Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers
Ming Luo *,Lunzheng Zhou ,Jianjun Cai ,Haiyan Zhang ,Chao Wang
1 School of Energy and Power Engineering,Jiangsu University,Zhenjiang 212013,China
2 State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering,Ningxia University,Yinchuan 750021,China
3 School of Environmental Science and Engineering and Key Laboratory of Municipal Solid,Southern University of Science and Technology,Shenzhen 518055,China
Keywords:SO2 Coal Iron ore Copper-based Chemical looping combustion
ABSTRACT Chemical looping combustion(CLC)is an energy conversion technology with high efficiency and inherent separation of CO2.The existence of sulfur in coal may affect the CO2 purity and the performance of oxygen carrier due to the interactions between sulfur contaminants and oxygen carrier.The migration of sulfur in Beisu coal during the in-situ gasification chemical looping combustion(iG-CLC)process using two oxygen carriers (iron ore and CuO/SiO2) was investigated respectively.The thermodynamic analysis results showed the formation of metal sulfides was thermodynamically favored at low temperatures and low oxygen excess coefficients,while they were obviously inhibited and the production of SO2 was significantly promoted with an increase in temperature and oxygen excess coefficient.Moreover,part of sulfur was captured and fixed in the forms of alkali/alkaline earth metal sulfate due to the high amount of alkali/alkaline earth metal oxides in the coal ash or/and oxygen carrier.The experimental results showed that the sulfur in coal mainly released in the form of SO2,and the sulfur conversion efficiency (XS) in the reduction stage were 51.04% and 48.24% when using iron ore and CuO/SiO2 respectively.The existence of metal sulfides was observed in the reduced oxygen carriers.The values of XS in the reoxidation process reached 3.80%and 7.64%when using iron ore and CuO/SiO2 respectively.The residue and accumulation of sulfur were also found on the surfaces of two oxygen carriers.
1.Introduction
Carbon capture and storage(CCS)has received increasing attention all over the world to mitigate the greenhouse effect.Nowadays,the atmospheric CO2concentration reaches 411.99 μmol·mol-1[1],while the corresponding value was only 280 μmol·mol-1before the world industrialization movement[2].The combustion of fossil fuels causes substantial emissions of CO2.It is reported that the proportion of power generation using fossil fuels has risen to about 1/3 of the total carbon emitted because of combustion[3].To capture pure CO2stream in a power plant,different technologies have been suggested including the pre-combustion,oxy-fuel combustion and post-combustion [2,4–7].However,those techniques are energy-intensive and therefore reduce the power generation efficiency[8].Chemical looping combustion (CLC) is a highly potential technology,because pure CO2can be sequestrated with no extra energy needed for the gas separation process.The global reserves and consumption of coal are abundant.In 2019,coal accounted for 57.64%of primary energy consumption in China [9].Therefore,it has obvious advantages if this technology can realize its industrial application.There are mainly two options for coal-direct combustionviachemical looping technology [10]:(1)in-situgasification CLC (iG-CLC);and (2)chemical looping with oxygen uncoupling (CLOU).
Sulfur is an inevitable impurity in coal[11],and its content varies from 0.2% (mass) to 11% (mass) in the forms of organic,inorganic and elemental sulfur [12–14].Sulfur in coal may has an negative effect on the CLC process,because it may not only degrade the quality of CO2captured,but also pollute the atmospheric environment [15].
The fate of sulfur in CLOU process has been investigated by different researchers [15–17],while only few investigations about sulfur evolution in theiG-CLC process have been reported.Wanget al.[18] investigated the sulfur emission in the reduction stage of theiG-CLC process of Liuzhi coal using NiFe2O4an oxygen carrier in a TGA apparatus.It was found that SO2was the main sulfurbearing compound,meanwhile,Ni3S2and NiFe2O4were detectedin the reacted oxygen carrier.Berguerandet al.[19]conducted theiG-CLC of petroleum coke in a 10 kWth(kilowatt-hours-thermal)circulated apparatus using ilmenite as an oxygen carrier and the emission of SO2from the fuel reactor was confirmed.The distribution of sulfur in coal during theiG-CLC process has also been investigated in circulated reactor systems ranging from 500 W·th to 100 kW·th [20–23].Results showed that most of the sulfur in coal emitted as H2S and SO2in the fuel reactor,while in the air reactor,SO2was the main sulfur-containing gas[20,21].Even though those circulated experiments showed the sulfur distribution in the air reactor and fuel reactor,the migration behavior of sulfur with reaction time when solid fuel was introduced into the reactor should be further investigated.Moreover,the forms of sulfurous gas are complex.Besides SO2and H2S,other sulfur-containing gases such as COS and CS2are also should be considered.
The distribution of sulfur may also be affected by the kind of oxygen carrier.Now Fe-,Cu-,and Ni-based materials have been investigated and used widely in CLC process [24].Considering the high price and hazardous characteristic of NiO,Fe2O3and CuO show more obvious advantages in the CLC process especially for the solid fuels.Now they have been applied successfully in circulated fluidized bed apparatuses [25–27].In this paper,a natural iron ore and a synthetic copper-based oxygen carrier (CuO/SiO2)were selected to investigate the sulfur transformation behavior in the CLC process.
To evaluate the feasibility of high-sulfur coal iniG-CLC process,it needs to understand the migration behavior of sulfur firstly.Therefore,in this work,the sulfur distribution was studied based on thermodynamic analysis and the sulfur migration behavior was investigated in a small batch fluidized bed reactor.The emission of gaseous sulfur-containing compounds,i.e.SO2,H2S,CS2and COS,with two different oxygen carriers (iron ore and CuO/SiO2)was compared and investigated.Moreover,the interactions between oxygen carriers and sulfur were also investigated.
2.Experimental
2.1.Materials
A high-sulfur coal,Beisu coal from Shandong province of China was selected in this paper.The proximate and ultimate analyses of Beisu coal (air dried basis) are listed in Table 1.After being ground and sieved,coal particles with the size ranging from 150 to 200 μm were used in each experiment.The coal ash was obtained by introducing air into a muffle furnace maintained at the temperature of 800 °C for 4 h.A similar production procedure can be found elsewhere in other literature [28,29].The chemical composition of coal ash is given in Table 2.As shown in Table 1,the total sulfur content in Beisu coal is as high as 4.58%.The forms of sulfur in Beisu coal were analyzed based on the Chinese National Standard GB/T 215-2003,and the contents of sulfate,pyrite,and organic sulfur were determined to be 1.68%,1.42%,and 1.48%,respectively.
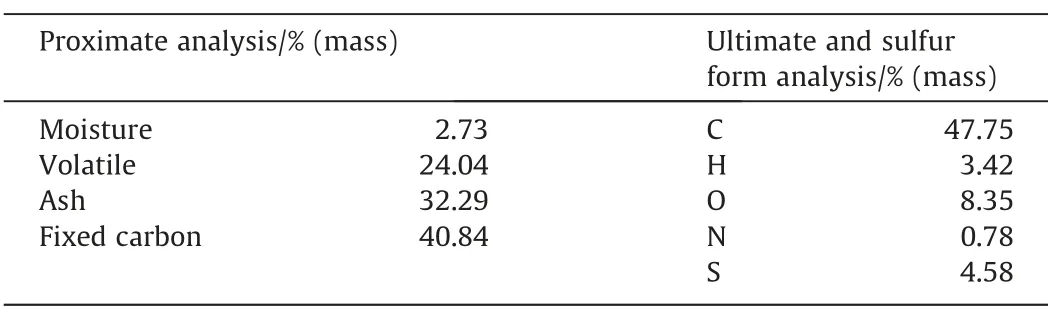
Table 1Proximate and ultimate analyses of Beisu coal
Two kinds of oxygen carriers were used in this paper.Iron ore was purchased from Australia and the composition analysis is shown in Table 3.A synthetic oxygen carrier,CuO/SiO2was prepared by the impregnation method [30].The mass ratio of CuO to SiO2was 1:1,and the particles in the size range of 75–180 μm were selected.The original materials including silica (SiO2) and copper nitrate trihydrate (Cu(NO3)2·3H2O),were directly purchased from the Sinopharm Chemical Reagent Co.,Ltd.

Table 2Ash chemical composition of high-sulfur coal

Table 3Chemical analysis of the iron ore sample/%
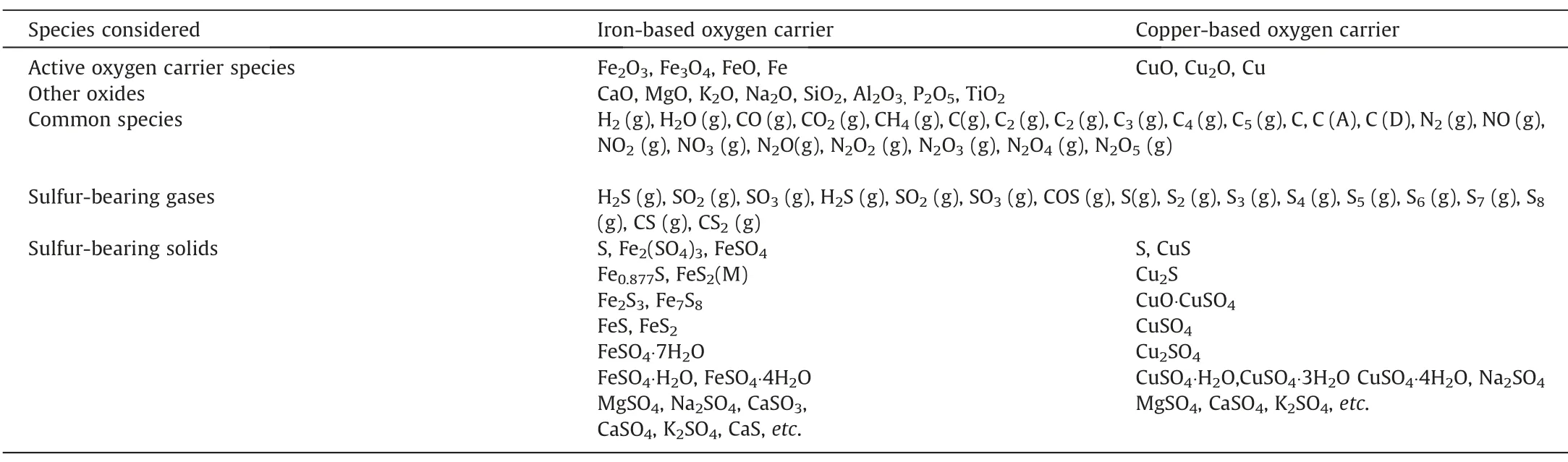
Table 4The substances contained in the simulation system
2.2.Experimental procedure
TheiG-CLC process was conducted in a small-batch fluidized bed (Fig.1).A detailed description of this experimental system can be found in our previous work [31].The reactor was made of stainless steel pipe with an inner diameter of 32 mm and a length of 1200 mm.A stainless steel porous distributor plate was placed 500 mm from the bottom.The reactor was heated with an electric kanthal wire.The temperature of system was measured by a K-type thermocouple and controlled by an electronic controller.The flow rates of N2and O2were measured and controlled by the mass flow controllers.Steam was used as the gasification agent.The distilled water was pumped into a pre-heating system by a water feeding pump (BL100-YZ15,Pre-Fluid) to generate steam,and then it was carried into the reactor by N2.The inlet and outlet lines of the reactor were surrounded and heated by electric heating bands to avoid the condensation of water vapor.
To control bed temperature and preheat the feeding gases,and to prevent the particles including the coal and oxygen carrier from leaking under the distributor plate,a small amount of quartz sand(1.0–1.5 mm)was put into the reactor.Then the oxygen carrier was placed on the quartz sand.The mass was 40 g for iron ore and 15 g for CuO/SiO2.Before each test,the oxygen carriers were heated in a temperature-programmed route(10°C·min-1)in flowing air to the reaction temperature.As temperature is crucial for the stability of oxygen carriers,the experimental temperature for iron ore was set to be 950°C,while considering the low melting point temperature of CuO,the corresponding temperature was set to be 800 °C for CuO/SiO2.Then the mixture of N2(600 ml·min-1) and steam(0.241 g·min-1)was introduced for 15 min to purge air and get an inert atmosphere.The molar concentration ratio of N2to steam was kept at 2:1 inside the reactor.Then 0.2 g coal was introduced and the reduction process began.In the oxidization process,5%O2in N2was used as the oxidizing agent.The time for oxidization process was set to be 20.5 min.N2was injected into the reactor and purged for 300 s to prohibit gas mixing between the two stages.After the oxidation process,the reactor was cooled with N2and then the particles were collected.
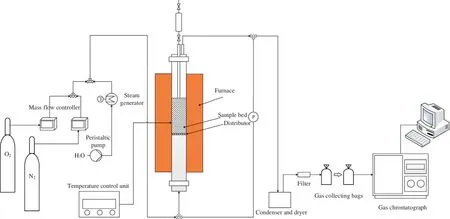
Fig.1.The schematic diagram of small fluidized bed reactor setup.
The relative atomic composition of coal can be represented as CαHβSγOδ(CO2)λ(H2O)μ(N2)ν,and the atom numbers can be obtained based on the proximate and ultimate analyses.Reaction(1) shows the balanced reaction between coal and MxOy:
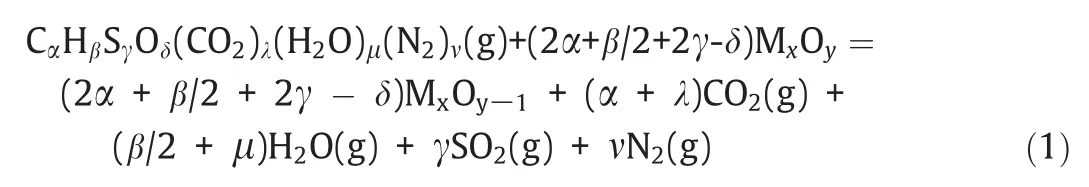
The definition of oxygen excess coefficientR,is as follows[32]:
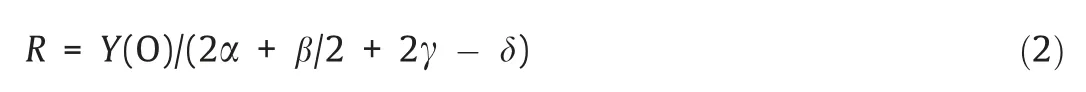
whereY(O) is the actual oxygen contained in the oxygen carrier.
Assuming the reduction form of Fe2O3and CuO is set to Fe3O4and Cu respectively,the stoichiometric amount of iron ore and CuO/SiO2was 15.02 g and 4.46 g respectively when 0.2 g Beisu coal was used as fuel.Therefore,Rwas 2.66 and 3.36 when using iron ore and CuO/SiO2respectively.
The gas products from the fluidized bed reactor after being dried were collected using gas sampling bags.Due to the fast reaction rate,the gas products were sampled every 30 s within the first 3.5 min,and every 1 min from the 3.5 min to 6.5 min,and every 3 min for the rest of time.The sampled gases were introduced to a gas chromatograph (GC-7820),and the sulfur-free gases (N2,O2,CO,CO2,H2,CH4,C2H4and C2H6) and sulfur-bearing gases(H2S,SO2,COS,and CS2) were measured.The detailed description can be found in our former literature [31].Note that the experiments were repeated three times to eliminate the influence of uncertainties.
2.3.Characterization of the oxygen carrier
The phase compositions of oxygen carriers were characterized by X-ray diffraction (XRD,ARL ADVANT Interlipower 4200,ThermoFisher) using Cu Kα radiation between 20° and 80°.Moreover,the chemical properties of the reacted samples were characterized by using energy-dispersive X-ray spectrometry (EDX,S-3400N).
2.4.Data evaluation
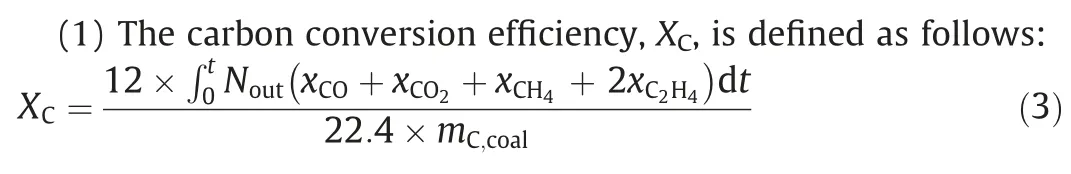
whereNoutis the total flow rate of gases out of the reactor,which can be obtained based on the mass conservation of N2;xiis the molar fraction of componenti,%;mC,coalis the mass of C in coal introduced into the system,g;
(2) The sulfur conversion efficiency
In the experiments,it is difficult to quantitatively estimate the sulfur contained in the solid components,therefore only the fraction of sulfur converted to gas components,XS,is calculated,and it is defined as follows:
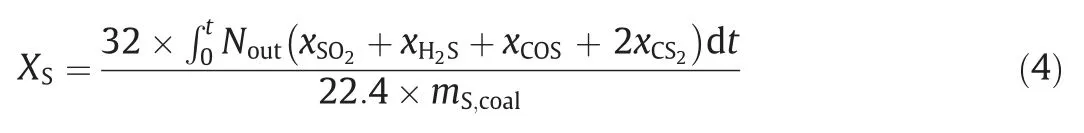
wheremS,coalis the mass of S in coal introduced,g.
3.Results and Discussion
3.1.Distribution of sulfur based on thermodynamic analysis
In this study,the distribution of sulfur in Beisu coal during the reduction process with two different oxygen carriers was first simulated using the HSC Chemistry software 5.0.The system was constructed,and calculated based on the principle of Gibbs free energy minimization [32].The similar simulation has been conducted byWanget al.[33] and Foreroet al.[34],however,the gasification agent was not included in the simulation systems.Here,steam was used as the gasification agent in this simulation system,and the water-to-carbon ratio was maintained at 1:1.
All the substances contained in the simulation system including active oxygen carrier species oxides,conventional species,and sulfur-bearing species are shown in Table 4.

Table 5Mass fractions of each element on the two oxygen carriers after one iG-CLC process
The influences of reaction temperature and oxygen excess coefficient on the distribution of sulfur were investigated.
3.1.1.Effect of temperature on sulfur distribution
The distribution of sulfur at different temperatures with two oxygen carriers is shown in Fig.2.The stoichiometric amount of oxygen carrier was added,and both of the oxygen excess coefficients were set to be 1 when using those two oxygen carriers.From Fig.2(a),it can be found that when the temperature ranged from 400 °C to 650 °C,iron sulfides (Fe2S3,Fe0.877S,and FeS) were observed.As shown in Fig.2(b),Cu2S was the main sulfurbearing component when the temperature was lower than 600°C.However,sulfur was more easily to be oxidized to form SO2at high temperatures (reaction (5)),which is in consistent with the results obtained by Jernalet al.[35].It should be noted that,part of sulfur was captured and fixed in the forms of alkali/alkaline earth metal sulfates such as K2SO4,Na2SO4,and CaSO4due to the high amount of alkali/alkaline earth metal oxides in coal ash or/and oxygen carrier.Therefore,the formation of metal sulfides was obviously inhibited and the production of SO2was significantly promoted with an increase in temperature.
Call it a glove-cleaner if you like, said the old man indifferently. Maybe it will clean gloves. I have never tried. One might call it a life-cleaner. Lives need cleaning sometimes.
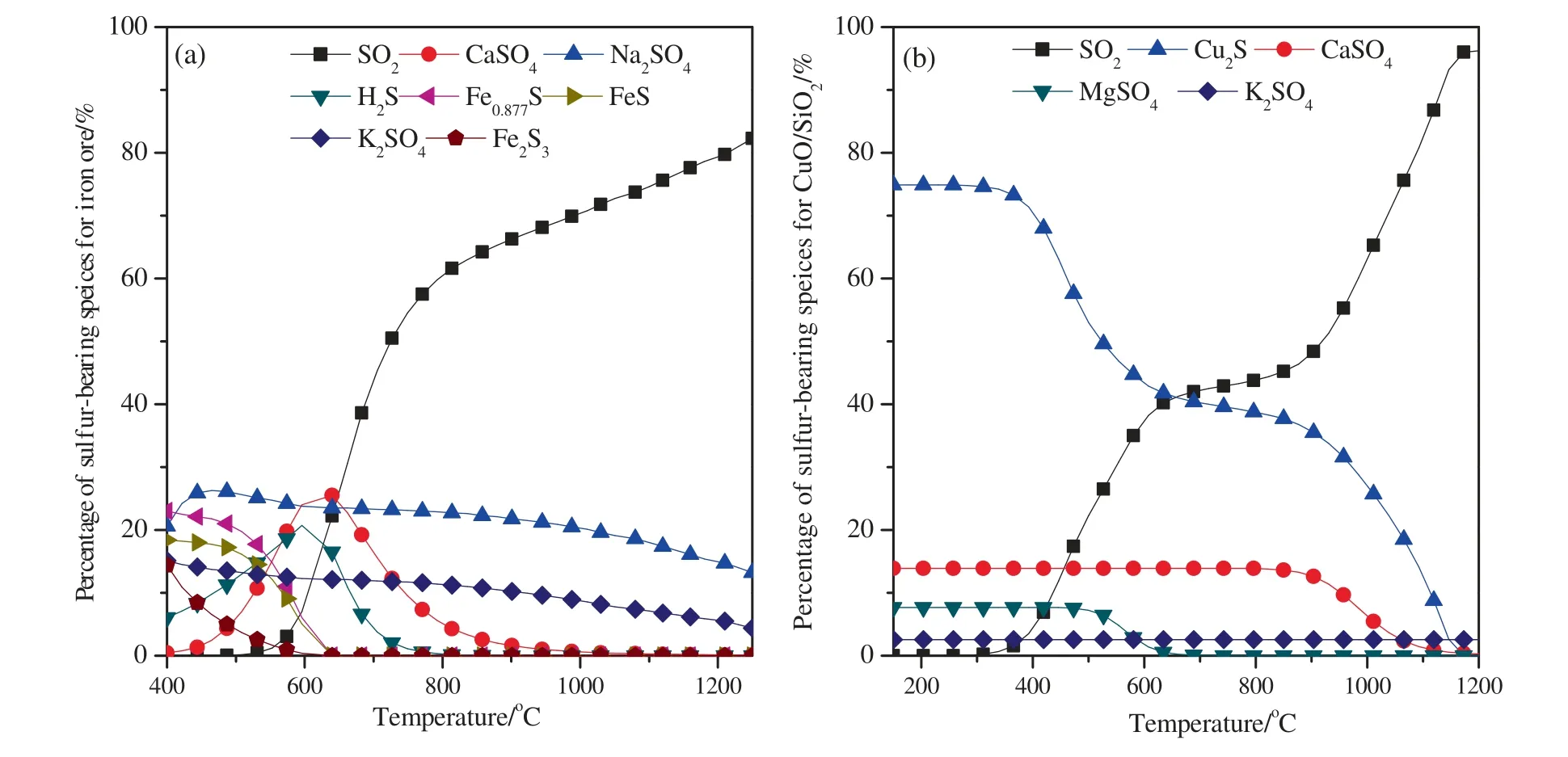
Fig.2.The distribution of sulfur at different temperatures:(a) Iron ore;(b) CuO/SiO2.
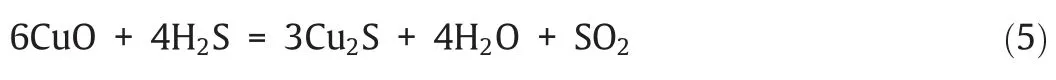
3.1.2.Effect of oxygen excess coefficient on sulfur distribution
The oxygen excess coefficient is a key parameter ofiG-CLC,which will also significantly affect the sulfur distribution.
The distribution of sulfur-bearing species iniG-CLC process as a function of oxygen excess coefficient is shown in Fig.3.The temperature was set to be 950 °C and 800 °C under iron-and copper-based oxygen carriers respectively.As shown in Fig.3(a),Fe0.877S and FeS were the main sulfides whenRwas lower than 0.6.This is reasonable because there was still a certain amount of reducible gases (H2,and CO) in the system and there was not enough lattice oxygen to oxide the sulfur to SO2.WhenRwas higher than 0.6,a small amount of Na2SO4,CaSO4,and K2SO4would be generated due to the reactions between sulfur and alkali andalkaline earth metal oxides in iron ore.A large amount of SO2was generated whenRwas higher than 0.6.This is because more lattice oxygen was applied from the oxygen carrier with the increase of oxygen excess coefficient,and a higher fraction of sulfur in coal would be fully converted into SO2.As shown in Fig.3(b),the formation of Cu2S was thermodynamically favored whenRwas lower than 1.1.However,whenRexceeded 0.9,its molar fraction decreased sharply and the fraction of SO2increased significantly.
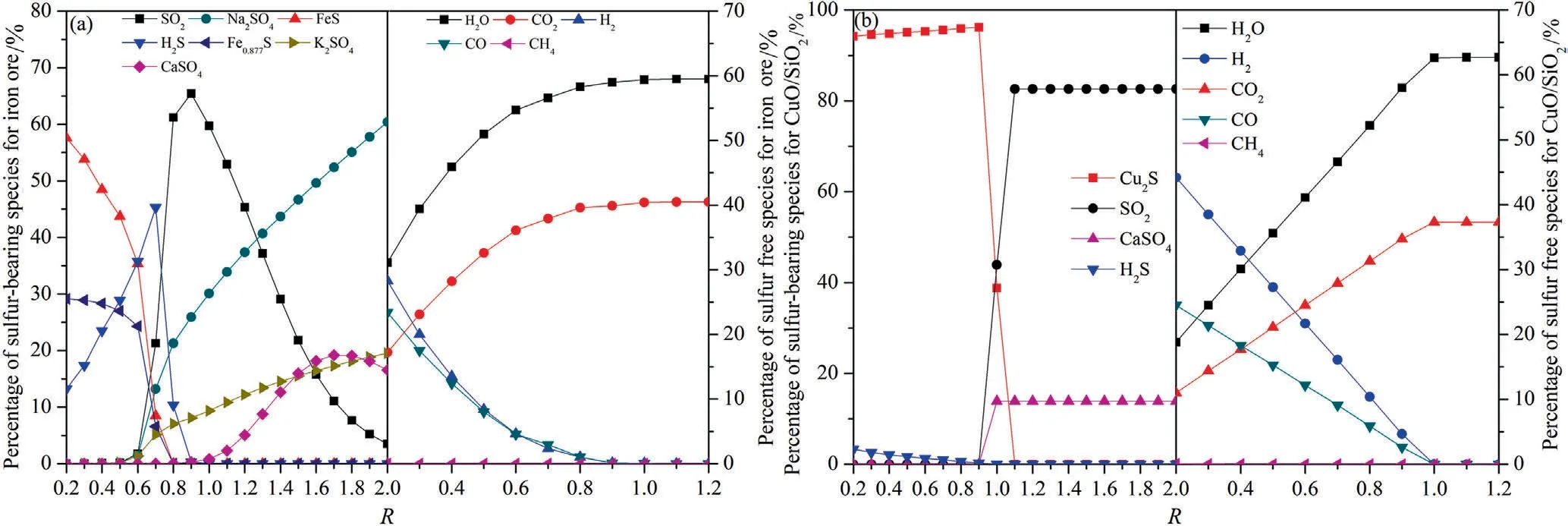
Fig.3.The distribution of different species as a function of oxygen excess coefficient:(a) Iron ore;(b) CuO/SiO2.
Therefore,it can be concluded that,the generation of iron sulfides was not thermodynamically favorable whenRwas higher than 0.8 and the temperature was higher than 650 °C,however,part of sulfur may be captured and fixed in the oxygen carrier because of the existence of alkali and alkaline earth metal oxides in iron ore.For copper-based oxygen carrier,the formation of copper sulfide (Cu2S) should to be considered especially whenRwas lower than 1.1.
3.2.Migration behavior of sulfur in iG-CLC process
The molar fractions of sulfur-free gases with different oxygen carriers in the reduction process ofiG-CLC process are shown in Fig.4.When coal was introduced,it can be found that a small peak of CH4appeared immediately.This is because the reaction rates between pyrolysis products and iron ore were not high enough to thoroughly convert the pyrolysis products,and therefore a certain amount of combustible gases was released out of the reactor.The following appeared peaks of CO and CO2were mainly due to the steam gasification process of coal char and the subsequential reactions between gasification gases and oxygen carrier particles.
There was no considerable difference in the migration behavior of sulfur-free gases when using two oxygen carrier materials.The gases generated were mainly CO2and part of CO and CH4.Only minor amount of H2was detected due to the high reducing reactivity.Those gases reached the peak concentrations rapidly within 3 min and then began to decline with the proceeding of the experiments.Comparing the CO2and CO peaks for iron-and copperbased oxygen carriers,it can be seen that the CO2peak value was higher and CO peak value was lower for copper-based oxygen carrier,which indicates that CuO had higher reactivity to oxidize CO to CO2than that of iron ore.The molar fraction of CO,CO2,and CH4in the gaseous products for iron ore accounted for 14.72%,82.73%,and 2.24% respectively,while the corresponding values were 11.43%,83.03%,and 4.54%for CuO/SiO2.From the perspective of carbon conversion,XCof Beisu coal in the reduction stage reached 79.32% when using iron ore while it increased to 84.1%when using copper-based oxygen carrier.The low carbon conversion rates were mainly due to the reasons as follows:1) part of solid fuel was carried out of the reactor by fluidizing agent without reacting with the oxygen carrier due to small particle size;2) part of solid fuel may be attached on the inner surfaces of the feeding tube or reactor tube.
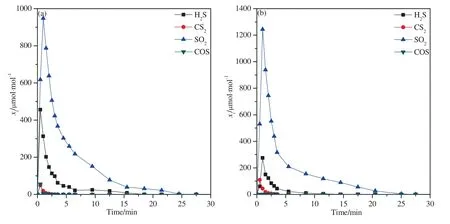
Fig.5.The molar fractions of sulfur-bearing gases in the reduction process:(a) Iron ore;(b) CuO/SiO2.
Fig.5 shows the release of sulfur-bearing gases in the reduction process.SO2was the main sulfur-bearing gas with a maximum concentration of 944 μmol·mol-1and 1243 μmol·mol-1respectively for iron-and copper-based oxygen carriers.This is reasonable because SO2can be produced by different reaction pathways:sulfur oxidation by oxygenated functional groups in coal [36–38],the reaction between the pyrite and steam (reaction(6)and(7))[39],or even the oxidation by oxygen carriers(reaction(5) and (8)) [40],etc.By comparing the two panels in Fig.5,it can be found that the peak concentration of SO2was higher when using copper-based oxygen carrier than iron ore.This is because CuO has higher reactivity to oxidize sulfur-bearing gas especially H2S to SO2.
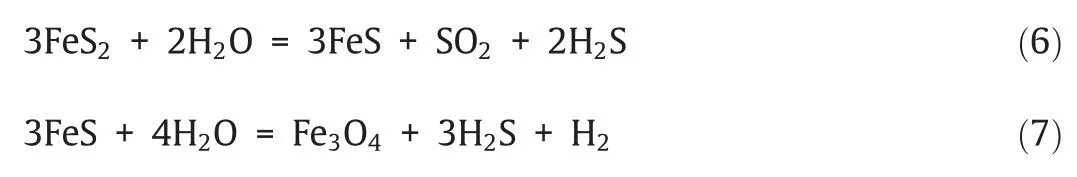
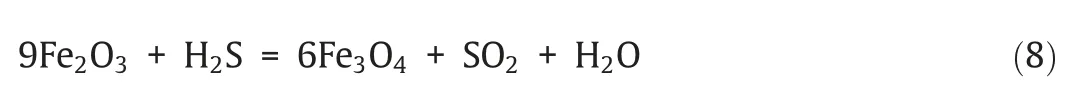
The content of COS was relatively low,which is because the COS-H2S shift reaction (reaction (9)) may happen at a high steam concentration atmosphere.The hydrogen generated in the gasification process may also promote the generation of H2S (reaction(10)),which happens when the temperature is higher than 450°C in the steam gasification process [41,42].

The release of sulfur-bearing gases in the reoxidation process is shown in Fig.6.Results showed that only SO2was released as the sulfur-bearing gas.Note that,SO2had a higher peak concentration when using CuO/SiO2(193 μmol·mol-1) than when using iron ore(89 μmol·mol-1).XSin this process reached 3.80% and 7.64% for iron-and copper-based oxygen carrier respectively.A higher amount of SO2was generated when using CuO/SiO2as an oxygen carrier in this stage,which may be due to the reasons as follows:1)higher amount of metal sulfides or metal sulfates was generated in the reduction process when using CuO/SiO2as an oxygen carrier,and they would be oxidized to be SO2and escaped from the reactor;2)part of fuel might be attached to the wall of the tube or reactor and did not react.The XRD analysis was performed to analyze the composition of the oxygen carriers after the reduction process.N2was introduced to cool the reactor to the ambient temperature at the end of the reduction period,and the XRD patterns of the oxygen carriers after reduction process are shown in Fig.7.
Fig.6.The relative concentration curves of sulfurous gas during the reoxidation process under two oxygen carriers:(a) Iron ore;(b) CuO/SiO2.
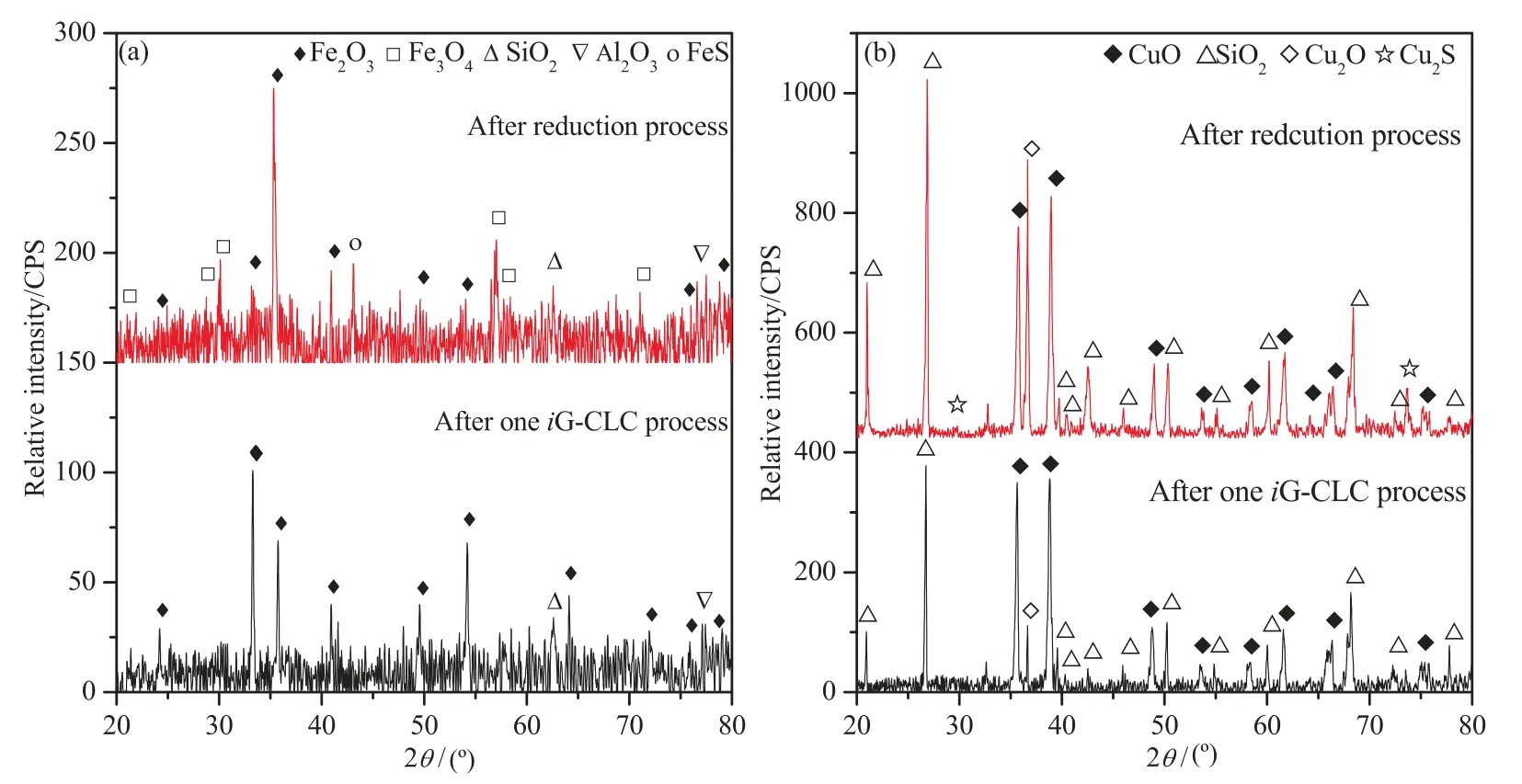
Fig.7.XRD patterns of the reduced oxygen carriers:(a) Iron ore;(b) CuO/SiO2.
It can be found from Fig.7(a) that Fe3O4and Fe2O3were the main active components in the oxygen carrier after the one reduction process.This is reasonable because the amount of oxygen carrier was excessive,and thus there was still part of Fe2O3unreacted.A small amount of FeS was detected in the reduced oxygen carrier,while in the reoxidized oxygen carrier,sulfur-bearing species were not found.Considering the high fraction of steam in the fluidizing agent,the appearance of FeS is mainly as an intermediate product of R3,rather than due to the sulfurization of the iron-based oxygen carrier.This has also been observed in Fig.7(b).A small amount of Cu2S was detected in the reduced oxygen carrier,which reflects that sulfur reacted with CuO and stored in solid phase.This maybe due to the reaction of CuO or/and Cu2O with sulfurous gases especially H2S and CS2(reaction(5),(11)–(13))][43].However,no Cu2S was detected after reoxidation process.This implied that those sulfur-bearing species generated can mostly be oxidized by the oxygen carrier into SO2.It is worth noting that sulfate sulfur was not detected due to its content was below the detection limit.
The value ofXSin the reduction process as a function of time and the distribution of sulfurous gases in theiG-CLC process are shown in Fig.8.As shown in Fig.8(a),the sulfur conversion fraction to gaseous species at the outlet of the reactor when using CuO/SiO2was lower than when using iron ore.The finalXSin the reduction process reached 51.04%when using iron ore,while the corresponding value was 48.24%when using CuO/SiO2as an oxygen carrier.By comparing the distribution of sulfur,it can be found that higherfraction of SO2and lower fraction of H2S were obtained in the flue gas exit when using CuO/SiO2.This is because of the limited oxidization property of iron ore with H2S [17].
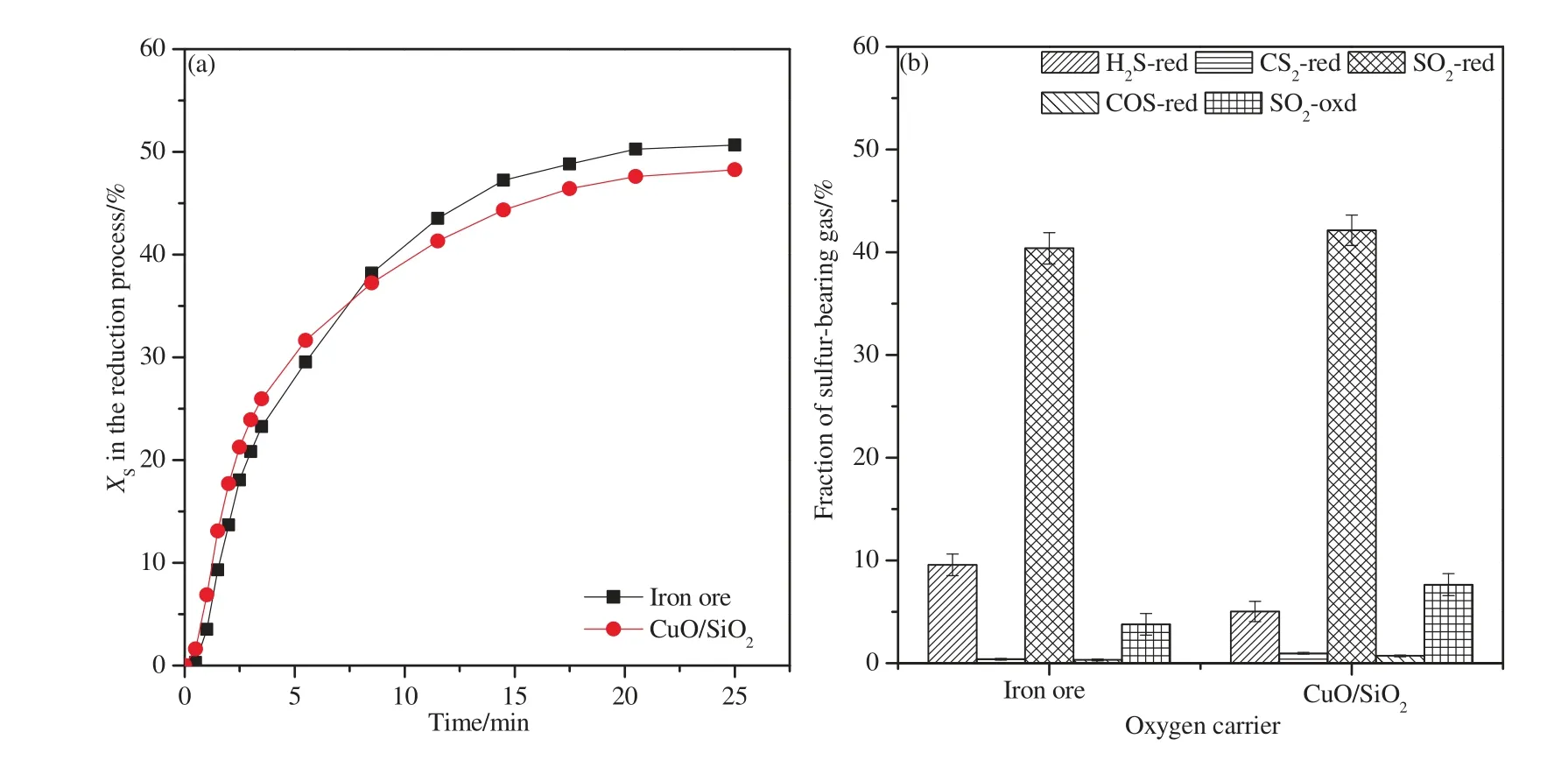
Fig.8. XS in the reduction process and the sulfur distribution in the iG-CLC process.
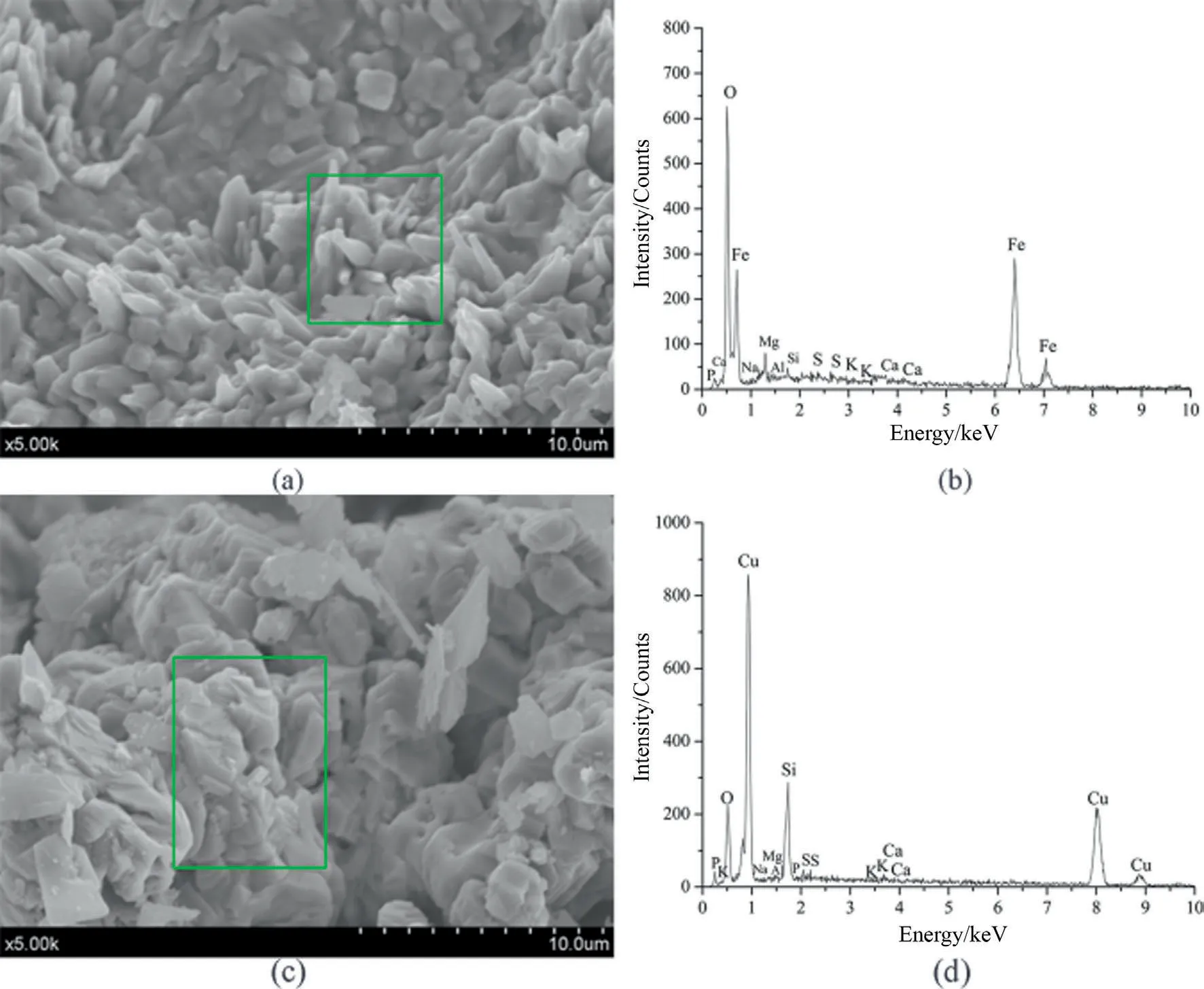
Fig.9.EDX patterns after one iG-CLC process:(a,b) Iron ore;(c,d) CuO/SiO2.
It is hard to thoroughly separate coal ash and unreacted carbon from the oxygen carrier iniG-CLC process of coal especially at high temperatures.Because of the high content of ash and high amount of sulfur in Beisu coal,those impurities may deposit on the surface of the particles or react with oxygen carrier.Therefore,it is necessary to conduct EDX analysis for the two oxygen carriers after theiG-CLC process.The spectra of the surfaces of the oxygen carriers are shown in Fig.9 and the results of the main elements on the surfaces of the two oxygen carriers are listed in Table 5.It is worth noting that the mass ratios of sulfur on the areal surfaces of iron ore and CuO/SiO2reached 0.91% and 1.61% respectively.This indicates that there was still a small amount of sulfur accumulated and stored in the oxygen carrier in solid phase even after one redox process.Moreover,the accumulation of alkali and alkaline earth metals were also detected on the surfaces of copper-based oxygen carrier.Considering those metals are not contained in the fresh oxygen carrier,their existence are mainly due to the adhering of coal ash.The reactivity and stability of those two oxygen carriers in a long term also need to be further studied.In order to alleviate the negative effects of sulfur on theiG-CLC process,desulfurization agents such as Ca-based additives can be added.However,it is difficult to separate the reacted desulfurization agents from the bed materials.Besides the desulfurization performance,the stability of solid desulfurization products and the interactions between the desulfurization agent and oxygen carrier should be considered in the future research process.
4.Conclusions
The sulfur emission in theiG-CLC process of Beisu coal with two different oxygen carriers (iron ore and CuO/SiO2) were studied based on the thermodynamic analysis and experimental investigation.The main results obtained are as follows:
(1) The formation of metal sulfides was thermodynamically favored at low temperatures and low oxygen excess coefficients,while they were obviously inhibited and the production of SO2was significantly promoted with an increase in temperature and oxygen excess coefficient.Moreover,part of sulfur was captured and fixed in the forms of alkali/alkaline earth metal sulfates due to the high amount of alkali/alkaline earth metal oxides in coal ash or/and oxygen carrier.
(2) The sulfur in Beisu coal mainly released in the form of SO2during theiG-CLC process,and the values ofXSin the reduction stage were 51.04%and 48.24%respectively for iron-,and copper-based oxygen carriers.Small amount of metal sulfides was observed in the reduced oxygen carrier by using XRD analysis.XSin the oxidation stage was 3.80% and 7.64% for iron-and copper-based oxygen carrier respectively.The deposition and accumulation of sulfur and coal ash especially the alkali and alkaline earth metals on the surfaces of oxygen carrier particles were detected by EDX analysis.Therefore,additional sulfur/ash removal or polishing methods should be adopted to avoid negative effects of sulfur on carbon purity and oxygen performance in theiG-CLC process of high-sulfur coal.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51606087),Start-Up Foundation of Jiangsu University (15JDG157).Foundation of State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering(2020-KF-07).
 Chinese Journal of Chemical Engineering2021年7期
Chinese Journal of Chemical Engineering2021年7期
- Chinese Journal of Chemical Engineering的其它文章
- Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
- Selective preparation of light aromatic hydrocarbons from catalytic fast pyrolysis vapors of coal tar asphaltene over transition metal ion modified zeolites
- The effect of hydrothermal pretreatment on the structure and fast pyrolysis behaviors of ShengLi lignite
- Formation and emission characteristics of VOCs from a coal-fired power plant
- Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition
- Kinetics of steam regeneration of SAPO-34 zeolite catalyst in methanol-to-olefins (MTO) process
