and 54.78%,respectively.Furthermore,the possible catalytic conversion mechanism of transition metal ion (Co and Mo) modified zeolites was proposed based on the distribution of products and the characterizations of catalysts.
1.Introduction
The potential application of coal tar (CT) in fuel oil possesses was limited because of its poor thermal and chemical stability,high-viscosity,high-toxicity,high hetero atom content and high oxygen content[1,2].Therefore,upgrading of CT is of great importance before it can be used as a basic raw material.Especially,it is not only necessary to eliminate the oxygen and hetero atoms,but also need preserve carbon and hydrogen in its components.Several techniques for transformation and upgrading of CT have been studied,such as pyrolysis [3],catalytic cracking [4] and hydrotreating mainly included hydrodeoxygenation (HDO) [5],hydrodenitrification(HDN)[6],hydrodesulfurization(HDS)[7]and catalytic hydrocracking[8]et al.Compared with other technologies,catalytic fast pyrolysis (CFP) is a well-developed technology to produce high quality oil from coal or CT owing to its observably advantages of mild reaction conditions,environmental friendliness,low cost and high yield,is of great interest to researchers due to its promising results [9,10].
In recent years,zeolite catalysts have been favored by many researchers due to its characteristics of low cost,anti-abrasion,anti-poisoning,anti-coking,and high shape selectivity [11],and widely used in the catalytic conversion of coal [12],coal tar [13],petroleum [14],biomass [15] and other fossil or bio energies[16].There are still some problems to be solved in the process of experimental research on the upgrading of the fossil energies.At present,there are few studies on rapid catalytic cracking of coal tar asphaltene (CTA),Liuet al.[17] used two kinds of natural zeolite catalysts(NMZ-A and NMZ-B)to upgrading the pyrolysis volatiles of CT.The results showed that when the ratio of CT to catalyst was 1:2,the yields of BTXN were increased by 143% and 99%respectively.Chenet al.[3]used a device combining the upper driptube and the lower moving bed to carry out catalytic upgrading of coal pyrolysis volatiles,and the light tar (boiling point lower than 360°C)in total pyrolysis tar can reach 57.0%,however,the cost for obtained result is the conversion of heavy tar into more gas and coke.Leckel [8] proposed a process based on a bench-scale fixedbed reactor combined with vapor-phase catalytic HDO,HDS and HDN,however,the hydrogenation experimental design was particularly challenging due to the high temperature (480 °C) and high pressures (up to 20 MPa) of hydrogen.Paloset al.[14] used two commercial equilibrium catalysts for upgrading the heavy coker naphtha by catalytic cracking in refinery FCC unit,which had a good effect on obtaining high yield light olefins and a gasoline fraction but its reaction process and evaluation system are complex.
Although vary studies on upgrading coal or coal tar (including HDO,HDN,HDS or chemicals preparation) have been carried out,while works on the experimental verification and theoretical research of CTor CTA catalytic conversion are relatively few.Moreover,it is most noteworthy that in the process of catalytic conversion of coal tar,there are still few reports on the extent of deoxygenation (Edeoxygenation) and lightweight (Elightweight) of coal tar by catalyst.Therefore,it is of great significance to establish an effective matching suitability system for studying the catalytic effect and mechanism of the catalysts during the CFP of coal or CT.At present,there are few reports on the specific evaluation system of HDO performance of catalystin the related research of pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS)experiments,and which needs to further improved.
Based on this,the principal purpose of this study were to apply a Py-GC/MS micro-reactor system and to compare vary temperatures(400,500,600,700 and 800°C)and catalysts for L-ArHs production during CFP process of CTA.USY,Mo/USY and Co/USY were investigated in the CFP process for its potential application on lowtoxicity,low-heteroatom,low-oxygen and high added value products from CTA catalytic pyrolysis.Moreover,theEdeoxygenation,Elightweightand possible reaction mechanism of CTA were also explored by the investigation of pyrolysis products composition and distribution by the CFP process.This study tries to combine the matching suitability system based on experimental results with the reaction mechanism of catalytic conversion,which hopes to provide ascientific guidance for the development of CFP technology for CT.
2.Experimental
2.1.CTA and catalysts preparation
CTA was part of CT which insoluble inn-hexane but soluble in hot benzene reagent and was used as the feed sample in this work,which was ground and sieved into give a particle size of 40–80 mesh (0.18—0.425mm).In addition,the proximate and ultimate analyses of CTA are shown in Table 1.

Table 1Ultimate and proximate analyses of CTA (% (mass),air dried basis)
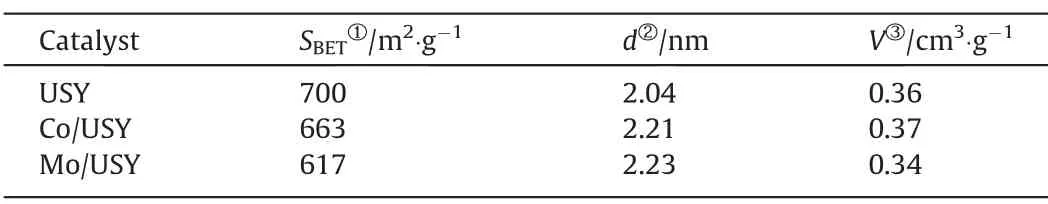
Table 2Textural properties of USY,Co/USY and Mo/USY
Commercial USY used here were purchased from the Catalyst Corp of Nankai University(China)with SiO2/Al2O3ratio of 5.4.Prior to the work,the catalyst was calcined in air at 550°C for 5 h with a muffle furnace to remove the adsorbed binding water,template agent and volatile impurities(ammonium salt,etc.)[18].Additionally,monometallic(5%(mass)Mo or 5%(mass)Co)introduction for USY catalyst carrier was achieved by equal volume impregnation,and modified USY are denoted as Mo/USY or Co/USY,respectively.
2.2.Characterization of catalysts
Powder X-ray diffraction (XRD) patterns of USY,Mo/USY and Co/USY were conducted by D8 ADVANCE(Bruker,Germany)using Cu Kα radiation at 40 kV and 150 mA,and the scanning measurement of 2θ was from 5° to 90° with a step of 0.02°.
Prior to the measurement,these catalysts were degassed in vacuum at 300°C for 15 h in order to remove the adsorbed water and gas.The N2adsorption/desorption isotherms were obtained in an Autosorb-1 apparatus (TriStar II 3020 M,Micromeritics,USA).Moreover,the methods of Barret-Joyner-Halenda (BJH) and Brunauer-Emmett-Teller(BET)were utilized to estimate of pore size distribution,pore volume and specific surface area,respectively.
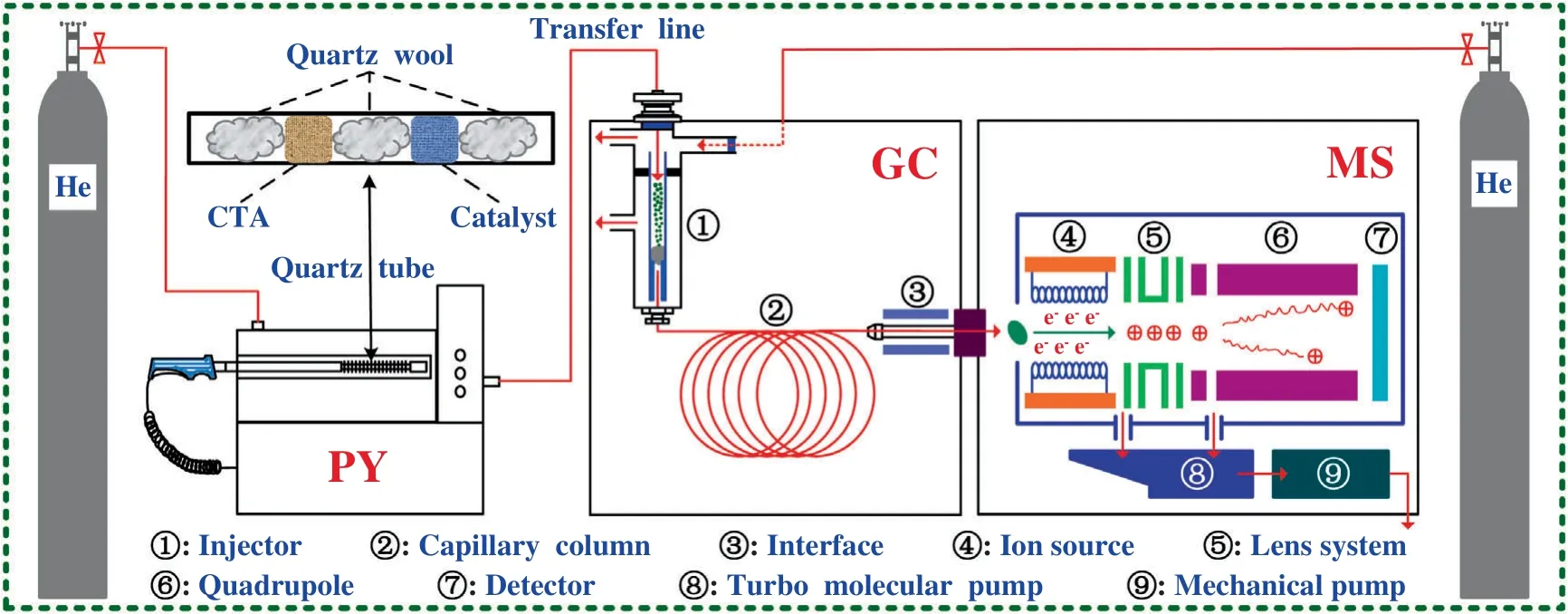
Fig.1.The device diagram of CFP experiment.
For each typical run,about 50 mg catalyst specimens were introduced in a quartztube,and heated from 100 °C and kept at 550 °C with a Helium steam for 1 h,and then cooled down to 100 °C.Finally,the catalyst samples were saturated by ammonia,and then the NH3-TPD curve was conducted with a fixed heating rate of 10 °C?min-1from 100 °C to 700 °C.
2.3.Experimental conditions and instruments
The equipment configuration for Py-GC/MS tests is shown in Fig.1.CFP experiments of CTA were conducted through an analytical CDS 5200 pyrolyzer (Py,America),and then the (catalytic)pyrolysis volatiles were identified by GC/MS (QP2010 plus,Shimadzu,Japan).In each experiment,a quartz tube was used to load the CTA (1 ± 0.01) mg and catalyst (1 ± 0.01) mg,and is shown in Fig.1.Moreover,the CTA sample in Py was initially kept at 50 °C,and then was heated at 20 °C?ms-1to the desired temperatures(400–800 °C),and the retention time was 20 s.High pure helium(99.999%) was utilized both as thecarrier gas and inert gas for Py-GC/MS studies.Furthermore,the related detail parameters of GC/MS as described elsewhere [17].The chromatographic peaks of CTA pyrolysis volatiles were identified by comparing the mass spectrum with NIST11 and NIST11s MS library database.Moreover,a semi-quantitative approach based on the comparison of peak area and its percentage of the chromatogram peaks was conducted to representing the yield and selectivity of the detected volatile products respectively,which is the same methodology recently employed by related studies [19].
2.4.Experimental data analysis and calculation methods
The selectivity of each detectable product was calculated by the peak area of certain compound and the total peak area of the all detectable products in the volatile tar,as shown in Eq.(1).WhereSirepresents the selectivity of each detectable product;PAimeans the peak area of a certain product;PAtotalmeans the total peak area of the all detectable products.

Additionally,in view of the diversity and complexity of the composition and distribution of CTA pyrolysis volatiles in this work,six major aromatics including benzene (B),toluene (T),oxylene(O-X),m-xylene(M-X)andp-xylene(P-X)and naphthalene(N) were quantitatively analyzed.In reality,the true yields of CTA pyrolysis volatiles cannot be obtained,because the Py-GC/MS experiment cannot collect the products,and the external standard method was used to determine the quantitative analysis of B,T,X(O-X,M-X and P-X) and N.A series of standard solutions of B,T,X and N with different concentrations were analyzed by GC/MS,and the corresponding chromatographic peak area values were obtained.Then,the standard curves have fitted according to these peak area values and the quantity of B,T,X or N in each standard solution,based on this,the actual yields of B,T,X or N in CTA pyrolysis volatiles was calculated.
Generally speaking,the deoxygenation effect of catalyst on tar is generally achieved by comparing the change of H/C and O/C molar ratios in pyrolysis volatiles before and after catalytic pyrolysis [20].In this study,the computation methods of H/C and O/C molar ratios of detectable products are shown in Eqs.(2) and (3),respectively.Where H/C and O/C are represent the molar ratios of H to C and O to C,respectively.Besides,the mass of detectable volatiles under the same conditions ismdv.The molecular formula of productidefined as CaiHbiOciNdiSei,among this,ai,bi,ci,diandeirepresent the atom number of C,H,O,N and S atom,respectively;in addition,Mirepresents the relative molecular mass of producti.
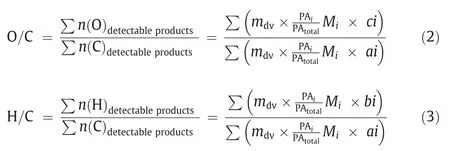
Moreover,the extent of deoxygenation (Edeoxygenation) and lightweight (Elightweight) were defined to measure the effect of deoxygenation and lightweight on the pyrolysis tar of CTA.According to the definition,in this study,the equations forEdeoxygenationandElightweightwere further modified which based on the previous study [21],as shown in Eqs.(4) and (5),respectively.

3.Results and Discussion
3.1.Characterization of catalysts
The XRD patterns of USY,Co/USY and Mo/USY of this study are shown in Fig.2.There are similar characteristic diffraction peaks at 6.1°,10°,11.9°,15.7°,18.7°,23.7°,27.1°,31.4°and no other obvious diffraction peaks were found of USY,Co/USY and Mo/USY,which may be due to the weak crystallization and low loading of metal oxides.On the other hand,it also suggests that very small metal oxide clusters are well dispersed on the external surface of zeolite[22].
The SEM mapping images (Fig.3(a)–(f)) revealed the relatively uniform distribution of Si,Al,O,Mo and Co throughout the crystal,additionally,the energy dispersive spectroscopy (EDS) results also showed that the element percentage of Mo and Co are 5.07% and 7.06%in Mo/USY and Co/USY respectively.It thus can be concluded that the results of EDS and SEM images further confirmed the presence and uniform distribution of Mo and Co in Mo/USY and Co/USY,which was also consistent with XRD results.
Fig.4(a) and (b) show the N2adsorption–desorption isotherms and pore size distributions of USY,Co/USY and Mo/USY.It can be found that the isotherm adsorption and desorption branch curves of these catalysts are not consistent,and obvious hysteresis loops can be observed,so they are all belong to the typical type IV isotherm adsorption and desorption curves.Moreover,in the range ofP/P0=0.5–1,the H4 type hysteresis loop appears,which indicates that these catalysts have typical narrow slit pore structure[23].Besides,it also can be seen that pore width of these catalysts are mainly composed of large quantities of micropores and small mesopores.
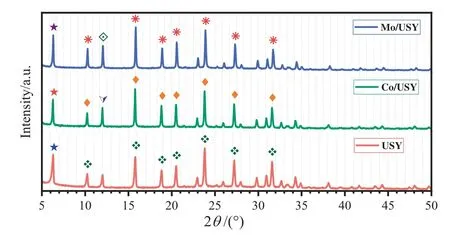
Fig.2.XRD patterns of USY,Co/USY and Mo/USY.
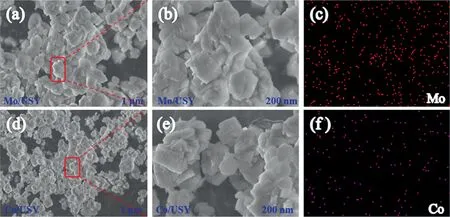
Fig.3.SEM images and element mapping of (a–c) Mo/USY and (d–f) Co/USY.
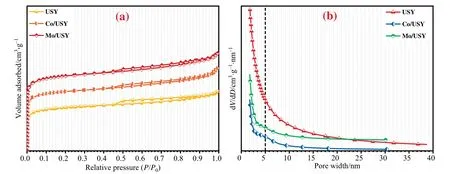
Fig.4.N2 adsorption–desorption isotherms (a) and the pore size distributions (b) of USY,Co/USY and Mo/USY.
The textural properties of USY,Co/USY and Mo/USY are shown in Table 2.It can be seen that the incorporation of transition metal Co and Mo into the USY carrier results in a decrease in the specific surface area(SBET),which may be due to the metal Co and Mo enter the pores of the USY and occupy a part of the pore volume [24].Furthermore,the average pore diameter (d) of Co/USY and Mo/USY is bigger than that of USY,which may be attribute to the formation of some mesoporous structures in the process of loading metal ions on USY support [25].
Figs.5 and 6 present the NH3-TPD curves and their corresponding fitting peaks of USY,Co/USY and Mo/USY,and as indicated,these catalysts hold two significant desorption peaks occurring at below and above 300 °C,corresponding to the weak and midstrong acid sites,respectively [26].Additionally,acid sites amountwas calculated by peak fitting (based on the integral peak area value of NH3-TPD desorption curve of USY at corresponding acid site) shown in Fig.7.It can be found that the order of total acid sites amount for these catalysts is:Mo/USY >USY >Co/USY,the order of mid-strong acid sites amount is:Mo/USY >USY >Co/US Y,and the order of weak acid sites amount is:Mo/USY >Co/USY >USY.
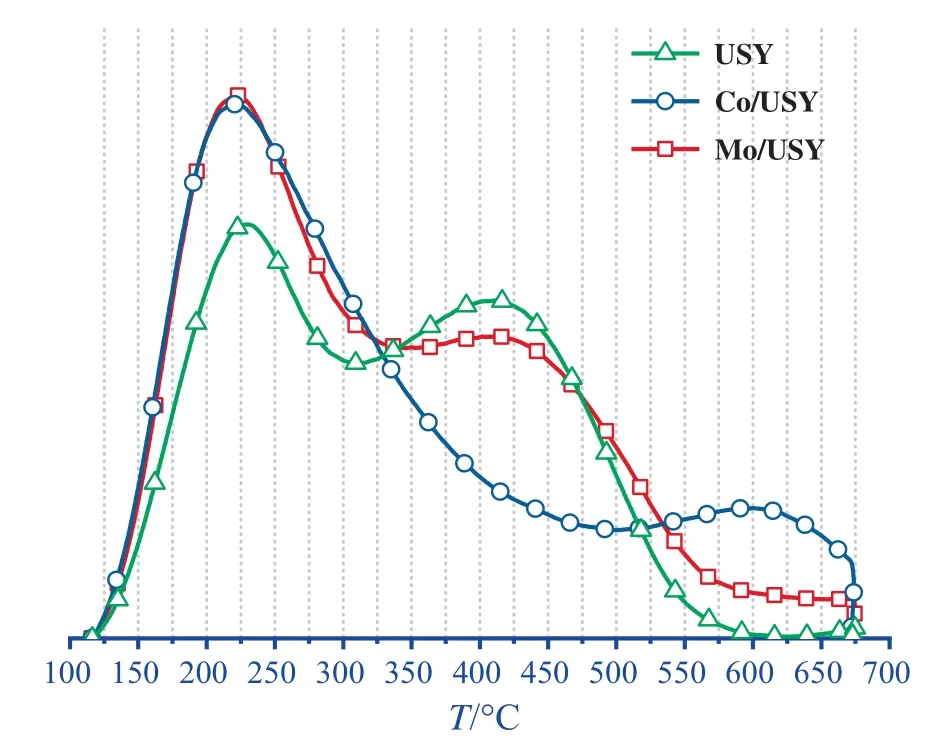
Fig.5.NH3-TPD curves of USY,Co/USY and Mo/USY.
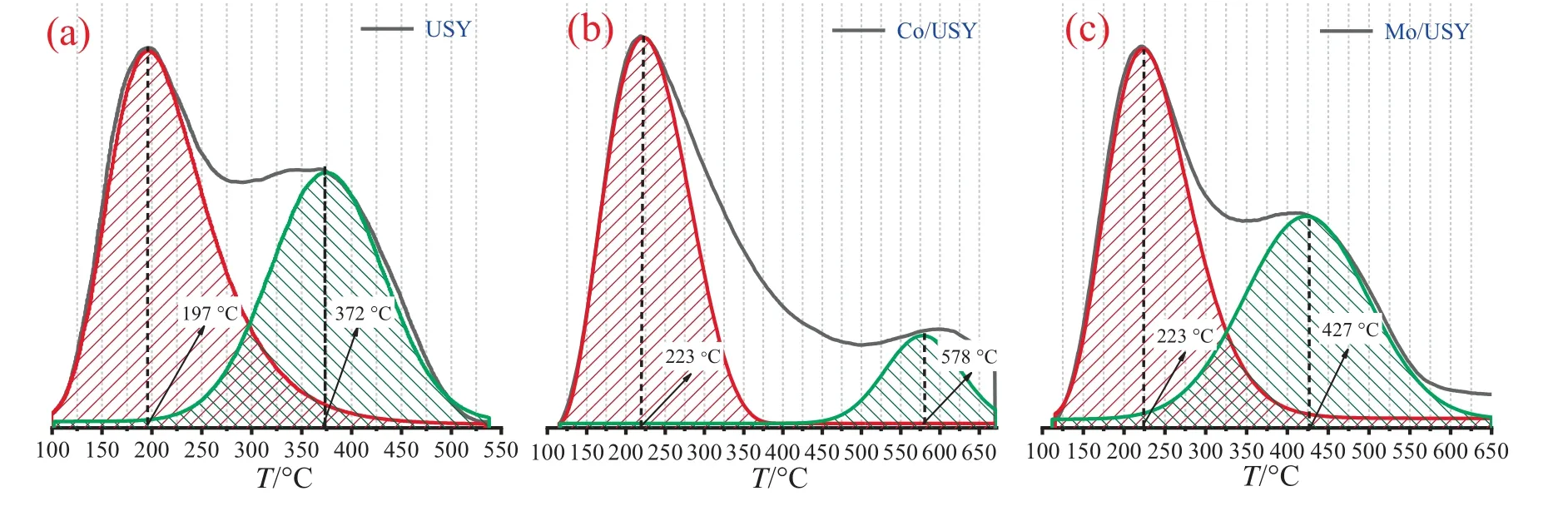
Fig.6.Fitting peaks of (a) USY,(b) Co/USY and (c) Mo/USY.

Fig.7.The acidy distribution of USY,Co/USY and Mo/USY.
Obviously,the participation of transition metal species has a significant effect on the distribution properties of acid sites [27].The number of weak acid sites in Mo/USY and Co/USY increased owing to the presence of metal oxides,this should be attributed to the exchange of proton by metal oxides during calcination process[28].These metal oxides could function as Lewis acidic centers[29],which are acting as acceptors of electron pairs and causing charge transfer processes,and plays an important role in catalytic processes[30].Besides,it can be observed that an additional strong acid adsorption peak around 600°C in both of Mo/USY and Co/USY,which may be attributed to the presence of low coordination aluminum.Low coordination aluminum was formed by zeolite catalyst dehydroxylation when calcination treatment temperature was high than 500 °C [31].In addition,it should be noted that the strong acid peak around 600°C of Mo/USY is smaller than that of Co/USY,which may be due to the electro negativity increases from Co to Mo causing the susceptibility of interaction increase between H atom of zeolite catalyst and metal species,so the Mo oxides prefer to deposition on the strong acidsites of zeolite catalyst surface or channels [32].
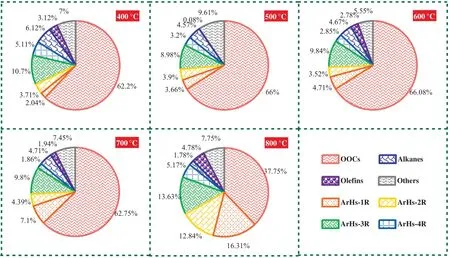
Fig.8.The selectivity of various pyrolysis volatiles derived from the fast pyrolysis processes of CTA at different FPTs.
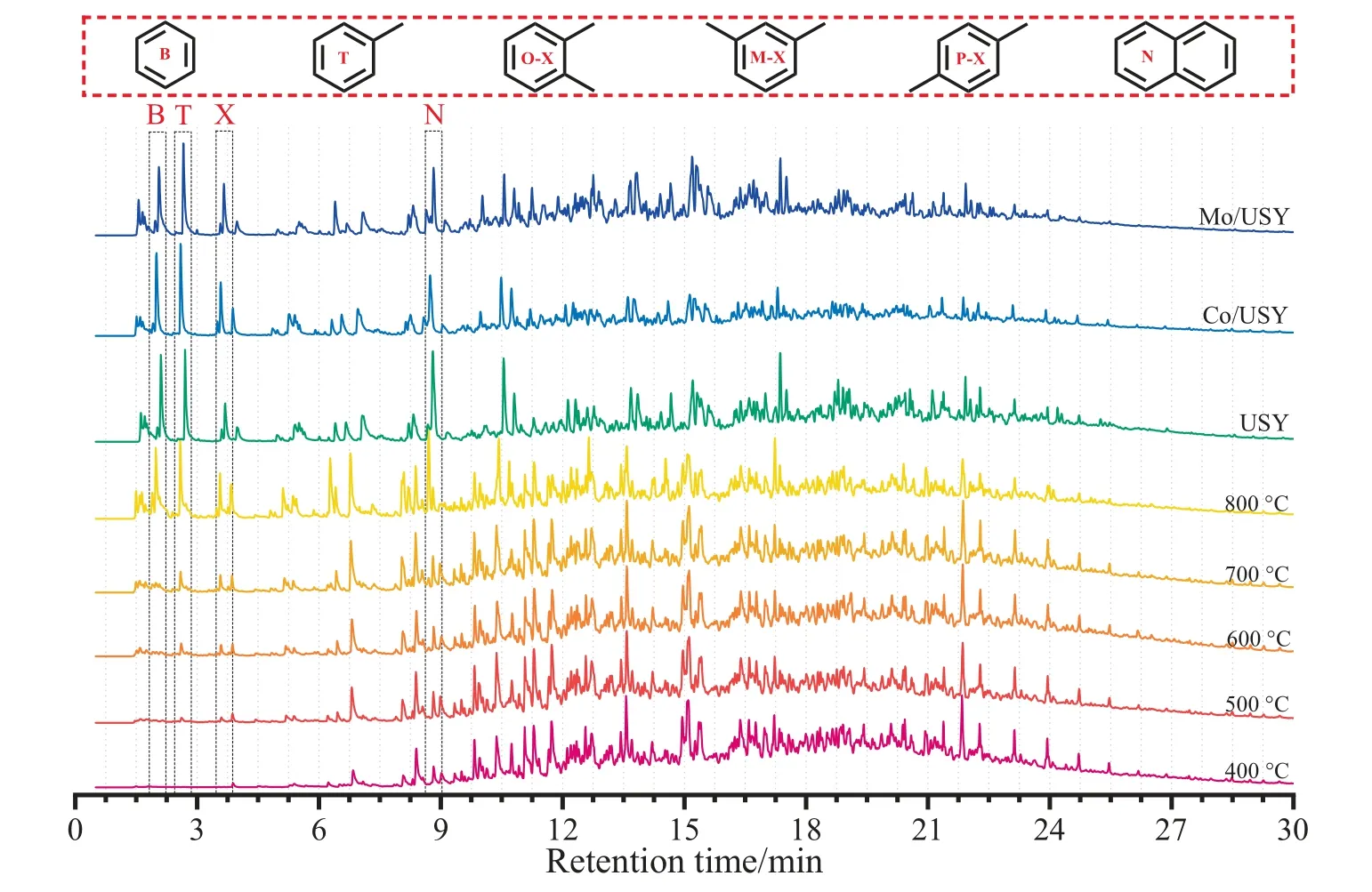
Fig.9.The total ion chromatograms of CTA during FP and CFP processes.
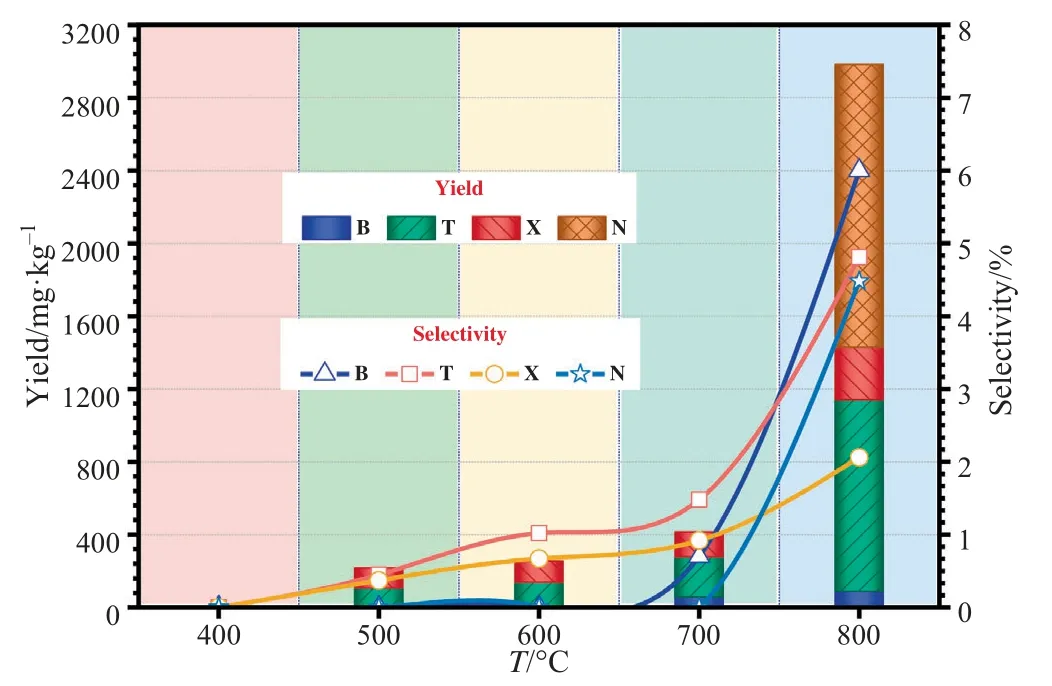
Fig.10.Effects of FPTs on the distribution of yield and selectivity of BTXN.
3.2.Characteristics of volatile products during fast pyrolysis of CTA
3.2.1.Pyrolysis volatile products distribution at different temperatures
It is known to all that the final pyrolysis temperature (FPT)plays a significant role in the yield and distribution of products during the pyrolysis processes of coal or coal tar[33].Fig.8 shows the selectivity of various pyrolysis volatiles derived from the fast pyrolysis (FP) of CTA at different FPTs.With the rising of FPTs,it can be seen that the basic change trends of selectivity for OOCs and ArHs,which are shown decreasing and then increasing,respectively.In addition,the selectivity of the light ArHs (L-ArHs,which containing ArHs-1 or ArHs-2) and polycyclic ArHs (P-ArHs,which containing ArHs-3 or ArHs-4) shows an increasing and decreasing trend,respectively.
Moreover,the total ion chromatograms of CTA at different FPTs from 400 to 800°C are shown in Fig.9.For a general overview,the composition and yield of the pyrolysis products were altered significantly after the FPTs increasing.In particular,the peak area(represents yield)and peak area percentage(represents selectivity)of BTXN shows a trend of gradually increasing.The effect of FPT on the distributions of yield and selectivity for BTXN also have investigated,its specific data are shown in Fig.10.It can be found that there are no generation of BTXN at 400 °C,then the total yield and selectivity of BTXN increased with increasing of FPTs and the most considerably increase was occurred at the range of 700–800°C,which resulted from the dramatic cracking reactions occur in CTA [34].Besides,the total yield and selectivity of BTXN were 2983 mg·kg-1and 17.35%,respectively.
It should be pointed that since the detectable products of different FPTs processes include a wide variety of compounds,the analysis is firstly focused on the top 8 high selectivity compounds(selectivity of each ≥1.90%) of each FP processes.Table 3 shows the top 8 high selectivity compounds in the detectable products of each FP processes of CTA.It can be observed that the top 8 major products are mainly composed of OOCs (percentage of OOCs≥88.67%,mainly naphthalenol and methyl-naphthalenolet al.) in the FPT range of 400 to 700 °C,while the top 8 major products are HCs (percentage of HCs is 63.47%,mainly BTNet al.) at 800 °C,among this,the selectivity of BTN are 6.00%,4.81% and 4.48%,respectively.
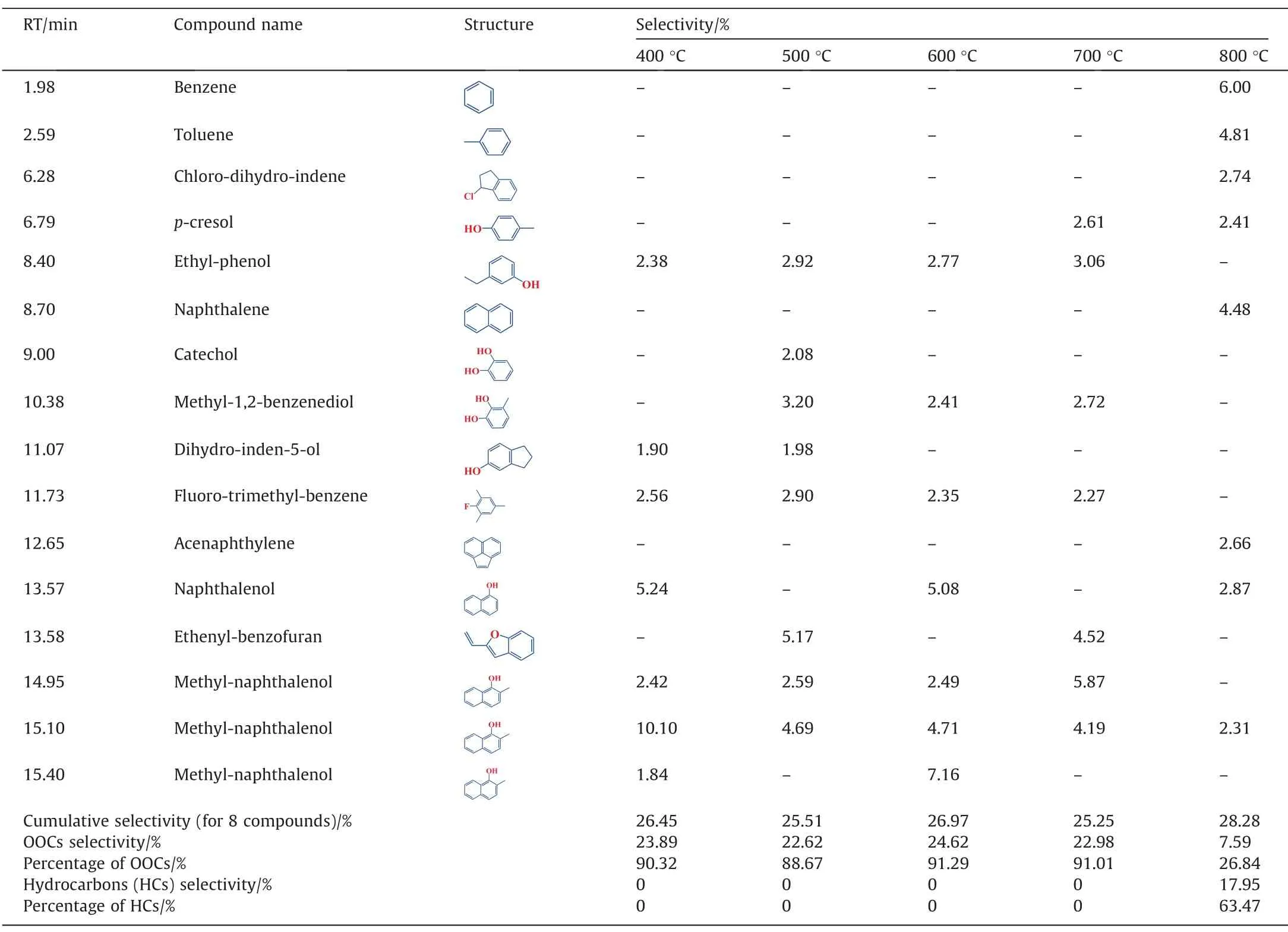
Table 3The top 8 selectivity compounds in the detectable products during FP of CTA
3.2.2.Effect of CTA structural on BTXN emission
CTA is a complex,heterogeneous,three-dimensional polymer consisting of irregular array of differently bonded hydroxyl and methoxy-substituted phenyl-propane units.A large numbers of molecular structures of coal-derived asphaltenes have been reported in the past literature.Aromatic carbons account for around 70%–90% in the complex structures of asphaltenes [35],and which is dominated by hydrogen and carbon.In addition,benzothiophenes,saturated sulfide and oxidized sulfur are the most common form of sulfur in asphaltene.As for nitrogen,it is mainly present in asphaltenes as the form of pyrrole,pyridine and saturated amine [36].
Additionally,the structural parameters of CTA have been explored in detail by XPS,1H-MAS-NMR and13C-MAS-NMR in our previous research[37],it can be known that the length of alkyl side chains in CTA is relative longer,and the types of carbon(C)in the CTA are mainly composed of protonated aromatic carbon(26.94%),methylene carbon bonded to methyl carbon (22.67%),carboxyl carbon (9.97%),aromatic bridgehead carbon (8.85%) and aromatic carbon bonded to oxy-aromatic (8.57%),et al.It also can be found that the bonding of oxygen (O) in CTA mainly including carbon–oxygen double bond(C=O,29.8%)and carbon–oxygen single bond (C-O,C-O-C and C-OH,40.8%).
As is known to all,the pyrolysis process is comprehensive effected by thermal cracking reactions and polycondensation reactions,and the major reactions are also different when the FPTs are different.From the results in section 3.1.1,it can be found that the selectivity of OOCs is decreased gradually with the FPTs increasing,while the selectivity of ArHs is increased.For example,when the FPT is 400 °C,the selectivity of OOCs and ArHs are 62.20% and 21.55%,respectively,while it is 37.73% and 47.94% respectively at 800 °C.Moreover,Fig.11 shows that the selectivity of phenols,LArHs and P-ArHs at different FPTs.It can be seen that the selectivity of phenols and P-ArHs presents a similar trend of slightly decreased respectively with the FPT increases from 400 to 700 °C,when the FPT further increased to 800 °C which presents an opposite trend.Furthermore,it is worth noting that the selectivity of both for L-ArHs and P-ArHs are increased with the increasing FPTs,which indicated that the thermal cracking reactions are the major reactions and the polycondensation reactions are also increased gradually with the FPTs increasing from 400 to 700 °C,and the polycondensation reactions are the major reactions at 800 °C.
3.3.Catalytic effect of transition metalon the volatiles from CTA pyrolysis
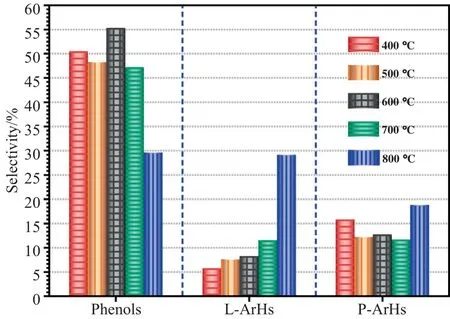
Fig.11.Effects of FPTs on the selectivity for Phenols,L-ArHs and P-ArHs.
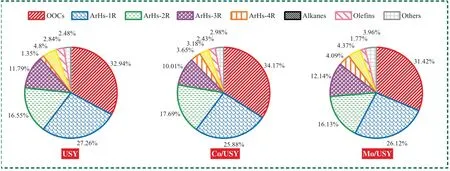
Fig.12.The selectivity of various pyrolysis volatiles derived from the CFP process of CTA.
Based on the analysis and exploration results in Section 3.2,it can be found that the highest yield of BTXN (2983 mg·kg-1) was obtained at the FPT of 800 °C during the fast pyrolysis process of CTA,thus the CFP experiment of CTA pyrolysis volatiles was carried out at the FPT of 800 °C.Besides,USY,Co/USY and Mo/USY were selected as the catalysts to further upgrade the quality of CTA pyrolysis volatiles.
Fig.12 shows the selectivity of various pyrolysis volatiles derived from the CFP process of CTA with different catalysts at 800°C.It is observed that the selectivity of OOCs,Aliphatic hydrocarbons (AlHs) and Others in CTA pyrolysis volatiles are all decreased after catalyzed by USY,Co/USY and Mo/USY,respectively.Table 4 shows the top 8 high selectivity compounds in the detectable products of each CFP processes of CTA.Different with the distribution results in Table 3,the top 8 major products are mainly composed of HCs (percentage of HCs ≥72.74%,mainly BTXNet al.) and OOCs (percentage of OOCs ≤27.26%,mainly phenol and methyl-phenolet al.)at different FPTs.Combined with the corresponding results shown in Fig.9,Fig.12 and Table 4,it thus can be concluded that all of USY,Co/USY and Mo/USY can effectively promote the conversion of OOCs and other compounds in CTA pyrolysis volatiles into L-ArHs.
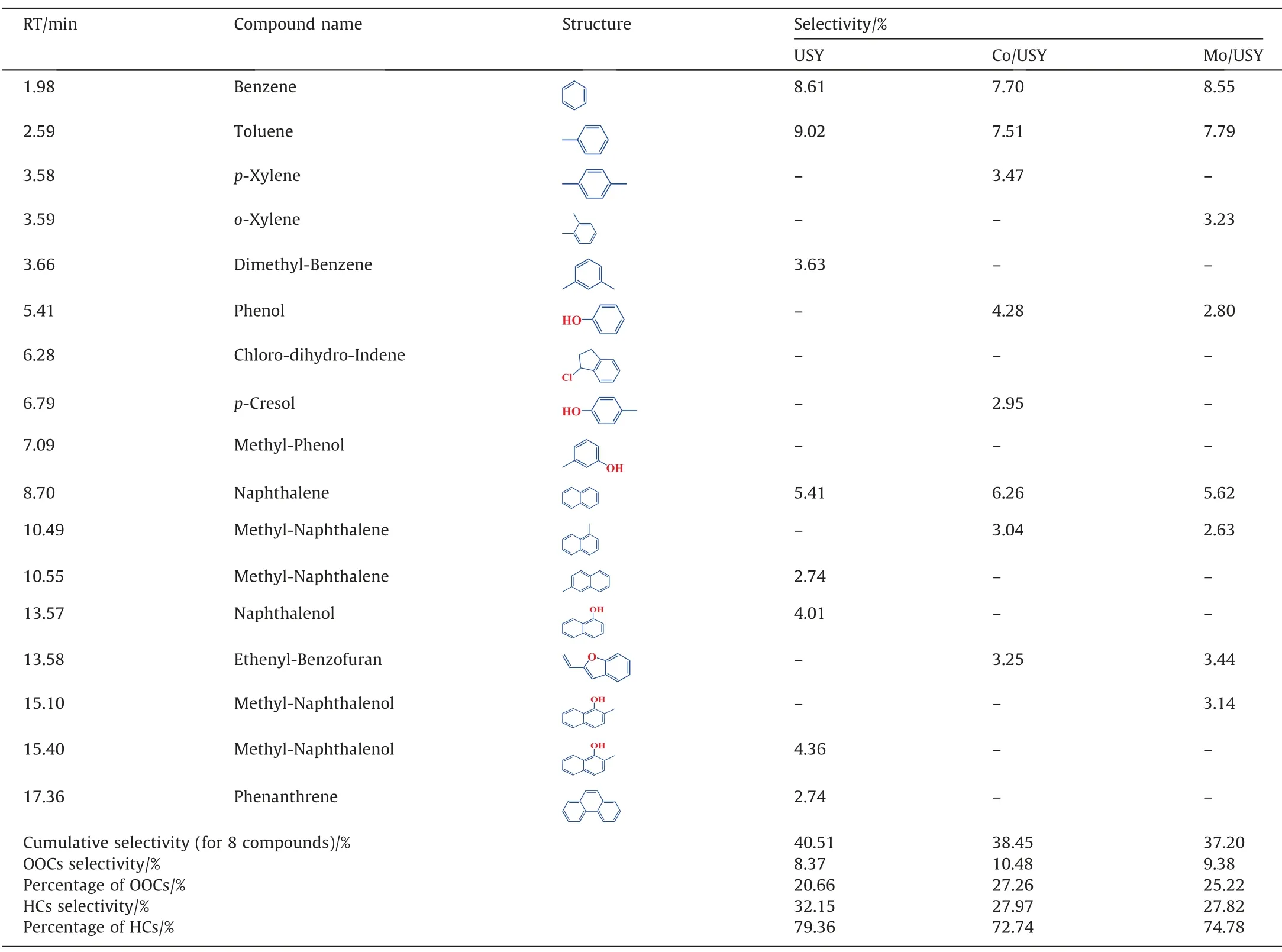
Table 4The top 8 selectivity compounds in the detectable products during CFP.
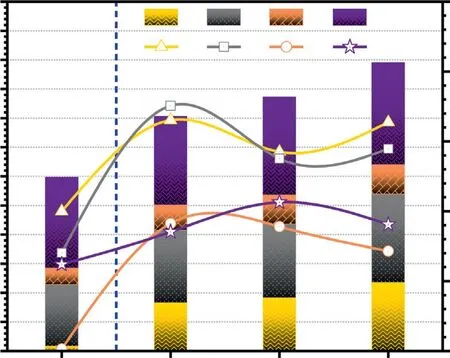
Fig.13.Effects of USY,Co/USY and Mo/USY on the yield and selectivity of BTXN.
The effects of USY,Co/USY and Mo/USY on the yield and selectivity of BTXN are shown in Fig.13.It is obvious that the effects of Co/USY and Mo/USY on improving the total yield of BTXN are better than USY.Compared with the non-catalytic FP of CTA,the total yield of BTXN in pyrolysis products catalyzed by Co/USY(4363 mg·kg-1) and Mo/USY (4953 mg·kg-1) are increased by 38.19% and 54.78% respectively,while it is 27.03% over USY(4032 mg·kg-1),which indicate that the Co/USY and Mo/USY have a better effect on improving the BTXN yield than USY.Additionally,Co/USY and Mo/USY have both two types of acidic and metal active sites,those active sites have a better effect on promoting the formation of BTXN by deoxygenation (including dehydroxylation,decarbonylation and decarboxylation),ring-opening,H transfer and aromatization reactions [29,38].On the contrary,it also can be found that the total selectivity of BTXN in pyrolysis products catalyzed by Co/USY (55.79%) and Mo/USY (54.58%) is lower than that catalyzed by USY (65.36%).It should be noted that the catalytic pyrolysis of CTA by Ni/USY was also carried out,but the results were not ideal.The relevant results are shown in Fig.S1 in the Supplementary Material,so it will not be repeated here.As mention above,the total yield and selectivity of BTXN show the different changing trend under same conditions.
Furthermore,Fig.14 shows the effects of USY,Co/USY and Mo/USY on the selectivity increase rate of BTXN.According to the Fig.14,it is observed that the highest increase rate of B,T and X are 43.50%,87.53% and 174.27% respectively,and are all catalyzed by USY rather than Co/USY and Mo/USY.While the lowest increase rate of N is also obtained by USY,and which is 20.76%.Besides,the increase rates of N are 39.73% and 29.45%,which are obtained by Co/USY and Mo/USY,respectively.
As shown in Table 2,it can be known that the surface area and total pore volume of USY(700 m2·g-1)is small than that of Co/USY(663 m2·g-1)and Mo/USY(617 m2·g-1),which indicated that there are more contact opportunities between reactant molecules and active sites of USY than that in Co/USY and Mo/USY[39].Moreover,the order of the average pore diameter of USY,Co/USY and Mo/USY is:Mo/USY(2.23 nm)>Co/USY(2.21 nm)>USY(2.04 nm),indicating that the larger molecular compounds are more likely to enter the channels of Co/USY or Mo/USY than USY for related catalytic reactions [40].As mentioned above,it can be found that more active centers of catalysts are more benefit to improve the total yields of BTXN,while the lager surface area and average pore diameter are suitable for deoxygenation on CTA pyrolysis vapors.
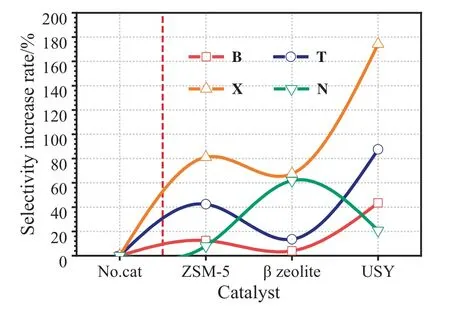
Fig.14.Effects of USY,Co/USY and Mo/USY on the selectivity increase rate of BTXN.
As is known to all,the total yield of the detectable volatile products would be depended on the following two approaches during the process of CFP.On the one hand,the acid sites on the catalysts would promote the thermal cracking of undetectable volatile products,which are heavy products with higher boiling point and difficult decomposed during FP process,and convert into light volatile products(which with lower boiling point and could be detected by GC/MS),which increased the total yield of the detectable volatiles.On the other hand,the strong acid sites on the catalysts would cause some volatile products to form chars or cokes through polycondensation,or promote them to decompose into gases which decreased the total yield of the detectable volatile products.
As mention above,it can be known that both of the Co/USY and Mo/USY have an appropriate effect to increase the yields of BTXN,while decrease its selectivity.In other words,both of the Co/USY and Mo/USY could increase the overall yield of pyrolysis volatile products while promoting the formation of BTXN.Besides,from the results of acid amounts distribution in the Fig.6,it is observed that the presence of transition metal increased the number of weak acid sites in Mo/USY and Co/USY compared with USY,which increased the active centers for the catalytic reactions.At the same time,the transition metal decreased the intensity of polycondensation reactions or the yield of the permanent gases,increased the overall yield of pyrolysis volatile products instead.
3.4.Evaluation of H/C and O/C molar ratios and catalytic conversion mechanism
3.4.1.Evaluation of H/C and O/C molar ratios
The H/C and O/C molar ratios of CTA,pyrolysis tar and catalytic upgraded tar obtained from the process of FP or CFP of CTA are shown in Fig.15.As presented in Fig.15,the O/C molar ratios of pyrolysis volatile products from the CFP process of CTA obtained over different catalysts (USY,Co/USY and Mo/USY) are lower than those from the FP process of CTA at different FPTs (from 400 to 800 °C),which indicating that all of those catalysts have a significantly catalytic effect on the deoxygenation of the CTA pyrolysis tar[41].Besides,the H/C molar ratios of pyrolysis volatile products obtained from the CFP process of CTA at 800°C by USY,Co/USY and Mo/USY are 1.06,0.99 and 1.01 respectively,while it is 0.97 obtained from the FP process of CTA at 800 °C.
Moreover,Fig.16 shows the simple average molecular formula and distribution of pyrolysis products under different conditions.As shown in Fig.16,from the comparison of the simple average molecular formula between the FP process and CFP process,it is evident that these catalysts have a remarkably effect of HDO on the products.As mentioned above,it thus can be concluded that those catalysts could be efficient to improve the H/C and O/C molar ratios of pyrolysis tar,although the quality of upgraded tar is still relatively low.
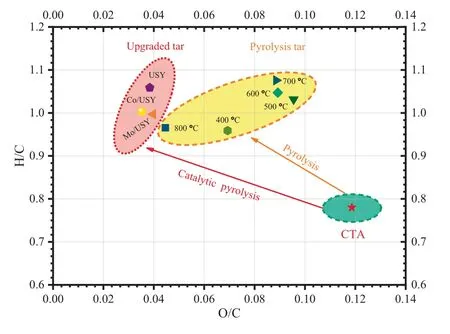
Fig.15.Comparison of the H/C and O/C molar ratios of CTA pyrolysis tar and catalytic upgraded tar obtained from the process of FP or CFP.
In addition,Fig.17 shows theEdeoxygenationandElightweight(based on pyrolysis tar at 800°C)for the catalytic upgraded tar.For a general overview,it can be obviously found that the percentage ofEdeoxygenation≥10.99% and theElightweight≥3.26% for these catalysts,and the order ofEdeoxygenationis:Mo/USY >USY >Co/USY.As shown in Table 2,the average pore size of Mo/USY is 2.23 nm which is the biggest among these all catalysts,which also means that it allows more oxygen-containing macromolecules to enter the pore and conduct deoxygenation at its acid sites[17].Additionally,the order ofElightweightis:USY>Mo/USY>Co/USY.From these results,it seems that the catalyst with more and larger pore sizes is more suitable for the deoxygenation reactions,while the catalyst with more acid amounts is more able to promote the degree of hydrogenolysis reactions.
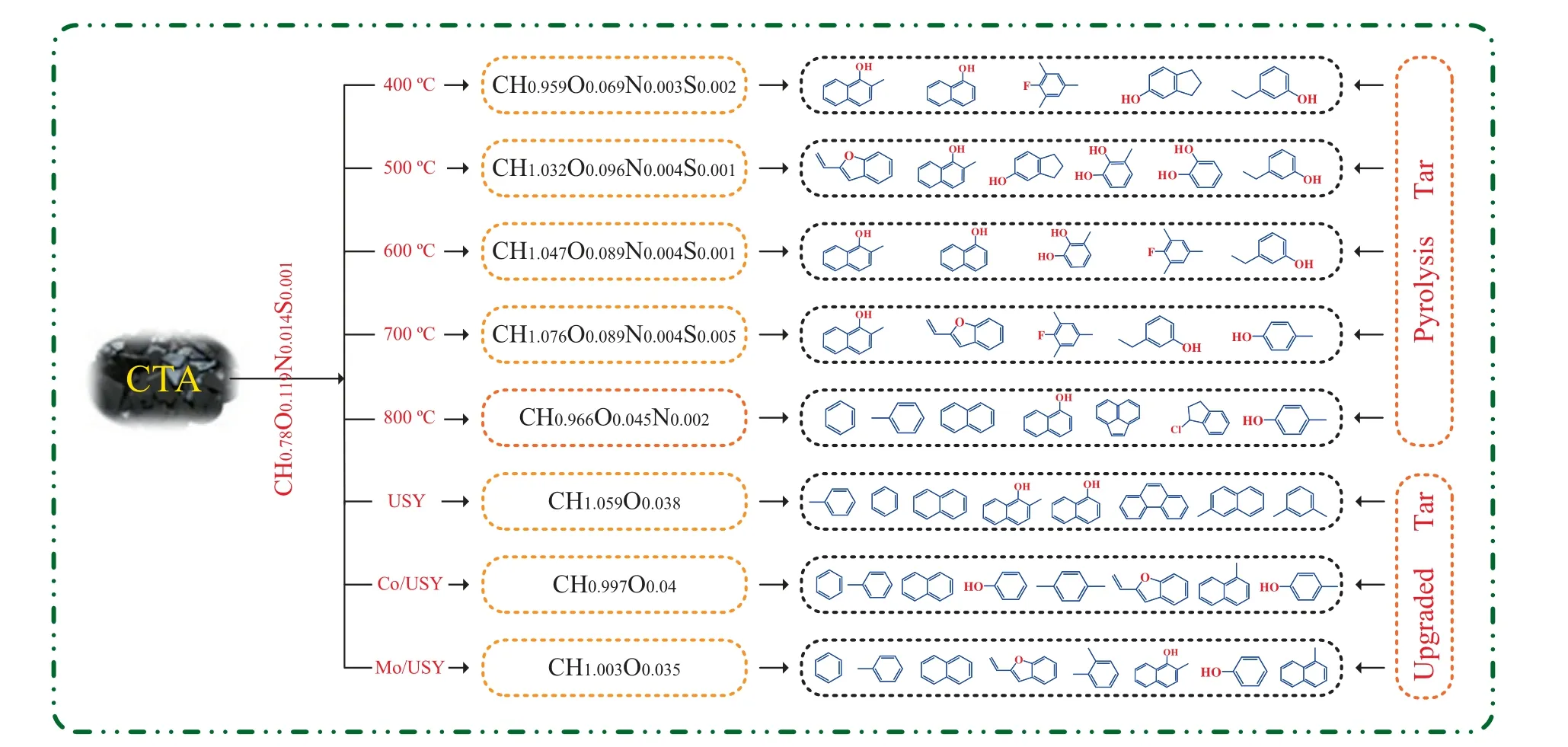
Fig.16.The simple average molecular formula and distribution of pyrolysis products under different conditions.
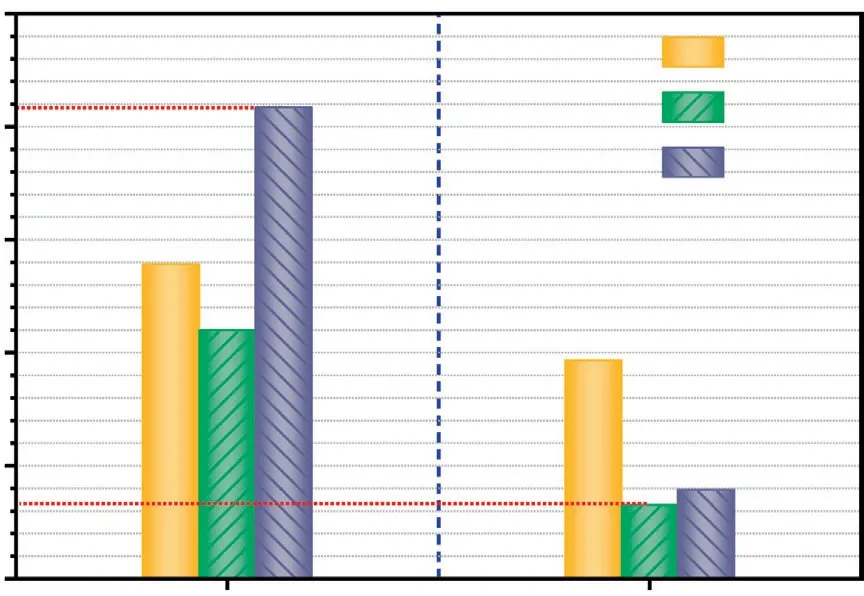
Fig.17.The Edeoxygenation and Elightweight of the catalytic upgraded tar.
As mentioned above,further combining with the contents of Figs.16,17 and Table 2,it can be concluded that the yield of the L-ArHs is mainly controlled by the specific surface area and acidic sites of these catalysts,while the effect of deoxygenation is determined by the size of their pore size.
3.4.2.The possible catalytic conversion mechanism
Fig.18 shows the total peak area (represents total yield) of (a)phenols,(b) P-ArHs,(c) AlHs and (d) others before and after catalyzed by USY,Co/USY and Mo/USY,respectively.Compared with the FP process of CTA,the total yields of those various compounds decreased in varying degrees during the process of CFP for CTA,while the total yields of L-ArHs such as BTXN increased at different levels (as illustrated in Fig.13.Besides,earlier studies also have found similar findings.Liuet al.[42]found that Y-type zeolite catalyst can effectively promote the cracking of P-ArHs into BTEXN in the volatiles of coal,and the pore size distribution and acidity characteristics of the catalysts are the main factors that affect its catalytic cracking activity.Liet al.[43] found that Mo/HZSM-5 is more effective than HZSM-5 on catalytic conversion of coal,including the dehydroxylation of phenols and the aromatization of AlHs.Additionally,previous studies also have shown that the removal rate of heteroatoms in coal liquefaction fraction can be significantly improved by adjusting the acidity and the contents of metal loading of catalysts [44].
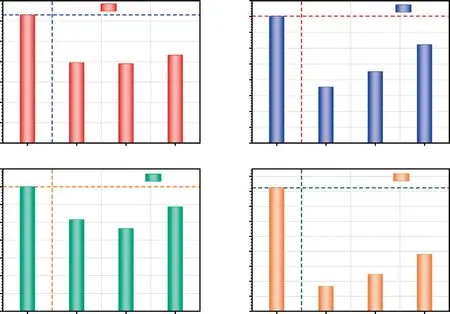
Fig.18.The total peak area of (a) phenols,(b) P-ArHs,(c) AlHs and (d) others before and after catalyzed by different catalysts at 800 °C.
Through the in-depth discussion and comparison of CFP process of CTA,the possible reaction catalytic mechanism was further proposed,as shown in Fig.19.It thus can be concluded that compared with the FP process of CTA,the three catalysts of USY,Co/USY and Mo/USY can effectively promote the dehydroxylation and dealkylation of phenols [43,45],the ring-opening and hydrogen-transfer reactions of P-ArHs[38,42],the aromatization of AlHs[43],as well as the HDO,HDN,HDS reactions of heteroatom compounds[44,46],and then the‘‘hydrocarbon pool” was formed.Moreover,it is important to note that the hydrocarbon pool is hydrogenpoor,and its activity determines the yield of the target products L-ArHs.Besides,its activity needs to be maintained in a hydrogen rich environment,otherwise the target product will undergo polycondensation to generate P-ArHs or carbon deposition [47].Additionally,it also can be seen from the results in Fig.13,which indicate that the Co/USY and Mo/USY have a better effect on improving the BTXN yield than USY.Combined with the characterization results of the catalysts from Fig.7 and Table 2,it can be founded that the modification by Mo and Co for USY can created the appropriate active sites and pore size,and enhance the production of L-ArHs by control the intensity of the reaction and the selective action of transition molecules.On the other hand,transition metal(Co and Mo)also created an appropriate synergistic effect with zeolites,regulating the product distribution toward L-ArHs [48].
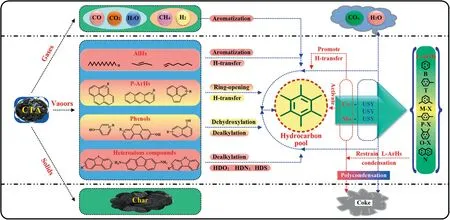
Fig.19.The possible catalytic mechanism of the catalyst for the volatiles of CTA.
4.Conclusions
Aiming at high yield and selectivity of L-ArHs together with low contents of OOCs and P-ArHs,three catalysts USY,Co/USY and Mo/USY were investigated for catalyzing CTA pyrolysis vapors into LArHs.Results showed that Mo/USY have a better ability to regulate the distribution of aromatics and selectively generate of L-ArHs than USY,Co/USY.Moreover,under the optimal FPT of 800 °C,the total yield of L-ArHs catalyzed over USY,Co/USY,Mo/USY were 4032,4363 and 4953 m2·g-1,respectively.Combined with the characterization results of the catalysts,it can be concluded that the modification of USY with Mo and Co can created the appropriate active sites and pore size to enhance the production of L-ArHs.On the other hand,transition metal (Co and Mo) also created an appropriate synergistic effect on promote the H-transfer,active hydrocarbon pool and restrain the polycondensation reactions of L-ArHs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financed by the projects of the National Natural Science Foundation of China(21776229,21908180,22078266),the National Key Research &Development Program of China(2018YFB0604603),the Key Research and Development Program of Shaanxi (2020ZDLGY11-02,2018ZDXM-GY-167).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.06.006.
 Chinese Journal of Chemical Engineering2021年7期
Chinese Journal of Chemical Engineering2021年7期
- Chinese Journal of Chemical Engineering的其它文章
- Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
- The effect of hydrothermal pretreatment on the structure and fast pyrolysis behaviors of ShengLi lignite
- Formation and emission characteristics of VOCs from a coal-fired power plant
- Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers
- Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition
- Kinetics of steam regeneration of SAPO-34 zeolite catalyst in methanol-to-olefins (MTO) process
