Kinetics of steam regeneration of SAPO-34 zeolite catalyst in methanol-to-olefins (MTO) process
Huaiqing An ,Hua Li ,Jibin Zhou ,Jinling Zhang ,Tao Zhang ,Mao Ye, *,Zhongmin Liu
1 National Engineering Laboratory for Methanol to Olefins,Dalian National Laboratory for Clean Energy,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
Keywords:Methanol to olefins (MTO)SAPO-34 zeolite catalyst Steam regeneration Regeneration kinetics
ABSTRACT Methanol-to-olefins (MTO) is industrially applied to produce ethylene and propylene using methanol converted from coal,synthetic gas,and biomass.SAPO-34 zeolites,as the most efficient catalyst in MTO process,are subject to the rapid deactivation due to coke deposition.Recent work shows that steam regeneration can provide advantages such as low carbon dioxide emission and enhanced light olefins yield in MTO process,compared to that by air regeneration.A kinetic study on the steam regeneration of spent SAPO-34 catalyst has been carried out in this work.In doing so,we first investigated the effect of temperature on the regeneration performance by monitoring the crystal structure,acidity,residual coke properties and other structural parameters.The results show that with the increase of regeneration temperature,the compositions of residual coke on the catalyst change from pyrene and phenanthrene to naphthalene,which are normally considered as active hydrocarbon pool species in MTO reaction.However,when the regeneration temperature is too high,nitrogen oxides can be found in the residual coke.Meanwhile,as the regeneration temperature increases,the quantity of residual coke reduces and the acidity,BET surface area and pore structure of the regenerated samples can be better recovered,resulting in prolonging catalyst lifetime.We have further derived the kinetics of steam regeneration,and obtained an activation energy of about 177.8 kJ·mol-1.Compared that with air regeneration,the activation energy of steam regeneration is higher,indicating that the steam regeneration process is more difficult to occur.
1.Introduction
Methanol-to-olefins (MTO) as one of the important routes for the production of light olefins has been successfully industrialized in China [1].In MTO reaction,SAPO-34 zolites with the eightmembered ring pore openings and CHA cavity exhibits satisfying catalytic performance and high selectivity to light olefins,attributing to the unique medium-strong acidity and hydrothermal stability[2–6].However,MTO reaction over the SAPO-34 zeolite catalyst suffers fast deactivation due to the coke formation [7–12],which results in a significant drop in methanol conversion and requires the continuous regeneration to restore catalytic activity.Over the past decades,researchers have been working on the reaction mechanism of MTO process and found that MTO reaction follows the hydrocarbon pool (HCP) mechanism [13–18].Based on the HCP mechanism,the active aromatic species,commonly referred to as HCP species,could act as cocatalysts to improve the light olefins selectivity and thus exert a noticeable effect on the product distribution.This suggests that,in either MTO reaction or regeneration process,regulating coke species on the catalyst should receive more attention.
Generally speaking,the deactivated catalyst can be regenerated by three approaches:oxidation,gasification and hydrocracking[11,19–21].Using different regeneration methods will result in different conversion paths for deposited coke on catalyst and thus different flue gas compositions in the regeneration process.The most frequently used method is the oxidation of coke with air or oxygen[1,22,23].This is the common practice for current industrial MTO process,which will however inevitably remove the active hydrocarbon pool species (HCPs).In addition,the main product of flue gas in the air regeneration process is carbon dioxide.In our previous work [24,25],we have proposed that partial regeneration of spent SAPO-34 catalyst by steam can selectively transform the deactivated coke into active HCPs to boost the light olefins selectivity,and with flue gas consisting of more than 88% high-value syngas and only 5% carbon dioxide.These advantages of steam regeneration make it an attractive approach for further developing advanced MTO process.One of the crucial step in developing an industrial MTO regeneration process is to establish the corresponding regeneration kinetics,which are expected to guide the design and stable operation of the regenerator in industrial plant.Despite its good application prospect,kinetics of steam regeneration of spent SAPO-34 zeolite catalyst in MTO process,however,remains blank.Zhaoet al.[26]studied the combustion kinetics of industrial spent MTO catalyst by thermogravimetric analysis,and obtained an average activation energy of 141.1 kJ·mol-1.This is lower than the combustion activation energy of spent fluid catalytic cracking(FCC)zeolite catalyst,which is 157 kJ·mol-1as reported by Dimitriadiset al.[27].
In one of our previous publications,Zhouet al.showed that the rate of steam regeneration is lower than that of air regeneration,and the H/C of the residual coke in the steam regeneration is relatively higher than that in air regeneration [24].Later on they further found that in the steam regeneration process the coke species can be directly transformed to the naphthalene cations,which are more stable than pyrenephenanthrene and other macromolecular cations,and the regenerated catalyst with rich the naphthalene cations can significantly promote the light olefins selectivity in MTO reaction [25].As our current work mainly focuses on the kinetics of steam regeneration and thus the discussion on the difference between the steam regeneration and air regeneration has not been documented in detail.Simultaneously,the nature of coke has an critical impact on the MTO regeneration and reaction.Therefore,before we carried out our kinetic study,we also investigated the evolution of the residual coke on the catalyst with different regeneration temperature during the steam regeneration process.In particular the crystal structure,acidity,residual coke properties and structural parameters of the regenerated catalyst under different regeneration temperature were characterized by XRD,NH3-TPD,TGA,GC–MS and N2physical adsorption–desorption.
2.Experimental
2.1.Catalyst preparation
Commercial SAPO-34 catalyst for industrial DMTO process was used,which was purchased from Catalyst &Catalysis Technology Co.Ltd.of CAS.To remove the template agent,all of the catalyst samples were calcined at 650 °C in air atmosphere for 6 h before the experiments.The catalyst powders were screened 80 mesh(180 μm) before the experimental tests.
2.2.Catalyst characterization
X-ray diffraction(XRD)patterns were obtained with a PANalytical X’pert PRO X-ray diffraction equipment,under the following conditions:CuKα ray (1.54187 nm),scanning voltage 40 kV,scanning current 40 mA,scanning speed 3 (°)·min-1,and scanning range 5-50°.
The temperature-programmed desorption of ammonia (NH3-TPD)was carried on a Micromeritics Autochem II 2920 instrument equipped with a TCD detector.The samples were first heated by a rate of 10°C·min-1from room temperature to 600°C in a stream of helium (99.99%,60 ml·min-1) and pretreated at this temperature and in atmosphere for 1 h.Then,the samples were cooled to 100 °C and subjected to ammonia-saturation in a stream of NH3/He.After purging with helium for 30 min,ammonia was desorbed by heating the sample up to 600 °C at a rate of 10 °C·min-1.
The N2adsorption/desorption measurements were carried out on a Micromeritics ASAP2020 setup.Prior to the measurements,the samples were degassed at 350 °C under vacuum for 4 h.The total surface area and pore volume were calculated based on the Brunauer-Emmett-Teller (BET) equation and t-plot method.
Thermogravimetric analysis was recorded on a SDT Q650 thermal analyzer.The samples with a weight of 15 mg were heated in flowing air from room temperature to 900 °C at a rate of 10 °C·min-1with an air flow rate of 100 ml·min-1.The formula for calculating the amount of coke is ω=(m250°C-m900°C)/m250°C×100%,where ω is the mass percentage of coke in the content of the fresh catalyst,andm250°Cis the mass of the catalyst when the temperature is programmed to 250°C.m900°Cis the mass of the catalyst when the temperature is programmed to 900 °C.
Gas chromatography-mass spectrometry(GC–MS)were used to identify the carbonaceous species[28].Typically,50 mg of samples were dissolved in 1.0 ml of 20% hydrogen fluoride in a screw-cap Teflon.The addition of 1 ml of CH2Cl2with 0.01% C2Cl6as the internal standard allowed extraction of the soluble fraction of coke,then addition of 7%NaOH solution neutralized excess acid and the organic phase were obtained by extraction.The extracted phase was detected with an Agilent 7890 A/5975C gas chromatography–mass spectrometry (GC–MS) instrument equipped with a HP-5 capillary column and FID detector.
2.3.Experimental setup
The experiments of MTO reaction and regeneration of spent catalyst samples were both performed in a fluidized-bed reactor made of quartz,which has an inner diameter of 0.019 m and a height of 0.35 m.For each experiment,5 g of catalyst was loaded into the reactor.The reaction products were in situ monitored with an Agilent 7890A gas chromatograph equipped with a flame ionization detector and a PoraPLOT Q-HT capillary column(25 m × 0.53 mm× 0.02 mm).
2.4.MTO reaction-regeneration cycle experiments
The MTO reaction experiments were carried out under 470 °C with a weight hourly space velocity (WHSV,i.e.,the ratio of the feed rate of methanol to catalyst loading) of 2 h-1and a ratio of water to methanol of 0.2.After the MTO reaction,the deactivated catalyst was heated to the regeneration temperature in a nitrogen purge atmosphere for aging the catalyst,and then achieved in situ partial regeneration with steam stream.After regeneration,the regenerated catalyst was cooled to 470 °C under a nitrogen purge atmosphere for next MTO reaction,and the reaction condition was unchanged.
3.Results and Discussion
3.1.Effects of regeneration temperature
3.1.1.XRD
Fig.1 shows the XRD patterns of regenerated SAPO-34 catalyst samples under different temperature and deactivated catalyst.It can be seen from Fig.1 that all of the catalyst samples display the characteristic peaks of a typical chabazite structure at 2θ values of 9.50°,13°,16.1°,17.53°,20.7° and 30.7° [29].
Table 1 lists the relative crystallinity of all samples.Compared with fresh catalyst,all of the regenerated catalysts show a relatively high crystallinity of 97%-100%,which indicates that the spent catalyst by steam regeneration does not damage the integrity of the crystalline structure,which can be attributed to the excellent hydrothermal stability of the SAPO-34 zeolite catalyst.
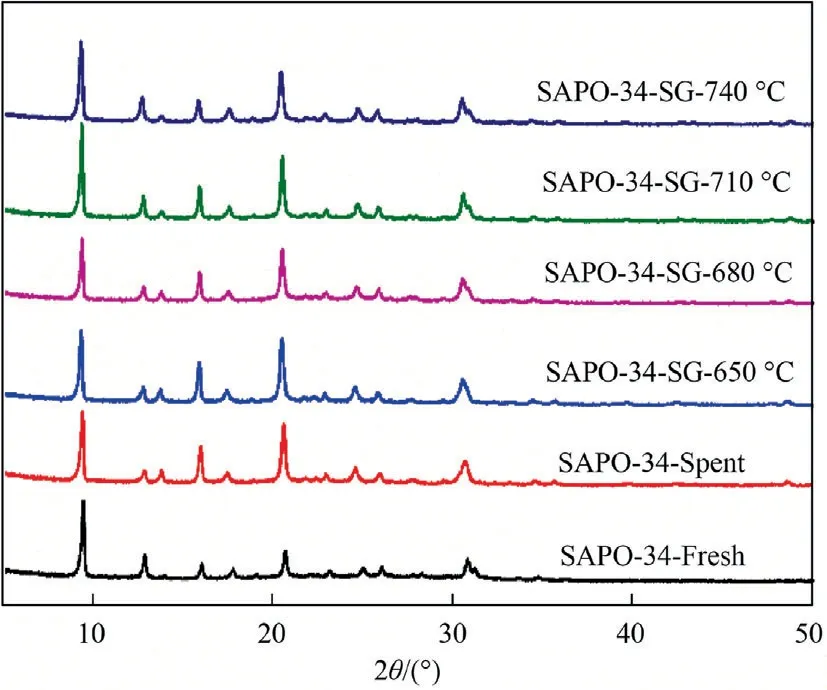
Fig.1.XRD patterns of SAPO-34 samples after regeneration under different temperature with regeneration time of 30 min.
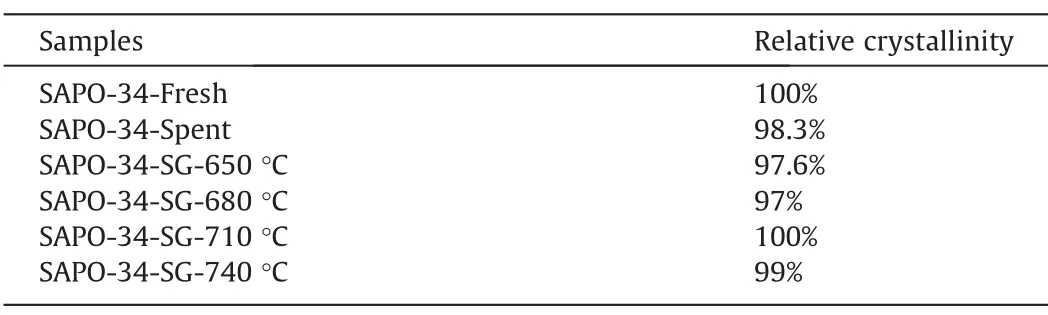
Table 1Relative crystallinity of SAPO-34 samples after steam regeneration under different temperature with regeneration time of 30 min
3.1.2.TGA
Fig.2 is the TG curve of regenerated SAPO-34 catalyst after regeneration under different temperature with regeneration time of 30 min.It can be seen from the figure that the TG curve of the regenerated catalyst can be divided into two stages.The first stage means that the temperature is within the range of room temperature to 250°C.At this time,the amount of coke deposit on the catalyst is reduced to a certain extent.The weight loss is mainly caused by the combustion of a small amount of water and some light volatile substances on the catalyst;the second stage refers to the temperature between 250 °C and 900 °C,at this time the amount of coke deposits on the catalyst decreases significantly,and this period of weight loss mainly due to the burning of coke deposits on the catalyst.Therefore,when calculating the residual coke content of the regenerated catalyst,only the weight lost in the second stage is considered.
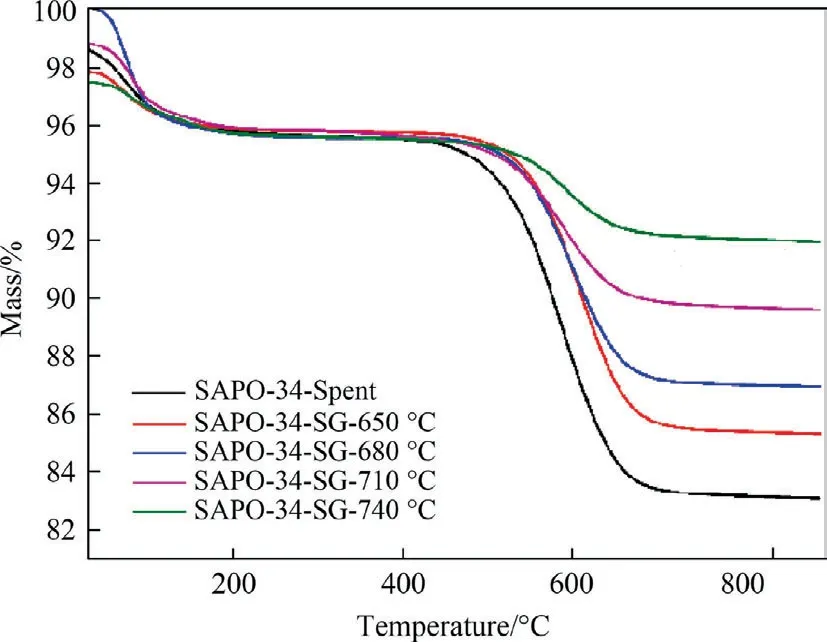
Fig.2.TG curve of regenerated SAPO-34 catalyst after regeneration under different temperature with regeneration time of 30 min.
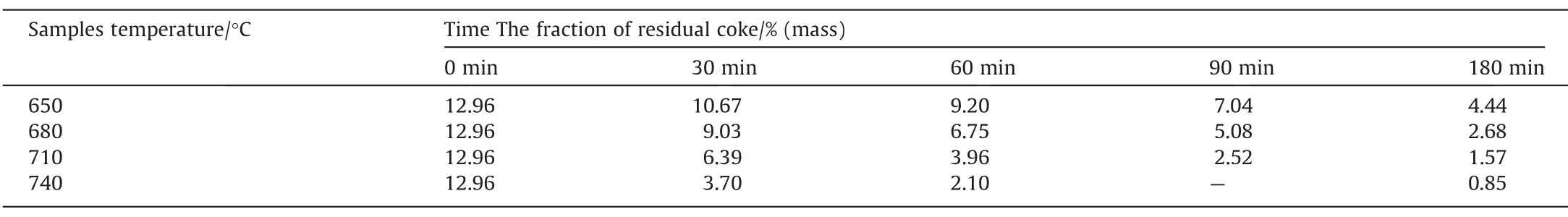
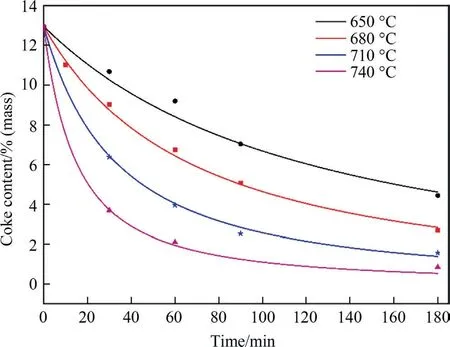
Table 2 lists the quantity of residual coke on the regenerated catalyst samples under different temperature.It shows that the quantity of residual coke on the deactivated catalyst is 12.96%,and the quantity of residual coke has been reduced after steam regeneration under different temperature.The higher the regeneration temperature,the lower the residual coke content,which means that the increase of regeneration temperature enhances the reaction rate and thus the degree of regeneration.In fact,for the same initial coke content,the decrease in the residual coke content of the catalyst suggests for the same regeneration duration the loss of coke content increases,i.e.the regeneration rate of coke increases,in other word,the degree of regeneration enhances with the increase in temperature.
3.1.3.GC–MS
In addition to the quantity of residual coke,the nature of the residual coke is also an important factor affecting the performance of the catalyst.Fig.3 shows the coke dissolving results of SAPO-34 regenerated catalyst samples under different regeneration temperature.In Fig.3,the samples from left to right are that obtained under 740 °C,710 °C,680 °C,650 °C and deactivated catalyst,respectively.It can be found that as the regeneration temperature increases,the color of regenerated catalyst samples after coke dissolving becomes lighter,indicating that the coke species or coke species content on the catalyst samples have been changed,which can be confirmed by GC–MS characterization results as shown in Fig.4.
GC–MS measurements are used to identify the residual coke species with molecular mass smaller than 200.Fig.4 shows that both the coke residue species and the corresponding fractions of these coke residue species on the regenerated catalyst samples have been changed.The coke residue species in the deactivated catalyst are mainly macromolecular organic compounds such aspolymethyl naphthalene,pyrene/phenanthrene,and methyl pyrene/phenanthrene.In particular,the fraction of pyrene and phenanthrene is relatively high,while the fraction of naphthalene is small.After regeneration,the fraction of soluble coke residue decreases,and the higher the regeneration temperature,the less the fraction of soluble coke residue.This can be attributed to the reduction of coke residue on the regenerated catalyst samples(Table 1).Furthermore,it can be found that the fraction of macromolecular organic compounds such as pyrene and phenanthrene has dropped after steam regeneration.Meanwhile the fraction of naphthalene,an active hydrocarbon pool species (HCPs) favoring light olefins formation in SAPO-34 catalyst,has increased significantly.Previous DFT calculations show that the among the intermediates confined in CHA cavity including benzenic (),naphthalenic(),phenanthrenic()and pyrenic()carbocations,naphthalenic cations are stable at high temperature [25].This explains that the fractions of pyrene and phenanthrene decrease and the fraction of naphthalene increases as steam regeneration proceeds.
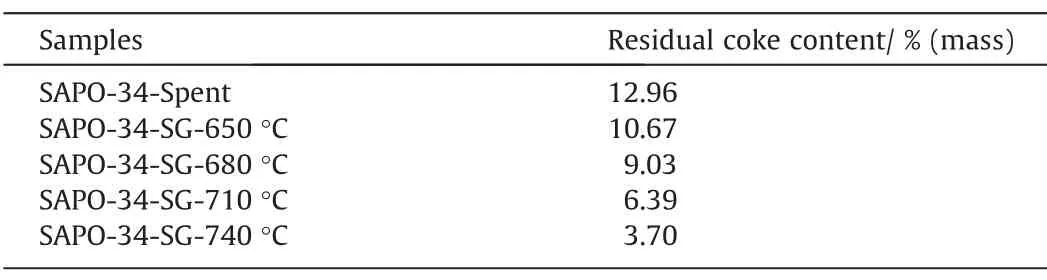
Table 2Residual coke content of SAPO-34 samples after steam regeneration under different temperature with regeneration time of 30 min

Fig.3.Coke dissolving of SAPO-34 samples after regeneration under different temperature with regeneration time of 30 min (from left to right:740 °C;710 °C;680 °C;650 °C;deactivated catalyst).
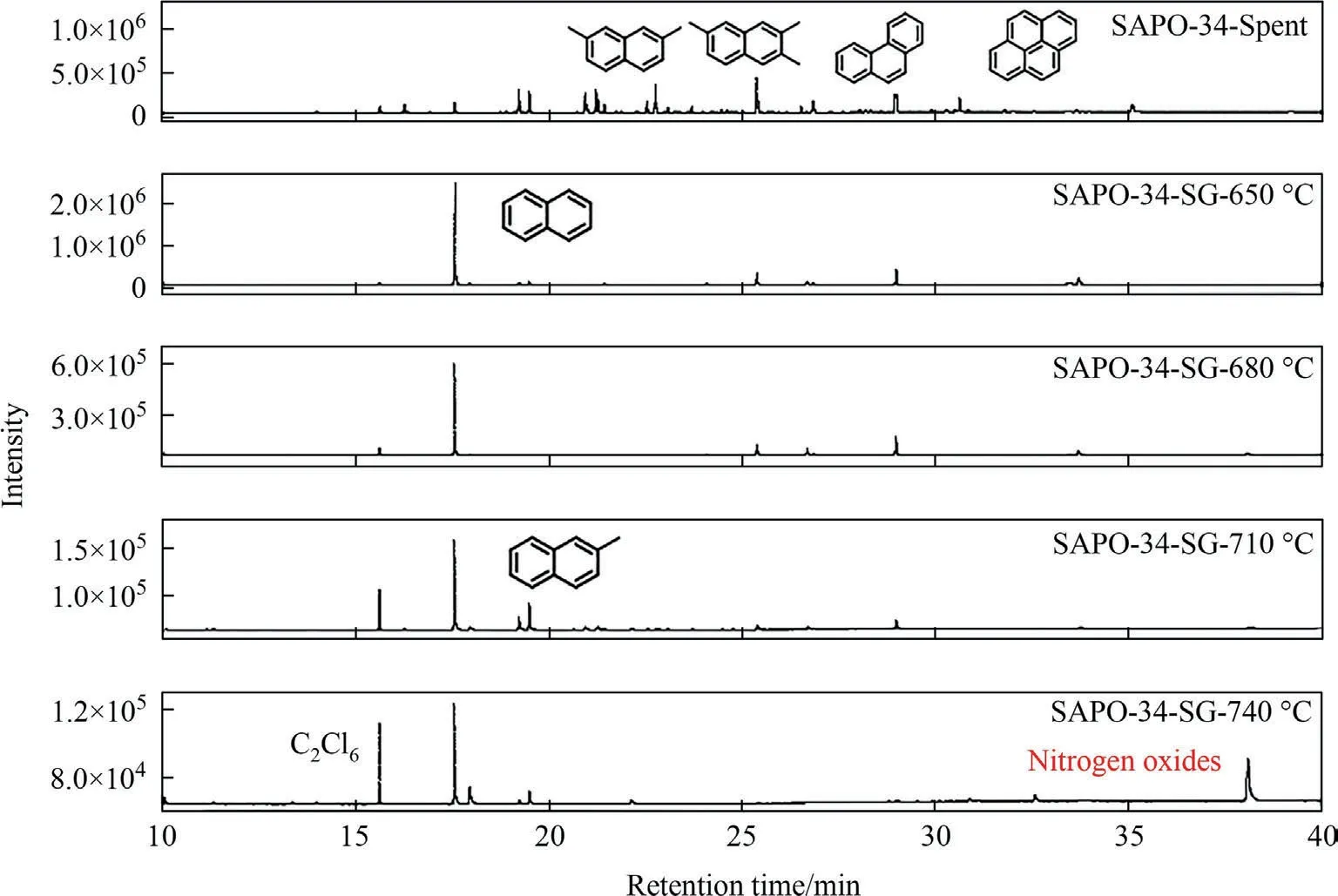
Fig.4.GC–MS results of SAPO-34 catalyst samples after regeneration under different temperature with regeneration time of 30 min.
At the same time,we noticed that the soluble residual coke species changed with the regeneration temperature.When the regeneration temperature is below 680 °C,the soluble residual coke species on the regenerated catalyst contain pyrene and phenanthrene in addition to the main product naphthalene.When the regeneration temperature rises to 710°C,the soluble residual coke species are mainly methylnaphthalene,with almost no pyrene and phenanthrene.This indicates that the increase of regeneration temperature would promote the hydrogen transfer and cracking reactions in the steam regeneration process,resulting in the hydrocracking of polycyclic aromatic hydrocarbons into light aromatic hydrocarbons and the increase of H/C ratio for residual coke.When the regeneration temperature is further increased to 740 °C,in addition to naphthalene and methylnaphthalene,nitrogen oxides also appear in the residual coke.It should be noted that nitrogen oxides would have a negative impact on the MTO reaction performance of SAPO-34 zeolite catalyst.Therefore,the steam regeneration should not be performed at too high temperature in order to avoid the formation of nitrogen oxides in the residual coke.
3.1.4.NH3-TPD
The acidity of SAPO-34 zeolite catalyst is an important factor affecting the MTO reaction.In this study,the NH3-TPD characterization has been used to measure the acidity of the SAPO-34 catalyst samples.Fig.5 shows the NH3-TPD results of the regenerated catalyst samples under different temperature and Table 2 lists the quantities of weak and strong acid sites.As can be seen from Fig.5,except for the sample of deactivated catalyst,all other regenerated catalyst samples show two obvious peaks.These two peaks,which correspond to either the low-temperature (LT,<250 °C) or high-temperature (HT,>250 °C),can be respectively ascribed to weak acid site and strong acid site [30,31].The evolution of catalyst acidity is presented in Table 2.
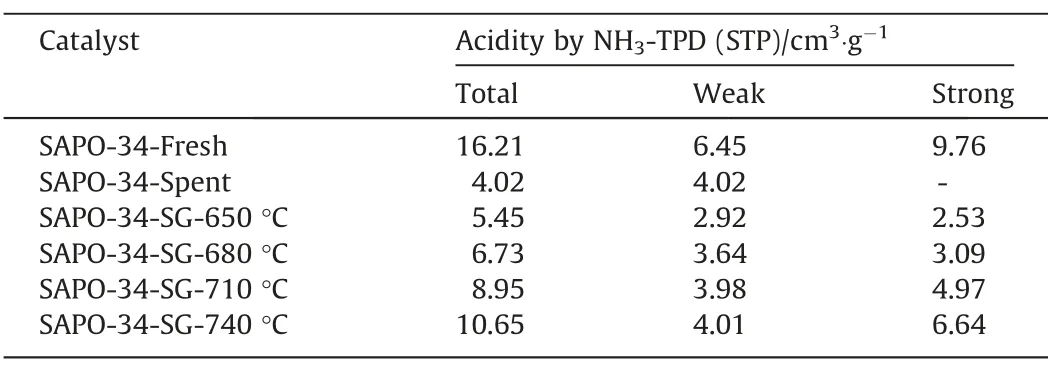
Table 3The quantity of acid on SAPO-34 samples after steam regeneration under different temperature with regeneration time of 30 min
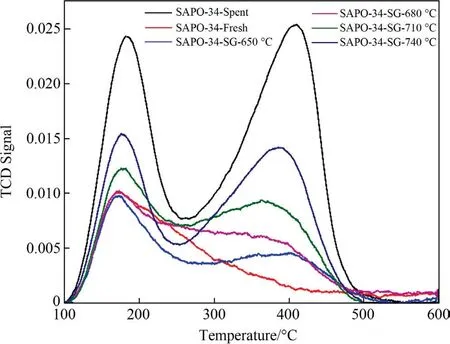
Fig.5.NH3-TPD results of SAPO-34 catalyst samples after regeneration under different temperature with regeneration time of 30 min.
It can be seen from Table 3 that,compared with that of the deactivated catalyst,the acidity of the regenerated catalyst samples have been restored to certain extent after steam regeneration,however,the acidity of the regenerated catalyst is lower than that of the fresh catalyst.The acid content of the regenerated SAPO-34 zeolite catalyst samples increases at a higher regeneration temperature in this work,which indicates that a higher regeneration temperature is favorable for the recovery of catalyst acidity in steam regeneration.The article published by Chenet al.shows that the recovery of the acidity of the regenerated catalyst is mainly due to the removal of the coke on the catalyst,because the coke depositions cover the acid sites of the catalyst [7].As the elevated regeneration temperature would lead to the quicker decrease of the coke residues,the acidic sites covered by the coke depositions can be readily restored at a higher temperature,which results in the enhanced acidity of the regenerated catalyst samples.
Years later, I learned that Coach Jordan had the same confidential5 talk with most of his players, but it didn t matter. He had inspired not only me; he inspired the whole team. We were all for playing under Coach Jordan, and because of that extra measure my dream came true: I was drafted out of Occidental by the Detroit Lions. I was a seventeenth-round pick, but it didn t matter. I had my chance to prove myself in pro football.
3.1.5.N2 physical adsorption/desorption
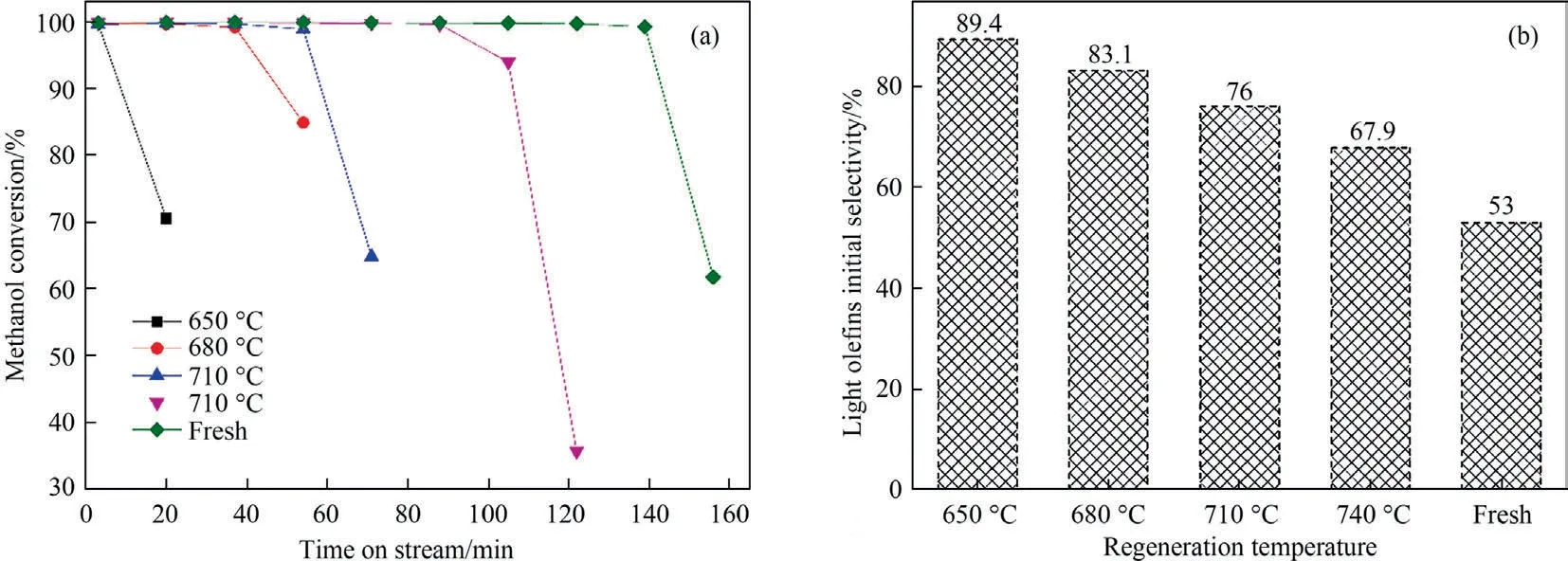
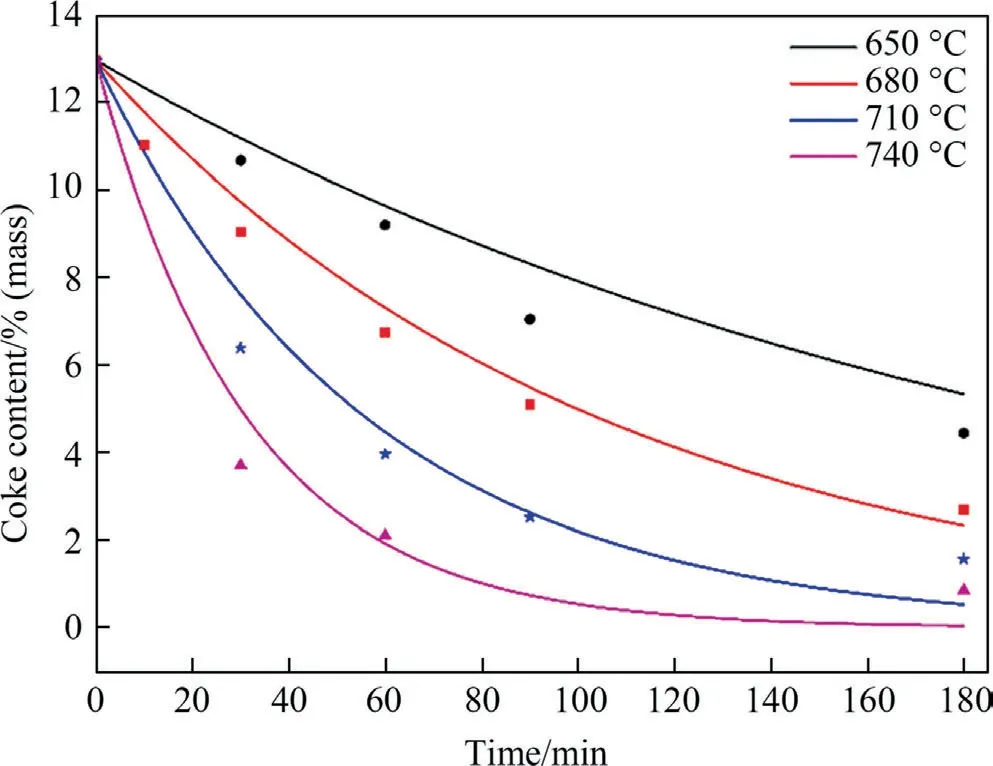
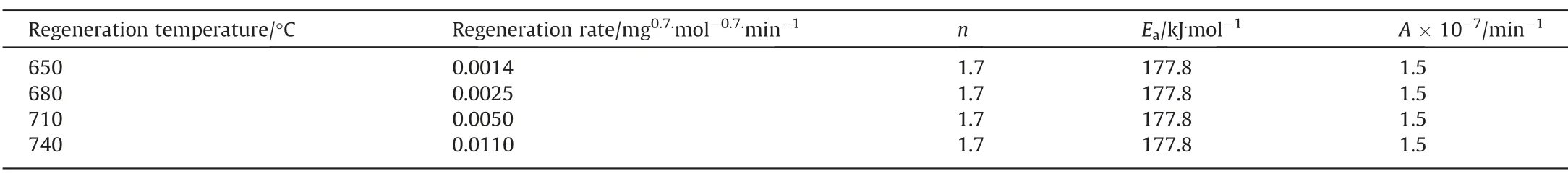
Fig.6 depicts the physisorption isotherms of SAPO-34 regenerated catalyst samples under different temperature by N2adsorption–desorption,with the corresponding textural properties (pore volume and BET surface area) showing in Table 4.It can be seen from Fig.6 that the adsorbed quantity in the regenerated catalyst samples has changed.The higher the regeneration temperature,the greater the adsorbed quantity.This means that as the regeneration temperature increases,the textural properties of the regenerated catalyst samples recover better.As can be seen from Table 4,the BET surface area (SBET) and micropore volume (Vmicro) of the SAPO-34 regenerated catalyst samples under different temperature have the following order:SAPO-34-Spent Table 4BET and pore volume of SAPO-34 catalyst samples after steam regeneration under different temperature with regeneration time of 30 min 3.1.6.Performance evaluation of regenerated SAPO-34 catalyst Fig.7 shows the methanol conversion and initial selectivity of light olefins under different regeneration temperature,which was analyzed based on the first data set obtained by online GC in MTO reaction and in this specified work the sampling for the first data set was carried out for time on stream of 3 min.It can be seen from Fig.7(a) that the higher the regeneration temperature,the longer the catalytic lifetime (i.e.for methanol to maintain a high conversion of 99%) of the regenerated catalyst in MTO reaction.This is because as the regeneration temperature increases,the quantity of coke residue on the regenerated catalyst decreases and the acidity and textural properties of the regenerated catalyst can be effectively restored,thereby the catalytic lifetime of the catalyst is extended.Fig.7(b) shows that the higher the regeneration temperature,the lower the initial selectivity of light olefins.According to the hydrocarbon pool mechanism,the active aromatic species could act as cocatalysts in the MTO reaction,thereby boosting the selectivity of light olefins.Zhouet al.[25] showed that naphthalenic species can promote the selectivity of ethylene.Therefore,combined with the GC–MS results,we can anticipate that the reasons why the initial selectivity of light olefins decreases with the increase of regeneration temperature can be attributed to the reduction of hydrocarbon pool species such as naphthalene on the regenerated catalyst. Above we discussed the change of catalyst properties during the steam regeneration process and the influence of regenerated catalyst on the MTO reaction performance.In the following section we try to establish a kinetics for steam regeneration of the spent SAPO-34 zeolite catalyst in MTO process.In order to calculate the kinetic parameters of the steam regeneration process,we also used our previous data obtained under different regeneration time.Table 5 shows the fraction of residual coke. Table 5The fraction of residual coke on SAPO-34 samples after steam regeneration 3.2.1.Linear fitting It is assumed that when deposited coke reacts with the steam,the change of the quantity of coke deposition satisfies whereCcrepresents the quantity of coke deposition on the catalyst,kthe reaction rate constant,andtthe time (the unit is min).From the initial condition,that is,the initial value of coke deposition isCcmax(12.96%),the change of quantity of coke deposition with time is: Fig.7.The methanol conversion (a) and initial selectivity of light olefins (b) under regeneration temperature with regeneration time of 30 min. By use of the Arrhenius formula together with formula (1) and (2),the linear fitting results are shown in Fig.8.It can be found that the linear fitting results show certain deviation with the actual values,especially for higher regeneration temperature and longer regeneration time.According to the error formula (Eq.(4)) the error is 0.68,and compared with the measured value,the deviation is larger,which indicates that the reaction between coke deposition and steam may not be the first-order reaction. 3.2.2.Nonlinear fitting We further assumed that the reaction between the coke deposition and steam is thenthorder reaction: Fig.8.Linear fitting results of kinetics of the steam regeneration. wherenrepresents the reaction order.The change of coke deposition with time can be given by: According to the Eqs.(3),(5),(6),the nonlinear fitting results are shown in Fig.9.It can be seen that the fitting results can well represent the actual values.According to the error formula (Eq.(4)),the calculated error value is 0.33.Compared with the linear fitting result,the error value is reduced by one time.The calculation results of kinetic parameters are listed in Table 6.It can be seen directly from Table 6 that the reaction rate of steam regeneration increases as the regeneration temperature increases.The reaction ordernis 1.7,and the activation energy is 177.8 kJ·mol-1. Table 6Kinetics parameters of steam regeneration In conclusion,the kinetics of steam regeneration of the spent SAPO-34 zeolite catalyst in MTO process can be expressed as Fig.9.Nonlinear fitting results of kinetics of the steam regeneration. withA=1.5 × 107min-1andEa=177.8 kJ·mol-1. 3.2.3.Comparison with kinetics of air combustion regeneration At present,the research on the regeneration kinetics of methanol to olefin process mainly focuses on the kinetics of air combustion of coke deposited in SAPO-34 zeolites.Zhaoet al.[26] used different methods to calculate the average activation energy of SAPO-34 catalyst in the air regeneration of MTO process and found the average activation energy is 141 kJ·mol-1.We calculated that the activation energy of the steam regeneration is 177.8 kJ·mol-1.It can be seen that the activation energy of steam regeneration is higher than that of air combustion regeneration,which indicates steam regeneration process has higher energy barrier.This also reflects that steam regeneration is more difficult to occur compared to the air combustion in MTO process. In this work,a kinetic study on the steam regeneration of spent SAPO-34 catalyst has been carried out.In doing so,we first investigated the effect of temperature on the regeneration performance by monitoring the crystal structure,acidity,residual coke properties and other structural parameters with XRD,NH3-TPD,TGA,GC–MS and N2physical adsorption–desorption.The results show that with the increase of regeneration temperature,the compositions of residual coke on the catalyst change from pyrene and phenanthrene to naphthalene,which are normally considered as active hydrocarbon pool species in MTO reaction.However,when the regeneration temperature is too high,nitrogen oxides can be found in the residual coke.Meanwhile,as the regeneration temperature increases,the quantity of residual coke reduces and the acidity,BET surface area and pore structure of the regenerated samples can be better recovered,resulting in prolonging catalyst lifetime. We have further derived the kinetics of steam regeneration.It is found that the reaction order of the steam regeneration reaction is 1.7,and the activation energy is about 177.8 kJ·mol-1.Compared that with air regeneration,the activation energy of steam regeneration is higher,indicating that the steam regeneration process is more difficult to occur. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements We are grateful to the National Natural Science Foundation of China (91834302).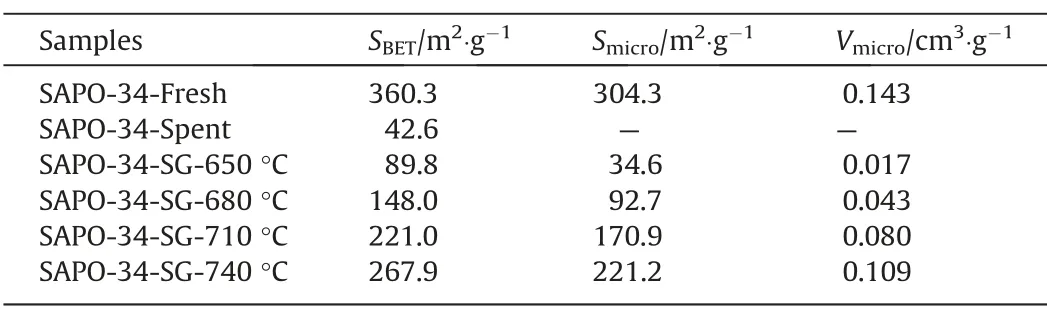
3.2.Kinetics of steam regeneration



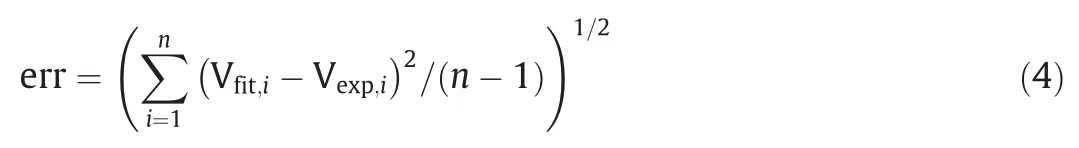



4.Conclusions
 Chinese Journal of Chemical Engineering2021年7期
Chinese Journal of Chemical Engineering2021年7期
- Chinese Journal of Chemical Engineering的其它文章
- Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
- Selective preparation of light aromatic hydrocarbons from catalytic fast pyrolysis vapors of coal tar asphaltene over transition metal ion modified zeolites
- The effect of hydrothermal pretreatment on the structure and fast pyrolysis behaviors of ShengLi lignite
- Formation and emission characteristics of VOCs from a coal-fired power plant
- Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers
- Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition
