Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition
Huaizhu Li ,Lingxue Kong *,Jin Bai *,Zongqing Bai ,Zhenxing Guo ,Wen Li
1 Key Laboratory of Coal Processing and Efficient Utilization,Ministry of Education,Xuzhou 221116,China
2 State Key Laboratory of Coal Conversion,Institute of Coal Chemistry,Chinese Academy of Sciences,Taiyuan 030001,China
Keywords:Coal gangue Coal rich in calcium and iron Ash fusion temperatures Viscosity-temperature characteristic Phase transformation
ABSTRACT Flow property of coal ash and slag is an important parameter for slag tapping of entrained flow gasifier.The viscosity of slag with high contents of calcium and iron exhibits the behavior of a crystalline slag,of which viscosity sharply increases when temperature is lowered than temperature of critical viscosity(TCV).The fluctuation in temperature near the TCV can cause an accumulation of slag inside the gasifier.In order to prevent slag blockage,it is necessary to adjust the ash composition by additive to modify the flow property of coal rich in calcium and iron.Main components of coal gangue are Al2O3 and SiO2,which is a potential additive to modify the ash flow properties of these coals.In this work,we investigated the ash flow properties of a typical coal rich in calcium and iron by adding coal gangue with different SiO2/Al2O3 ratio.The results showed that the ash fusion temperatures(AFTs)firstly decreased,and then increased with increasing amount of coal gangue addition.Chemical composition of coal ash rich in calcium and iron moved from gehlenite primary phase to anorthite,quartz and corundum primary phases.The slags with coal gangue addition behaved as a glassy slag,of which the viscosity gradually increased as temperature decreased.Besides,a high SiO2/Al2O3 ratio of coal gangue was beneficial to modify the slag viscosity behavior.Addition of coal gangue with a high SiO2/Al2O3 ratio impeded formation of crystalline phases during cooling.This work demonstrated that coal gangue addition was an effective way to improve the ash flow properties of the coal rich in calcium and iron for the entrained flow gasifier.
1.Introduction
Coal gasification is a feasible way to realize the clean and efficient utilization of coal resources.Compared with other gasification technologies,the advantages of entrained-flow coal gasification are high carbon conversion,low pollution and good flexibility of feedstock[1,2].Due to the high operating temperature(>1300 °C) of the entrained-flow coal gasifiers,the ash is discharged as liquid slag from the bottom of the gasifier.In order to ensure smooth tapping of the slag,the ash flow properties of coal including ash fusion and slag viscosity properties must meet requirement for slag tapping.It is generally accepted that flow temperature (FT) of ash should be less than 1400 °C and the slag viscosity should be 2.5–25 Pa·s within the tapping temperature[3,4].Therefore,the flow property of coal ash and slag plays an important role in the entrained flow gasification.
The ash flow property is largely influenced by its chemical composition.Relationship between ash flow property and chemical compositions can be explained as follows:the acid oxides (SiO2,Al2O3and TiO2) are prone to increase ash fusion temperatures(AFTs) and slag viscosity at high temperature,while the basic oxides(Fe2O3,CaO,MgO,K2O and Na2O)lower the AFTs and slag viscosity [5].Ash of Yibei coal from Xinjiang province has high contents of calcium and iron(Fe2O3+CaO>35.00%(mass)),which results in low FT and slag viscosity at high temperature[6].In addition,the slag exhibits as the behavior of a crystalline slag,of which viscosity sharply increases when temperature is lowered than temperature of critical viscosity (TCV).The fluctuation in temperature near the temperature ofTCVcan cause an accumulation of slag inside the gasifier.Therefore,Yibei coal cannot be directly used in the entrained-flow coal gasification.
In order to achieve coal that can be processed in entrained flow gasifiers,it is usually blend the coal with additives to produce a feed with appropriate ash flow property[7].For the ash rich in calcium and iron,additives with high acid oxide content like SiO2,Al2O3should be selected,such as sand,silica or kaolin.Konget al.[8] found that the AFTs of coal with high contents of calcium and iron (Fe2O3+CaO=64.63% (mass)) firstly decreased with increasing amount of kaolin,then reached a minimum value before increasing again.Slag viscosity at high temperatures increased with increasing kaolin addition,and the slag behaved as a glassy one when kaolin addition was 12%.Huet al.[9] found that sand addition to coal could improve the slag viscosity property of the Jinjitan coal with high contents of calcium and iron (Fe2O3+CaO=38.49% (mass)).When sand addition were 20% and 40%,the slag transformed from a crystalline one to a glassy one.Liuet al.[10]found that silica addition could be used to adjust viscosity fluctuation behaviors of the coals YL and YCW1# with high content of calcium and low content of silicon by adding 10.62%and 6.30% of SiO2.
Coal gangue is one of the solid waste in the process of coal production,the annual yield is about 10%of the coal output.The accumulated stacking amount of coal gangue has reached more than 5 billion tons,and the amount of annual emission is about 100 million tons in China.Spontaneous combustion and pollution of air,water and soil due to coal gangue stacking have become an urgent issue to be solved.Because main components of coal gangue are Al2O3and SiO2[11–13],it can be used as an additive to modify the ash flow properties of Yibei coal,which can not only solve the problems caused by coal gangue stacking but also realize the utilization of coal gangue.However,no studies have been reported on modification of ash flow properties of coal rich in calcium and iron by coal gangue addition.
In this work,we investigated the method of coal gangue addition to modify the ash flow properties of Yibei coal.Two coal gangues were selected.The AFTs and viscosity-temperature curves of slag with coal gangue addition were measured by ash fusion detector and high-temperature rotating viscometer respectively.X-ray diffraction(XRD)was carried out to detect the crystalline minerals.Thermodynamic calculation was used to analyze effect of coal gangue addition on phase transformation of ash and slag.
2.Materials and Methods
2.1.Coal and coal gangue
One coal sample from Yibei,Xinjiang province,was selected and denoted as YB.Two coal gangues with high contents of SiO2and Al2O3were selected to blend with YB coal,and denoted as Gangue-A and Gangue-B,respectively.The samples were ground to a particle size of less than 0.200 mm.Proximate and ultimate analyses of the samples are shown in Table 1.
The coal and coal gangues were ashed in the muffle furnace at 815 °C according to the Chinese standard GB/T 212–2008.Chemi-cal compositions of ashes were analyzed by X-ray fluorescence(XRF)and shown in Table 2.Gangue-A has a high SiO2/Al2O3ratio,and Gangue-B has a low SiO2/Al2O3ratio.
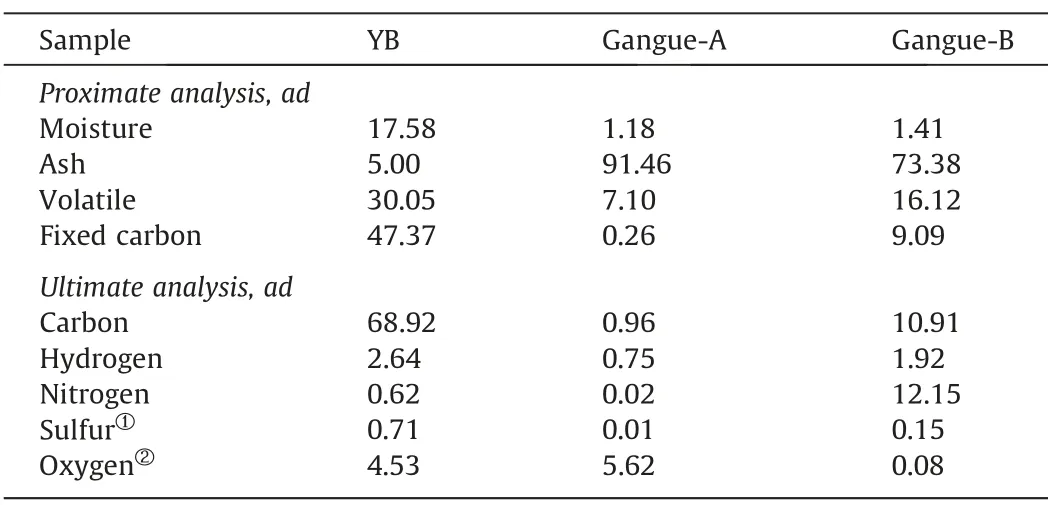
Table 1Proximate and ultimate analyses of coal and coal gangue (% (mass))
2.2.Measurement of AFTs
The AFTs of ash with coal gangue addition were measured by a 5E-AF4115 fusion analyzer under CO/CO2(6:4,volume fraction)atmosphere according to the Chinese standard GB/T 219-2008.The ash cone was heated to 900 °C at 15 °C·min-1,and then increased at 5 °C·min-1to FT.Four characteristic temperatures were recorded:deformation temperature(DT),softening temperature (ST),hemispherical temperature (HT),and flow temperature(FT).The AFTs of the coal and coal gangue are shown in Table 3.The ash of YB coal has high contents of CaO and Fe2O3(CaO+Fe2-O3=37.35%) and low FT (FT=1300 °C).However,ash of coal gangue has high contents of SiO2and Al2O3(SiO2+Al2O3>94.00%),DT of the coal gangue was higher than 1550 °C.

Table 2Ash chemical composition of coal and coal gangue (% (mass))

Table 3Ash fusion temperatures of coal and coal gangue (°C)
2.3.Slag viscosity measurement
The viscosity-temperature curves of YB coal ash slag with coal gangue addition were measured by Theta high-temperature rotating viscometer under CO/CO2(6:4,volume fraction) atmosphere.For the viscosity measurement,the ash was firstly pre-melted in a high temperature furnace,and the final temperature was usually set to 200°C higher than FT to ensure that the slag can melt completely and form homogeneous liquid.
The pre-melted slag was crushed to less than 5 mm,and placed into the crucible.The sample was heated at 10°C·min-1to 1400°C,then at 5°C·min-1to the target temperature and held for 20 minutes under CO/CO2(6:4,volume fraction)atmosphere.The spindle was slowly immersed into the molten slag.The viscosity and temperature were recorded as the temperature decreased at a cooling rate of 3 °C·min-1,which was proved to be a suitable cooling rate for continuous viscosity measurements [14].When the viscosity was over 200 Pa·s or the torque was beyond the limits,the spindle was raised out from the slag and the experiment was stopped.
2.4.Mineral characterization
The slag after a viscosity test was dried and crushed to less than 0.075 mm for XRD analysis.To detect the crystalline minerals in the slag,the XRD patterns were recorded by a Bruker D2 X-ray powder diffractometer with Cu Kα radiation.
2.5.Thermodynamic calculation
FactSage (version 6.4) was used to calculate the phase equilibrium,mass fraction of the solid phases,and diagram of mineral phases in a multi-component ash system.In this work,the ash system consisted of SiO2,Al2O3,Fe2O3,CaO[15,16].Databases,FACTPS and FToxid,were selected in the calculation.The calculations were performed from 1100°C to 1700°C with an interval of 20°C under CO/CO2(6:4,volume fraction) atmosphere.
3.Results and Discussion
3.1.Properties of YB coal ash
Fig.1 shows the viscosity-temperature curve of the YB coal ash.It can be seen that the slag viscosity gradually increased till the temperature decreased toTCV.The temperatures corresponding to 25 Pa·s and 2.5 Pa·s were 1356 °C and 1439 °C,respectively.However,the slag viscosity abruptly increased rapidly when thetemperature lower thanTCV,which demonstrated the behavior of crystalline slag.In addition,TCV(1355°C)was close to the temperature at 25 Pa·s,which was not good for the slag tapping.
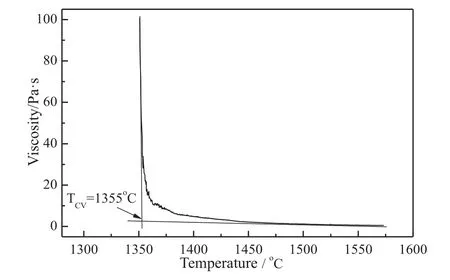
Fig.1.Viscosity-temperature curve of YB coal ash.
In order to explain the slag viscosity of YB coal ash,Fig.2(a)shows the solid phase formation of YB coal ash.The spinel was firstly separated solid phase when the slag was below 1417 °C.The amount of spinel increased as the temperature decreased.The viscosity of YB increased rapidly below 1355 °C,which indicated the precipitation of spinel led to increase of the viscosity.Kondratievet al.[17] found that formation of spinel lowered Fe,Mg and Al in the liquid phase,and the slag started to show non-Newtonian behavior with spinel precipitation.Fig.2(b) shows XRD patter of YB slag after viscosity test.Ohet al.[18] found that formation of crystalline phases during cooling was the main reason for the crystalline slag.It can be seen that gehlenite and hercynite were the crystalline minerals in the slag.That explains why the slag of YB coal ash exhibits the behavior of a crystalline one.Besides,Liet al.[19]found that precipitation of gehlenite can cause the slag viscosity fluctuation.It should avoid the formation of gehlenite in the slag to eliminate the viscosity fluctuation problem[20].
In slagging gasifiers with crystalline slags,the fluctuation of operation temperature nearTCVcan rapidly increase slag viscosity and cause the slag blockage inside the gasifier[21].Once the slag is blocked,the temperature in gasifier has to be increased to melt the blocked slag.In the worst case,the gasifier has to be shut down so that the blocked slag can be removed by mechanical means.Therefore,it is necessary to add additives to change the slag from a crystalline one to a glassy one.
3.2.Chemical composition of slag vs addition of coal gangue
Silica and alumina are the dominant compositions in coal ash with addition of the coal gangue.Therefore,total of SiO2+Al2O3and SiO2/Al2O3ratio are regarded as the important factors which influence mineral transformation and ash flow properties.
Fig.3 illustrates effect of coal gangue addition on the total content of SiO2+Al2O3and SiO2/Al2O3ratio of YB ash.When the coal gangue-A (or B) additions were 2%,4%,8% and 10%,the total content of SiO2+Al2O3of the ash with gangue-A was close to that with the same proportion of that with the coal gangue-B,while the SiO2/Al2O3ratio obviously changed,which became the main factor that affect the composition of the ashes with the coal gangue-A(or B)addition.SiO2/Al2O3ratio was also one important factor affecting slag tapping in the gasifier[22].For the ash slag with same content of SiO2+Al2O3,an increase of SiO2/Al2O3ratio usually prevents the crystallization of slag and is beneficial to increase the viscosity.
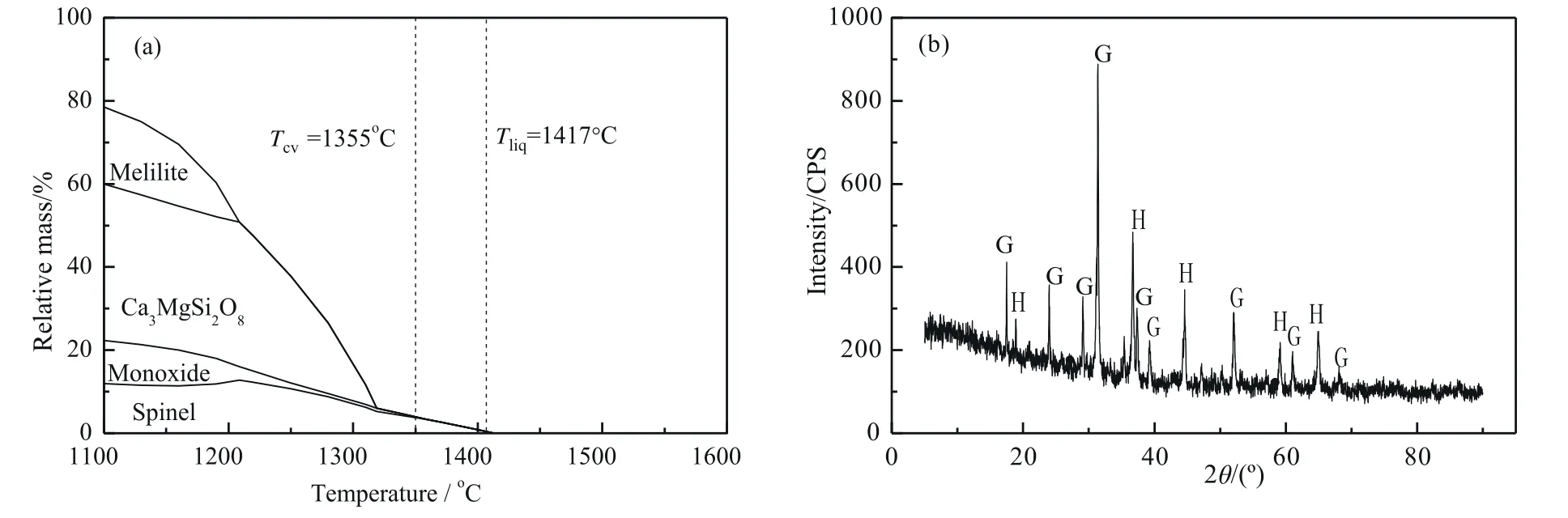
Fig.2.(a) Phase transformation of YB coal ash and (b) XRD pattern of YB slag after viscosity test.G:Gehlenite (Ca2Al2SiO7);H:Hercynite (FeAl2O4).
Fig.3.Total content of SiO2+Al2O3 and SiO2/Al2O3 ratio of YB ash with coal gangue-A (or B) addition.
3.3.Effect of coal gangue on AFTs of YB
As shown in Fig.4,the AFTs firstly decreased with increasing coal gangue addition,and then reached to the minimum value when the coal gangue-A (or B) addition increased up to 2% before increasing once more.When the coal gangue addition was 2%,the FT of ash with Gangue-A(or-B)addition was 1170°C and 1211°C,respectively.In addition,when the coal gangue addition more than 2%,the AFTs increased slowly with increasing of gangue-A addition ratio,while they increased rapidly with increasing of gangue-B addition ratio.The FT of ash with 10% gangue-A addition was 1242 °C,while it was 1390 °C with the same proportion of gangue-B.Because coal gangue-A with a high SiO2/Al2O3had the higher content of SiO2,it was beneficial for low eutectic with silicate.This demonstrated that addition of gangue B showed a more significant effect on the AFTs of YB coal.
Fig.5 shows primary phase of YB coal ash with addition of coal gangue by Factsage calculation.The chemical composition of YB coal ash was located in the gehlenite (Ca2Al2SiO7) primary phase.When the coal gangue-A (or B) addition was 2%,chemical composition of the ash moved to the anorthite (CaAl2Si2O8) primary phase.As for the coal gangue-A addition increased up to 14%,it moved into the quartz (SiO2) primary phase.However,as for the coal gangue-B addition was 10%,it moved into the corundum(Al2O3) primary phase.The melting point of gehlenite was 1593 °C,which was higher than that of anorthite (melting point:1550°C)but lower than that of quartz(melting point:1750°C)and corundum (melting point:>2000 °C).Therefore,the AFTs firstly decreased,and then increased with increasing coal gangue addition.
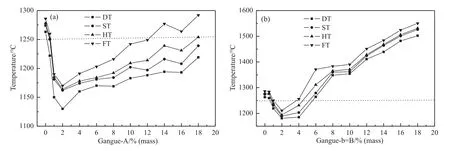
Fig.4.AFTs of YB with different additions of Gangue-A (or -B).
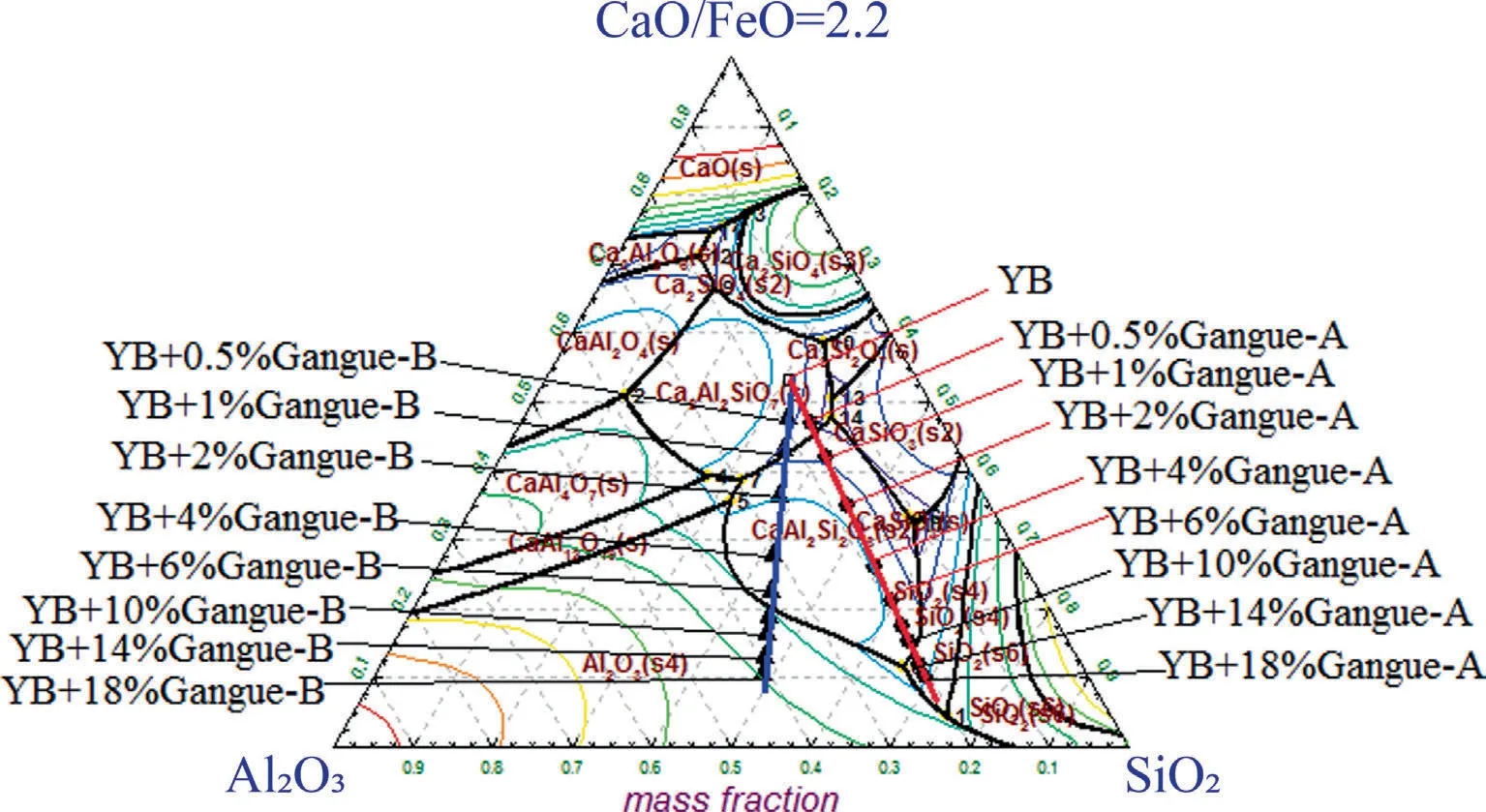
Fig.5.SiO2-Al2O3-(CaO/FeO) phase diagram calculated by FactSage.
3.4.Effect of coal gangue addition on slag viscosity
The viscosity-temperature curves of samples with different gangue addition are shown in Fig.6.It can be seen that the slag viscosity increased with coal gangue of adding proportion.Coal gangue addition that is beneficial for the slag exhibited the behavior of a glassy slag,particularly coal gangue-A.The slag viscosity increased gradually with temperature decreasing,which was beneficial for smooth slag tapping in the gasifier.
Fig.6 also shows that the lowest operating temperature when the viscosity was 25 Pa·s increased with increasing coal gangue addition,which was lower when the same addition of gangue-B.When the coal gangue-A addition was 2%,the temperature was 1248 °C,while it was 1204 °C for coal gangue-B.The results indicated that the operating temperature of slag tapping with coal gangue B was lower than that with the same proportion of coal gangue-A.
3.5.Effect of coal gangue on phase transformation
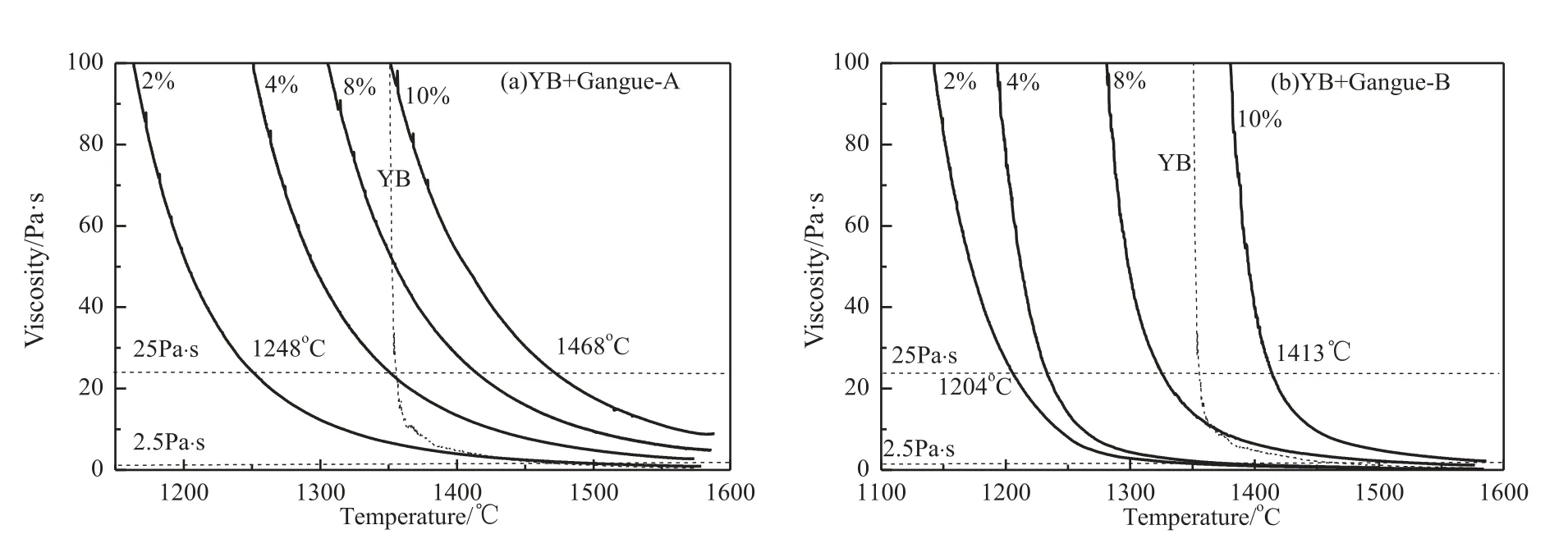
Fig.6.Viscosity-temperature curves of YB with coal gangue-A (or -B) addition.
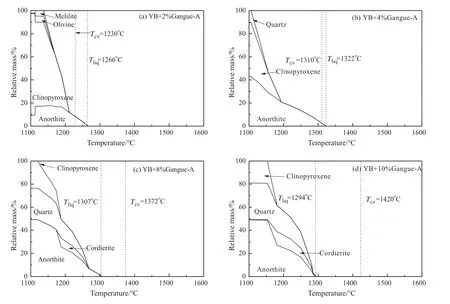
Fig.7.Phase transformation of YB with different additions of Gangue-A.
To account for the effect of coal gangue addition on slag viscosity of YB coal,Factsage was used to calculate the solid phase and liquid phase of slag.Fig.7 gives phase transformation of YB coal ash with coal gangue-A addition.The solid started to deposit when the temperature lowered than the liquidus temperature (Tliq),theTliqat which the slag was a fully liquid without solid phase.As for the coal gangue-A addition,the anorthite was firstly the separated solid phase instead of spinel.In Fig.7(a)and(b),the anorthite started to form at 1266 °C and 1322 °C,the viscosity increased rapidly below 1230 °C and 1310 °C respectively,which indicated the separation of anorthite was the main reason for increase of the viscosity.In Fig.7(c) and (d),the viscosity increased rapidly without solid phase,which indicated change of the slag bulk composition led to increase of the viscosity [23].
Fig.8 presents phase transformation of YB coal ash with coal gangue B addition.When coal gangue B addition was 2% (Fig.8(a)),the spinel was firstly separated solid phase when the temperature was below 1415 °C,but theTCVwas lower than before with the coal gangue-B addition.The anorthite started to form at 1250 °C,which was coincided with theTCV.This implied that the precipitation of anorthite led to increase of the viscosity.In Fig.8(b),the viscosity increased due to separate of anorthite largely.In Fig.8(c) and (d),the first solid phase became mullite when the coal gangue-B addition 8% and 10%.TheTCVincreased due to increase of the mullite with increasing of the coal gangue-B addition.This demonstrated that formation of mullite was the reason for increasing of the viscosity when the coal gangue-B addition more than 8%.
Fig.9 shows XRD patterns of the slags with addition of coal gangue after viscosity test.In Fig.9(a),there was no crystallinemineral in the slags with coal gangue-A addition,which accounted for formation of the glassy slag.However,in Fig.9(b),the crystalline minerals,calcium aluminum iron oxide and magnesioferrite,were detected in the slag with 2% coal gangue-B addition.The crystalline mineral was anorthite when coal gangue-B addition was 8%.All of these crystalline minerals contributed to the formation of the crystalline slags with coal gangue B addition.
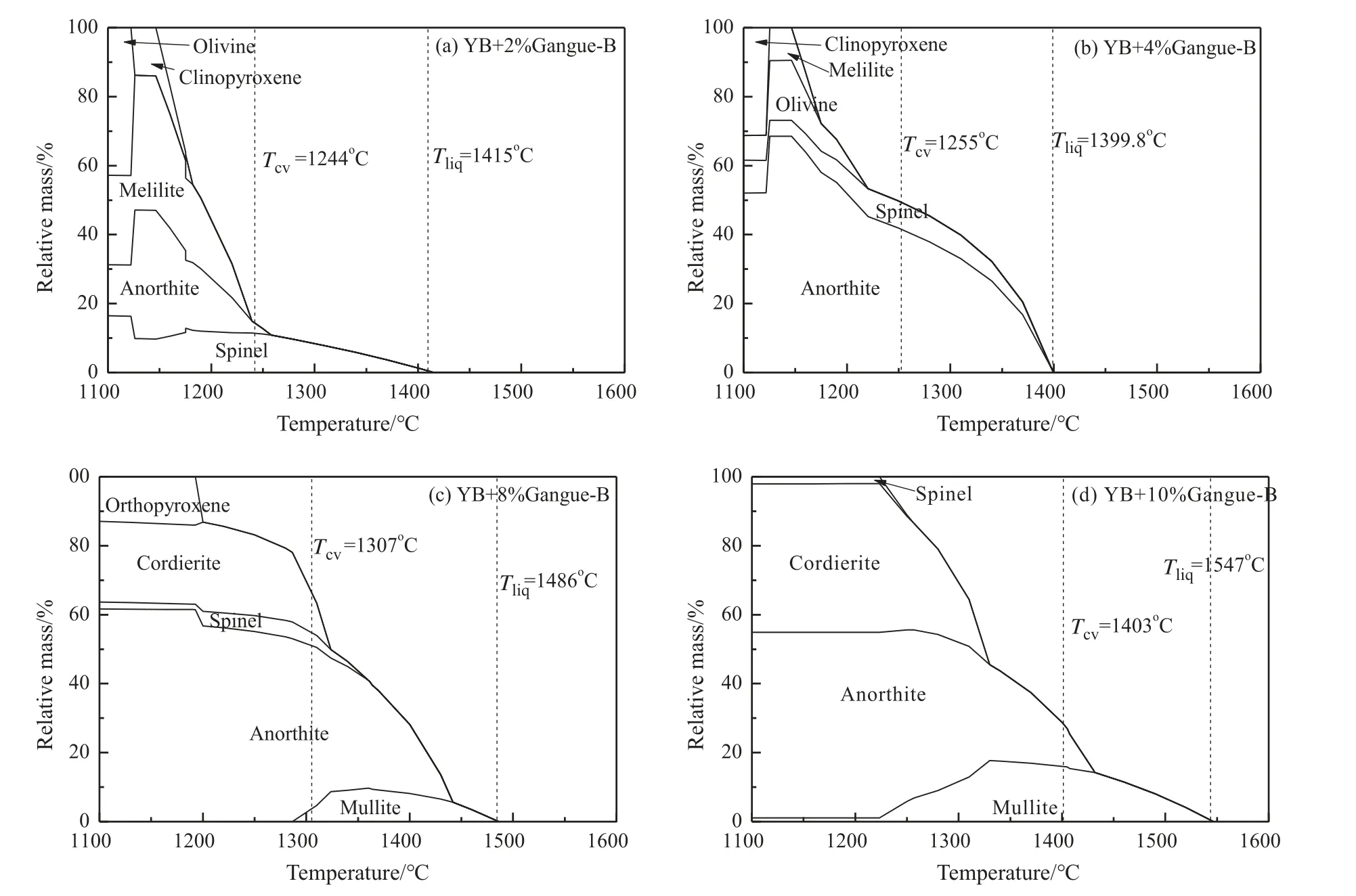
Fig.8.Phase transformation of YB with different additions of Gangue-B.
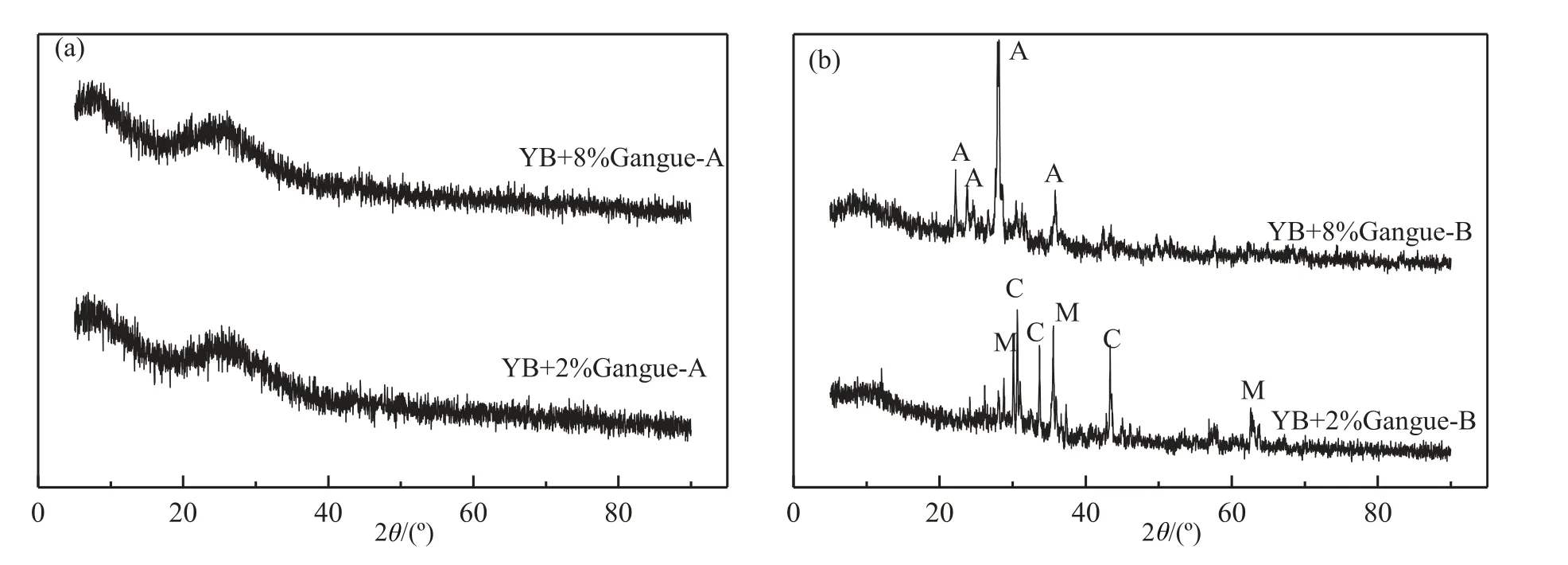
Fig.9.XRD patterns of the slags with coal gangue-A(or-B)addition after viscosity test.A:Anorthite(CaAl2Si2O8);M:Magnesioferrite(Fe2MgO4);C:Calcium Aluminum Iron Oxide (Ca2Fe1.4Al0.6O5).
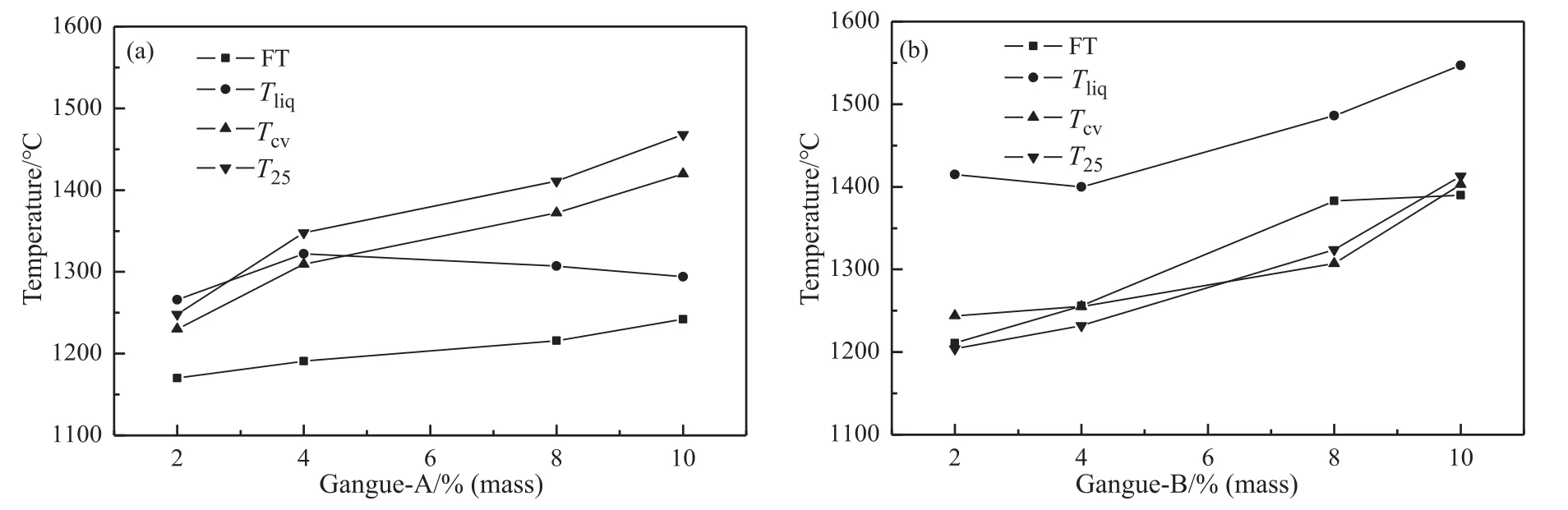
Fig.10.Slag tapping characteristic temperatures of YB coal with coal gangue addition.
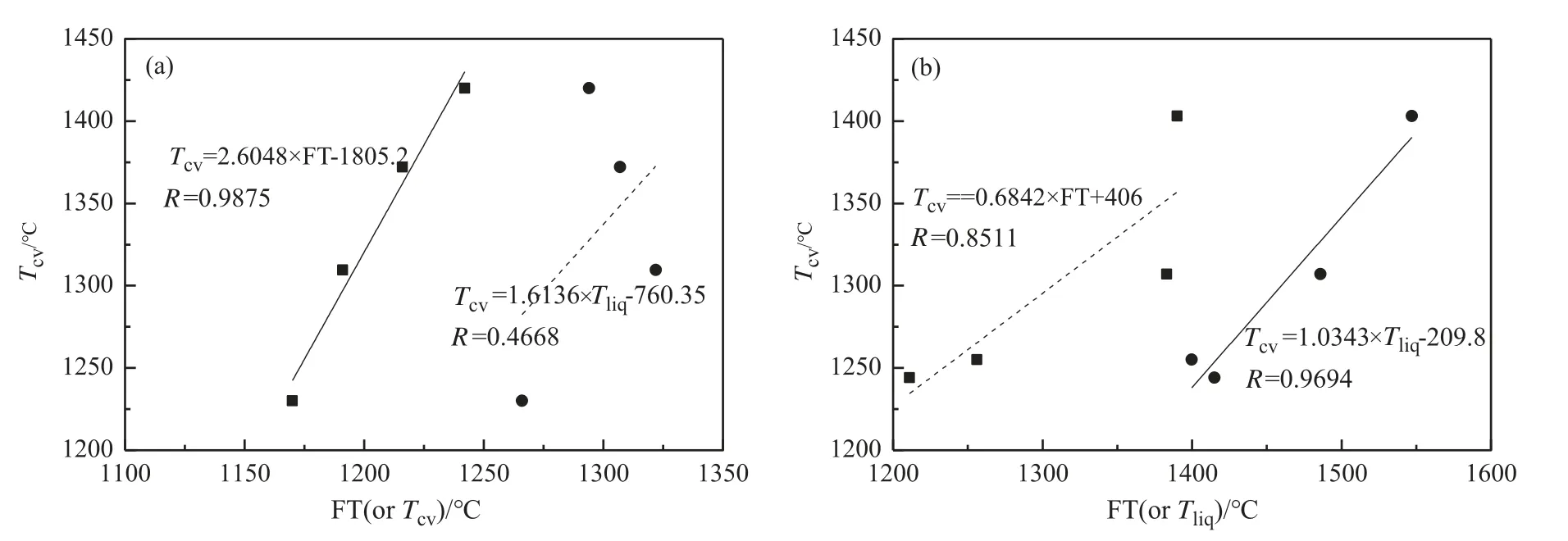
Fig.11.Fitting of TCV with FT or Tliq.
3.6.Effect of coal gangue on characteristic temperatures of slag tapping
TCVandT25(the temperature at which the viscosity was 25 Pa·s)are two important temperatures to determine operating temperature of slag tapping and gasification temperature.However,the measurement of slag viscosity is high cost and not available in most laboratory.In hence,a lot of researches have correlatedTCVwith ash fusion temperatures and theTliq[24].
Fig.10 shows the FT,Tliq,TCVandT25with different additions of Gangue-A (or -B).The trend of FT,TCVandT25with increasing gangue-A addition was similar.TheTCVwas higher thanTliq,which indicated the viscosity of liquid phase may have a stronger effect on the viscosity.However,theTCVandT25with increasing gangue-B addition were close to that ofTliq.TheTCVwith gangue-B addition was far belowTliq,which indicated increase of viscosity was mainly due to solid precipitation.
Fig.11 shows the fitting result ofTCVwith FT(orTliq),and theRvalues which were used to analyze the correlations between theTCVand FT(orTliq).Yanet al.[22]found thatTCVof the glassy slags can be predicted with FT,whileTCVof the crystalline one can be predicted withTliq.Generally,anRvalue of 0.9 or higher is excellent for predicting theTCVvalues of slag.It can be seen in Fig.11 that theTCVcan be well correlated with the FT for the slag with gangue-A addition,while theTCVof slag with gangue-B addition showed a good relationship withTliq.
4.Conclusions
Yibei coal ash was rich in calcium and iron,which exhibited the behavior of a crystalline slag.As a result,the coal was not suitable directly for the entrained-flow gasifier.In this work,we used coal gangue with different SiO2/Al2O3ratio to modify the ash flow properties.The results demonstrated that coal gangue addition was an effective way to improve the ash flow properties of YB coal.The main conclusions are as follows:
(1) The AFTs decreased firstly,and then increased with increasing coal gangue addition.The coal gangue with the low SiO2/Al2O3ratio showed a more significant effect on the AFTs of YB coal due to existence of high melting point Al2O3.Chemical composition of YB coal ash moved from gehlenite primary phase to anorthite,quartz and corundum primary phases.
(2) The slag viscosity of Yibei coal ash increased with increasing coal gangue addition.The slag which behaved as a crystalline slag changed to that which exhibited as a glassy one with addition of coal gangue A.
(3) Addition of coal gangue with a high SiO2/Al2O3ratio impeded the crystallization of anorthite during cooling.A low SiO2/Al2O3ratio of coal gangue was favorable for the crystallization of calcium aluminum iron oxide,magnesioferrite and anorthite.
(4)TCVof slag can be correlated with FT andTliq,which was largely dependent on the viscosity behavior of slag with coal gangue addition.TheTCVof the glassy slag should be correlated with the FT,whileTCVprediction of the crystalline one should be usedTliq.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2017CXNL04).
 Chinese Journal of Chemical Engineering2021年7期
Chinese Journal of Chemical Engineering2021年7期
- Chinese Journal of Chemical Engineering的其它文章
- Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
- Selective preparation of light aromatic hydrocarbons from catalytic fast pyrolysis vapors of coal tar asphaltene over transition metal ion modified zeolites
- The effect of hydrothermal pretreatment on the structure and fast pyrolysis behaviors of ShengLi lignite
- Formation and emission characteristics of VOCs from a coal-fired power plant
- Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers
- Kinetics of steam regeneration of SAPO-34 zeolite catalyst in methanol-to-olefins (MTO) process
