The effect of hydrothermal pretreatment on the structure and fast pyrolysis behaviors of ShengLi lignite
Boyang Bai,Luyao Qiang,Suisui Zhang,Zhiwei Peng,Hang Mu,Xiaoxun Ma*
School of Chemical Engineering,Northwest University,Xi’an 710069,China
International Scientific and Technological Cooperation Base of the Ministry of Science and Technology (MOST) for Clean Utilization of Hydrocarbon Resources,Xi’an 710069,China
Chemical Engineering Research Center of the Ministry of Education (MOE) for Advanced Use Technology of Shanbei Energy,Xi’an 710069,China
Shaanxi Research Center of Engineering Technology for Clean Coal Conversion,Xi’an 710069,China
Collaborative Innovation Center for Development of Energy and Chemical Industry in Northern Shaanxi,Xi’an 710069,China
Keywords:Hydrothermal pretreatment Lignite Physicochemical structure Fast pyrolysis
ABSTRACT The structure and composition of coal determine its fast pyrolysis characteristics,and the study of the relationship between them can play an important role in the efficient and clean utilization of coal.So,in this work,hydrothermal pretreatment was used to artificially change the structure and composition of ShengLi (SL) lignite,which was used to investigate the influence of structural changes on pyrolysis.The physicochemical structure and composition of samples were characterized by X-ray diffraction,specific surface area and porosity analyzer,solid-state 13C nuclear magnetic resonance,Fourier transform infrared spectroscopy,and elemental analyzer.Pyrolysis experiments were carried out in a powderparticle fluidized bed reactor,and the distribution and composition of the pyrolysis products were analyzed.The gasification activity of char was investigated by thermogravimetric analysis with a CO2 atmosphere.The results show that hydrothermal pretreatment(HTP)can destroy the cross-linking structure of SL lignite,and affect its aromaticity,pore structure,functional group,and carbon structure to change the distribution and composition of pyrolysis products of SL lignite,especially the composition of tar.Finally,the structure–activity relationship between the structure,composition,and pyrolysis characteristics of coal was comprehensively studied.
1.Introduction
Energy is a necessary condition for a country’s economic development and an important basis for social progress.Coal,China’s main resource,still accounts for 57.7% of the country’s total primary energy consumption in 2019 [1].Low-rank coal accounts for about 55% of China’s coal reserves,of which lignite accounts for about 13%,however,due to the low energy density of lignite,its direct utilization is difficult [2].With the large consumption of medium and high-rank coal and the shortage of energy,how to use a large amount of lignite efficiently and cleanly is a key point.
Middle-or low-temperature pyrolysis technology can convert lignite into syngas,char,and tar [3,4],the tar from pyrolysis can be used to process fuel oil or other chemicals [5,6].Meanwhile,the char can be gasified or directly combusted to generate electricity due to its moisture was removed [7],it is observed that lignite pyrolysis technology not only increasing the added value of lignite but also solving the problem of an energy shortage,with obvious economic benefits.Therefore,it is considered to be an effective way to use lignite.A large number of studies have shown that the pyrolysis characteristics of coal are affected by temperature,reactor,pressure,catalyst,atmosphere,coal structure,and other factors [8–11].The structure and composition of coal have a vital influence on the pyrolysis characteristics,the structure–activity relationship between coal structure and pyrolysis characteristics can be established by artificially changing the coal structure and then examining its pyrolysis characteristics.Some researchers try to use various pretreatment methods to change the physical and chemical structure of lignite,such as metal catalyst load,thermal pretreatment,acid pretreatment,ionic liquid pretreatment,and organic solvent thermal pretreatment,etc.[12–16],these pretreatment methods can effectively change the structure of coal.Thermal pretreatment can effectively remove moisture and oxygen content in lignite,but it will increase its cross-linking degree;ionic liquid can efficiently damage the cross-linking structure of coal and increase the yield of naphthalene compounds;Organic solvent thermal pretreatment can efficiently remove oxygen from coal and advance the hydroliquefaction reactivity,but it will reduce the pyrolysis reactivity.
Hydrothermal pretreatment (HTP) is another widely used method in coal pretreatment [17].During the HTP,water can be thought of as a solvent for dissolving oxygen-containing functional groups or as a reactant for reacting with coal,and some thermally stable covalent bonds at high temperatures will break under hydrothermal conditions[18].Compared with the above methods,the HTP operation process is simple and the separation of products is much easier [19–21].The researchers found that hydrothermal processes can destroy the pore structure of coal and remove some oxygen-containing functional groups to remove water from the coal[22],so HTP has an improvement in the slurry forming capacity of lignite [23,24] and reduce its critical temperature of spontaneous combustion [25].Due to the strong solubility of subcritical water,HTP also can effectively remove metal elements such as K,Na,and even Hg in coal [19,26,27].Moreover,researchers [28,29]found that HTP can effectively improve the calorific value and the combustion performance of low-rank coal.Recently,previous researches on lignite hydrothermal pretreatment mainly focus on its influence on physical properties,slurry capacity,fluidity,liquefaction,and combustion performance of coal [23–25].Some researchers [20,30] have investigated the influence of hydrothermal pretreatment on coal structure and changes in the yield of pyrolysis products,however,the composition and characteristic changes of pyrolysis products were not investigated.
In this work,the effects of HTP on the structure,pyrolysis characteristics,pyrolysis product distribution,and composition of ShengLi(SL)lignite were studied.The HTP of SL lignite at different temperatures was performed from 200 to 300°C,the fast pyrolysis experiment of samples was conducted in a powder-particle fluidized bed reactor at a temperature of 600 °C,pyrolysis gas and tar were analyzed using gas chromatography (GC) and gas chromatography-mass spectrometry (GC–MS),respectively.The structure and composition changes of coal samples were characterized by Brunauer-Emmet-Teller (BET),Fourier transform infrared spectrometer (FT-IR),Element analyzer,13C nuclear magnetic resonance (NMR),X-ray diffraction (XRD),and inductively coupled plasma optical emission spectrometry (ICP-OES).Based on the above research,the structure–activity relationship between SL lignite structure,composition,and pyrolysis characteristics was comprehensively studied.
2.Experimental
2.1.Material preparation
The lignite from ShengLi coalfield in Inner Mongolia is used as raw material,it was sieved into the size of 40–100 μm,dry for 12 h at 105 °C,then sealed and stored in a cabinet filled with N2,named as SL.
The HTP was carried out in an autoclave (500 ml).30 g of coal and 90 ml of deionized water was added to the autoclave for each reaction (The ratio of coal to water is 1:3),then purge with nitrogen for 10 min.The autoclave then heats up to the specified temperature at a rate of 3.5 °C?min-1(200,220,240,260,300 °C,respectively) and held for 1 h,with a stirring rate of 300 r?min-1and 0.1 MPa pressure of N2.After the reactor is cooled to room temperature,the total volume of the gas in the reactor is calculated by the method of exhaust saturated sodium bicarbonate solution,and the solid product was filtered and dry for 12 h at 105 °C.The treated coal samples were named SL-200,SL-220,SL-240,SL-260,and SL-300.The proximate and ultimate analyses of samples are shown in Table 1.
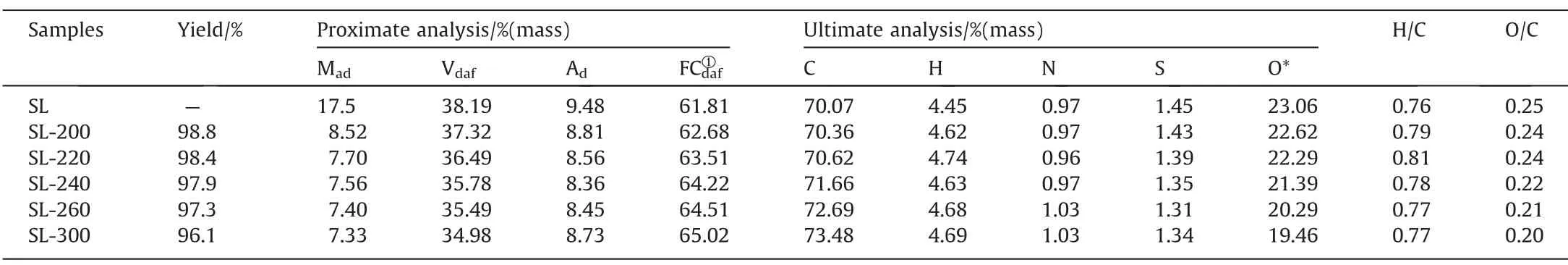
Table 1Proximate and ultimate analysis of coal samples
2.2.Experimental equipment
The pyrolysis experiments were carried out in a powderparticle fluidized bed reactor with silica sand (180–360 μm) as the fluidized medium,the powder-particle fluidized bed reactor is shown in Fig.1.To ensure the smooth progress of the experiment,in the process of the experiment,the space velocity is larger than the carrying velocity of the coal,but less than the carrying velocity of quartz sand,the minimum fluidization velocity and carrying velocity of the fluidized medium were obtained by theoretical calculation,which were 0.17 and 2.84 m?s-1,and the fluidization height is 13.5 cm [3].In each experiment,(6 ± 0.05)g of the sample was pyrolyzed at 600°C in a nitrogen atmosphere,and the flow rate of feeding gas and fluidizing gas were 1.2 and 0.6 L?min-1,respectively.When the reactor temperature stabilized at 600 °C,the coal sample was blown into the reactor through the feeding gas.After a short period of pyrolysis,the pyrolysis products are taken out of the reactor,and the char was first separated into collection bottles at the cyclone separator,the tar was then condensed in a condenser and collected,after the experiment,the tar in the condensing tube was cleaned and collected with a certain amount of dichloromethane.Finally,the uncondensed gas was quantitatively analyzed by an on-line GC analyzer (Micro 3000,Agilent,America).To ensure the accuracy of the experimental results,each group of experiments was repeated three times and averaged.
The pyrolysis yield is calculated by the following formula:
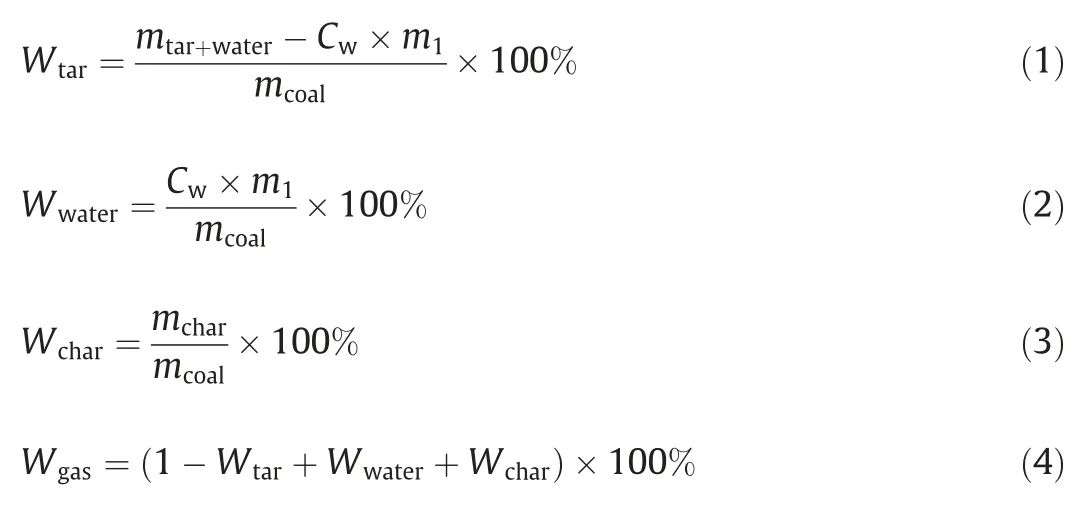
wheremtar+wateris the mass of liquid products,g;m1is the mass content of dichloromethane solution of liquid product,g;Cwis the water content of dichloromethane solution,g,which was measured by a micro moisture meter based on the K-F Coulomb method;andmcoalandmcharare the mass content of coal and char on a dry and ash-free basis,respectively,g.
The gasification experiments of char were carried out on a thermogravimetric analyzer (TGA) (STA-449F3,Netzsch,Germany).In each experiment,(8±0.1)mg samples were taken and heated from 30 to 1000°C at a heating rate of 10°C?min-1kept for 25 min with a CO2flow for 30 ml?min-1.The gasification conversion (x) and gasification reactivity index (R0.5) are calculated by the following formula:

wherem0,mt,m∞are the initial mass of char,the mass of char at timet,and residual mass,respectively,g.τ0.5is the gasification time when the gasification conversion reaches 50%.
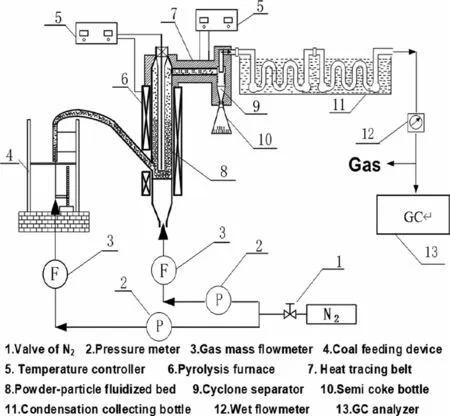
Fig.1.Powder-particle fluidized bed equipment for pyrolysis.
2.3.Analysis method
The ultimate and proximate analyses of coal were measured by SDTGA5000a (Sundy Ltd.,China) and EL-III analyzer (Vario Ltd.,Germany),respectively.The designated metals content in coal was analyzed by ICP-OES (PerkinElmer Company,America).
A specific surface area and porosity analyzer(ASAP 2020-HD88-3FLEX,Micromeritics,America) was used to determine the change in the pore structure of each sample.The samples were measured at–196 °C using liquid nitrogen as the adsorbate.The pore structure parameters,such as the specific surface area,pore size,and pore volume,were calculated using the BET model and BJH model.
An X-ray Diffractometer (D/max 2200 PC03030502,Rigaku,Japan) was used to analyzed the microcrystalline structure of the sample,which used Cu radiation,scanning range 2θ=10°–80°,a continuous scan type,and measurement accuracy of 0.02.
The functional groups in samples were tested by FT-IR spectroscopy (Bruker Company,Germany) at the range of 400–4000 cm-1,and the resolution rate is 4 cm-1,32 scans.Before testing,samples were mixed with KBr in the proportion of 1:100,then grinded into powder evenly and tabletting.
The solid-static13C NMR was carried out by a CP/MAS NMR spectrometer (Bruker AVANCE III 600,Germany),which frequency for carbon and frequency for proton is 150.9 MHz,and the probe length was 4 mm.The rotation rate of magic-angle (MAS) was 10 kHz,the contact time of cross-polarization (CP) was 3 ms,and the circulation delay time was 3 s.
A Shimadzu GC–MS-QP 2010 Plus equipment was used to analyze the composition of tar samples.The flow rate was 1.0 ml?min-1and the split ratio is 10:1.The program was set as follows:began at 40°C for 4 min,then heated to 70°C at 4°C?min-1(keep for 2 min),then heated to 200 °C at 10 °C?min-1(keep for 3 min),finally heated to 300°C at 4°C?min-1(keep for 5 min).The injection temperatures were 300 °C and the ion-source temperatures were 230 °C.The mass spectrometer was set in the electron ionization mode at 70 eV withm/zfrom 50 to 500.The compounds were identified in the library dataviaa probability matching method.The peak area normalization method was used to calculate the relative content of compounds.
3.Results and Discussion
3.1.Influence of HTP on SL lignite composition
Table 1 shows the proximate and ultimate analysis of coal samples.It can be seen that HTP effectively reduces the moisture content and volatile in SL lignite,because it effectively removes some organic functional groups in coal,which contains a large number of hydrophilic functional groups,such as carboxyl and hydroxyl groups [13].Meanwhile,the H/C atomic ratio showed a trend of first increasing and then decreasing,and reached its maximum at SL-220.That’s probably because the H+and OH–in water transferred into coal [31],and the decomposition of the aliphatic compound before 220 °C is relatively slow,so the H/C atomic ratio was increased;after 220 °C,the aliphatic compound begins to decompose in a large amount,leading to a significant decrease H/C atomic ratio.The O/C atomic ratio decreased with the increase of temperature,which shows that oxygen-containing functional groups have been decomposing during HTP.
Meanwhile,as the temperature of HTP up,ash and sulfur content decreased because some metals and polluting elements dissolve in the water.The composition of the designated metals in the ash was analyzed by ICP-OES,as shown in Table 2.The data indicate that HTP can effectively remove part of alkali metals and alkaline earth metals (AAEM),especially calcium ions,thus reducing the cross-linking points of SL coal and effectively inhibit the occurrence of pyrolysis crosslinking reaction [32],conducive to the generation of tar.
Table 3 shows the composition of gases released during the HTP.CO is released due to the fracture of the methoxy and aldehyde functional groups.CO2is produced by carboxyl decomposition,and its content increase from 10.41 × 10–3mmol?g-1of SL-200 to 12.38 × 10–3mmol?g-1of SL-300,indicating that the decomposition of the carboxyl group is continuously intensifying.The release of small molecules of hydrocarbons is due to the fracture of the aliphatic chain,and the release of C1–C3is less before 220 °C,possibly because the fracture of the aliphatic side chain is slight.
3.2.Influence of HTP on SL lignite physicochemical structure
3.2.1.XRD
The XRD patterns of samples over the examined 2θ range were curve-fitted with 3 bands by using PeakFit v4.12,which was shownin Fig.2,R2of the XRD fitting curve is shown in Table S1.The result shows that after HTP treatment,the peak of FeSiO3at about 29°disappeared obviously,which proves again that HTP can remove the metal elements in coal.
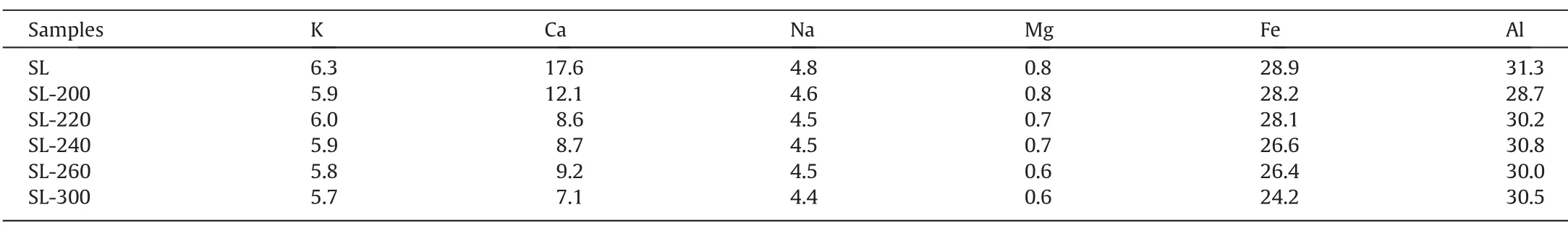
Table 2Composition of designated metals (mg?g-1) in coal samples
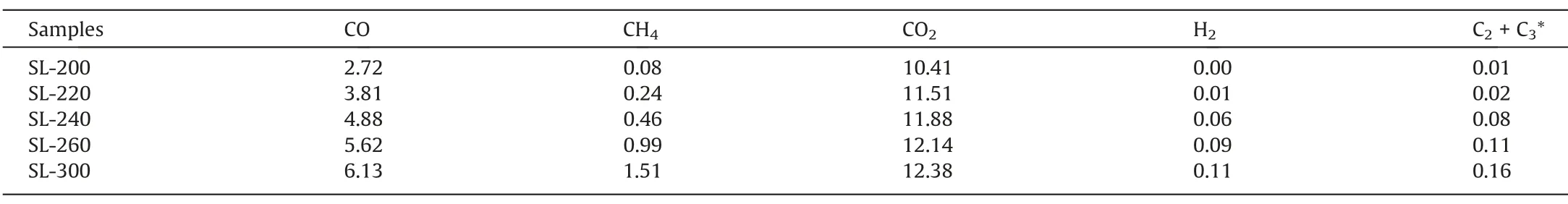
Table 3Composition of gases released during HTP(×10–3 mmol?g-1,dry ash free basis)
Meanwhile,three parameters of carbon crystallite structure were calculated by using the Bragg equation and Scherrer equations [26,33]:

where thed002is the interplanar spacing of two aromatic layers of microcrystalline,LcandLaare average crystallize height and average diameter of microcrystalline,λ=0.154 nm,θ(002)and θ(100)are the corresponding scatting angles,β(002)and β(100)are the full widths at half-maximum of (002) and (100) peaks,respectively.
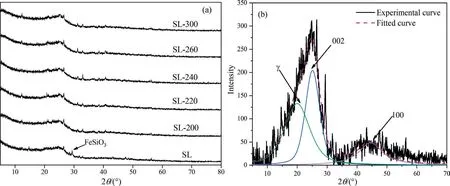
Fig.2.XRD patterns (a) and fitting diagram (b) of samples.
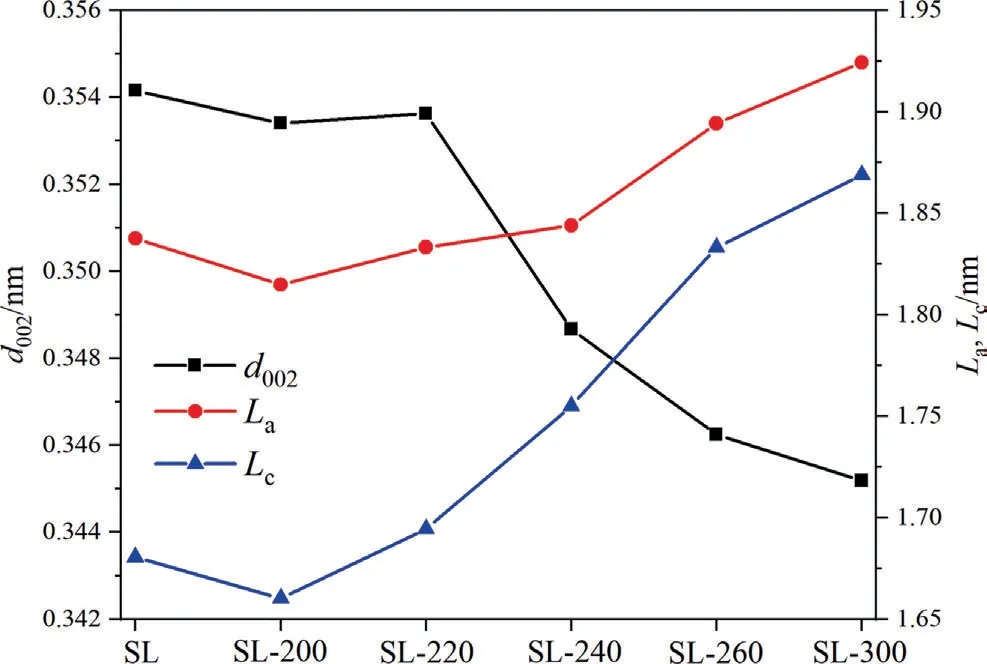
Fig.3.Microcrystalline parameters of samples.
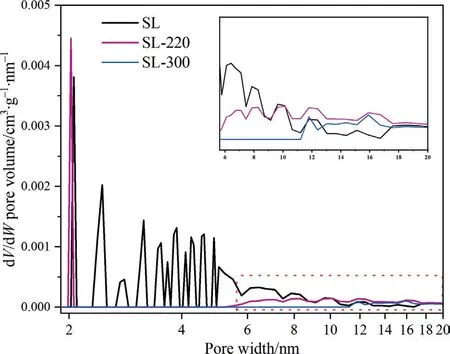
Fig.4.Pore size distribution of samples.
The microcrystalline parameters of samples were shown in Fig.3.Before 220 °C,three characteristic parameters have little change.After 220 °C,as the HTP temperature goes up,the value ofd002is significantly reduced.On the other hand,bothLaandLcare gradually rising.This means that HTP had little influence on the coal microcrystalline structure before 220 °C.However,with the further increase in temperature,the carbon microcrystal size increased.This may be because,before 220 °C,the dehydration and decarboxylation reaction occurred mainly,so it had little influence on the structure of the carbon microcrystalline.However,after 220°C,the aliphatic compound begins to decompose in large quantities,H/C atomic ratio began to reduce,and a small degree of polycondensation may occur,leading to an increase in the carbon microcrystal size.In a word,too high HTP temperature will lead to the increase in aromaticity of SL lignite,which is unfavorable for pyrolysis.
3.2.2.BET
Fig.4 shows the pore size distribution of samples.It can be seen that SL lignite has a large number of mesoporous pores between 2–6 nm,and HTP significantly changes the pore size distribution of SL coal.When the HTP temperature reaches 220 °C,the distribution peaks of 2.1 nm and 10–18 nm increased obviously while other mesoporous pores with pore diameter between 2–6 nm basically disappear.The distribution peak of 2.1 nm increased could be attributed to the releases of small molecular gases during the HTP,which decomposition of functional groups.Meanwhile,some holes collapsed and merged in HTP,this is the reason for the increase of the distribution peaks of 10–18 nm in SL-220 and is conducive to the release of volatiles in the pyrolysis process.When the HTP temperature is further raised to 300 °C,the distribution peak began to concentrate between 11.2 and 18 nm,and the peak strength significantly decreased,indicating that the holes continued to collapse.
The changes of surface areas,pore volume,and pore size of samples are shown in Table 4.The surface areas and pore volume show a trend of decreasing with the rise of HTP temperature,and average pore diameter is the opposite,it is further proved that HTP causes partial holes to collapse and merge,and the distribution of holes is concentrated to the place with larger pore width.However,too high HTP temperature will cause a large number of holes to collapse,seriously damaging the pore structure.
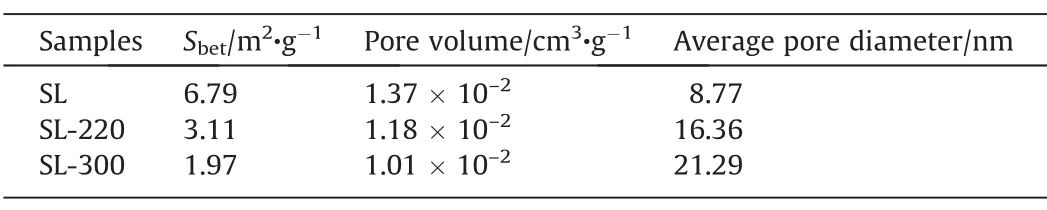
Table 4Pore structure parameters of samples
3.2.3.FT-IR
The FT-IR analysis of samples is depicted in Fig.5.Previous studies have shown that the infrared spectrum of coal is mainly divided into four parts [34–37]:hydroxyl groups stretching vibration (3600–3000 cm-1),aliphatic C–H group stretching vibration(3000–2800 cm-1),oxygen-containing functional group stretching vibration (1800–1000 cm-1),and aromatic H-substituted groups bending vibration (900–700 cm-1).
During HTP,the decomposition of oxygen-containing functional groups and the cleavage of aliphatic side chains mainly occurred,so the oxygen-containing functional group and aliphatic C–H group were curve-fitted by using PeakFit v4.12,as shown in Fig.6,R2of the FT-IR fitting curve is shown in Table S2.The absorption peak at 1615 cm-1in the infrared spectrogram represents aromatic carbon (Car),and HTP has almost no influence on its content[34,38,39].So,the following parameters are used to describe the relative content of different functional groups.The ratio of COOH/Car(A1701cm-1/A1615cm-1),R–O–R’/Car(A1110cm-1/A1615cm-1),and Ar–OH/Car(A1310cm-1+1178cm-1+1150cm-1/A1615cm-1) respectively represents the relative content of the carboxyl group,aryl ethers and phenolic hydroxyl in the samples. The CH2/CH3(A2921cm-1/A2959cm-1) ratio measures the length of aliphatic side chains and bridge bonds.
Above parameters are presented in Table 5.Among oxygencontaining functional groups,the thermal stability of carboxyl groups is poor,so COOH/Carcontinually decreases with the rise of HTP temperature,it is further proved that HTP can decompose carboxyl group with release CO2,and reduce the water content of SL lignite.The aryl ethers do not decompose easily at low temperatures,whereas the ratio of R–O–R’/Cardecreases from 0.37 of SL to 0.30 of SL-300.This is due to the hydrolysis of ether bonds during HTP.Meanwhile,the ratio of Ar–OH/Carincreased from 1.67 of SL to 1.80 of SL-300,there are two possible reasons for the increase of phenolic hydroxyl:one possibility is that in the hydrothermal environment,part of hydrogen and hydroxyl radical are crosslinked to benzene or phenoxy through electrophilic substitution reaction,forming phenolic hydroxyl group;another possibility is that when the aryl ether hydrolyzes,it combines with some of the hydrogen radicals to form phenolic hydroxyl groups.The value of CH2/CH3was unchanged before 220°C but showed a significant downward trend after 220 °C.It proved that the aliphatic side chains had fewer breaks before 220°C;and after 220°C,the weak covalent aliphatic side chains began to break significantly,longer aliphatic side chains become short and some small molecules of hydrocarbons are released.This conclusion is consistent with the analysis results of gases released during HTP.
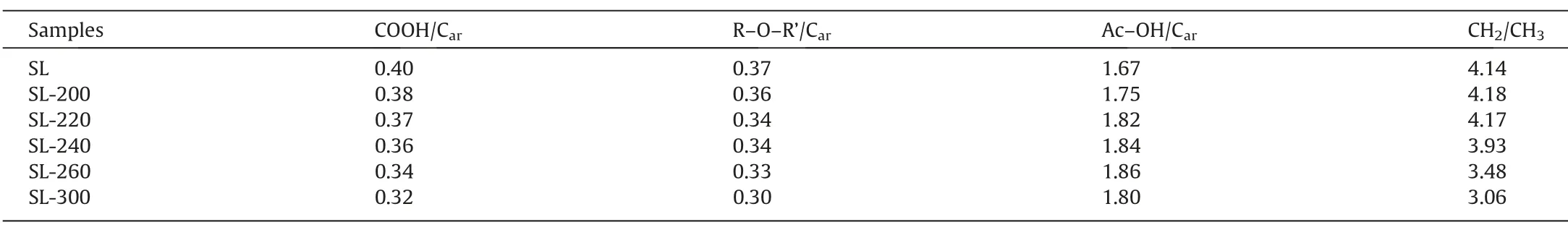
Table 5Parameters derived from the FT-IR analysis of samples
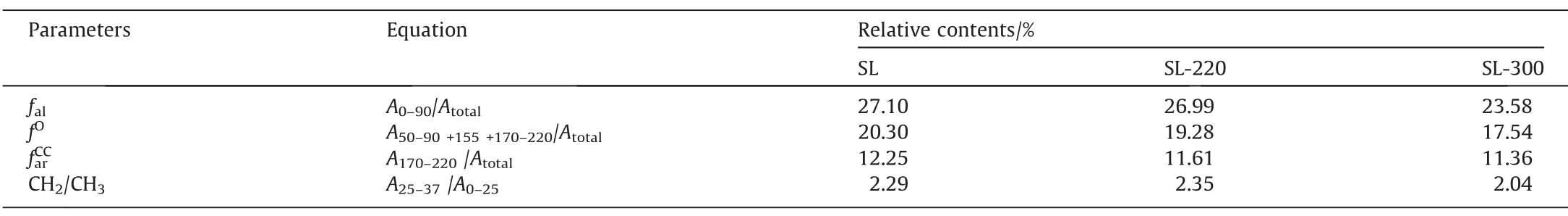
Table 6Carbon groups parameters of samples
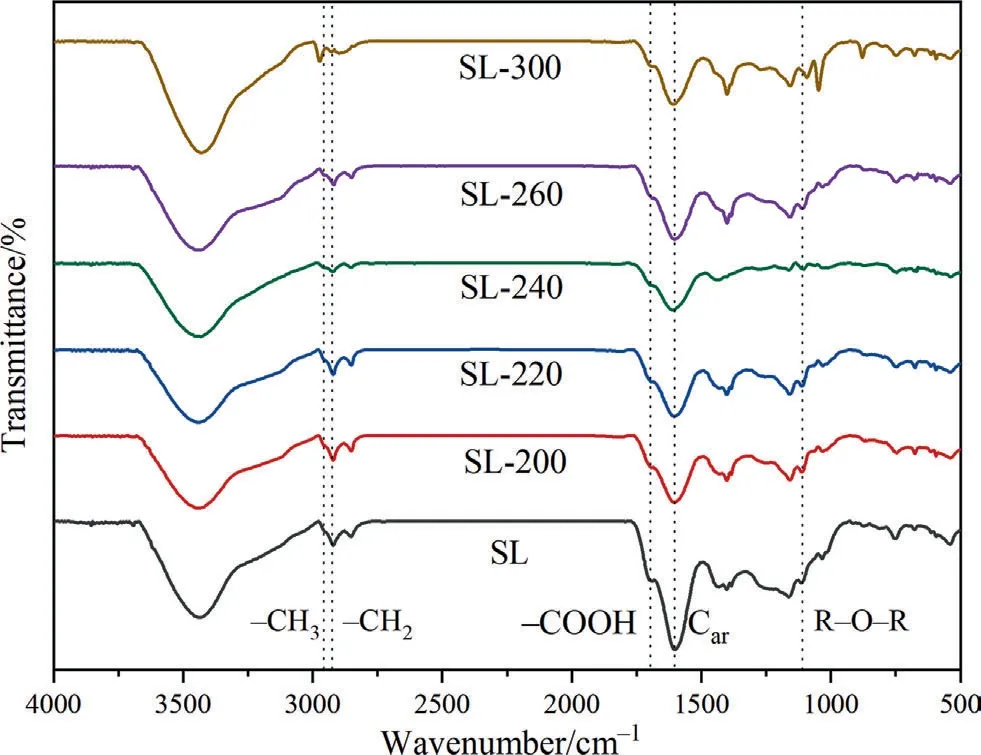
Fig.5.FT-IR spectra of samples.
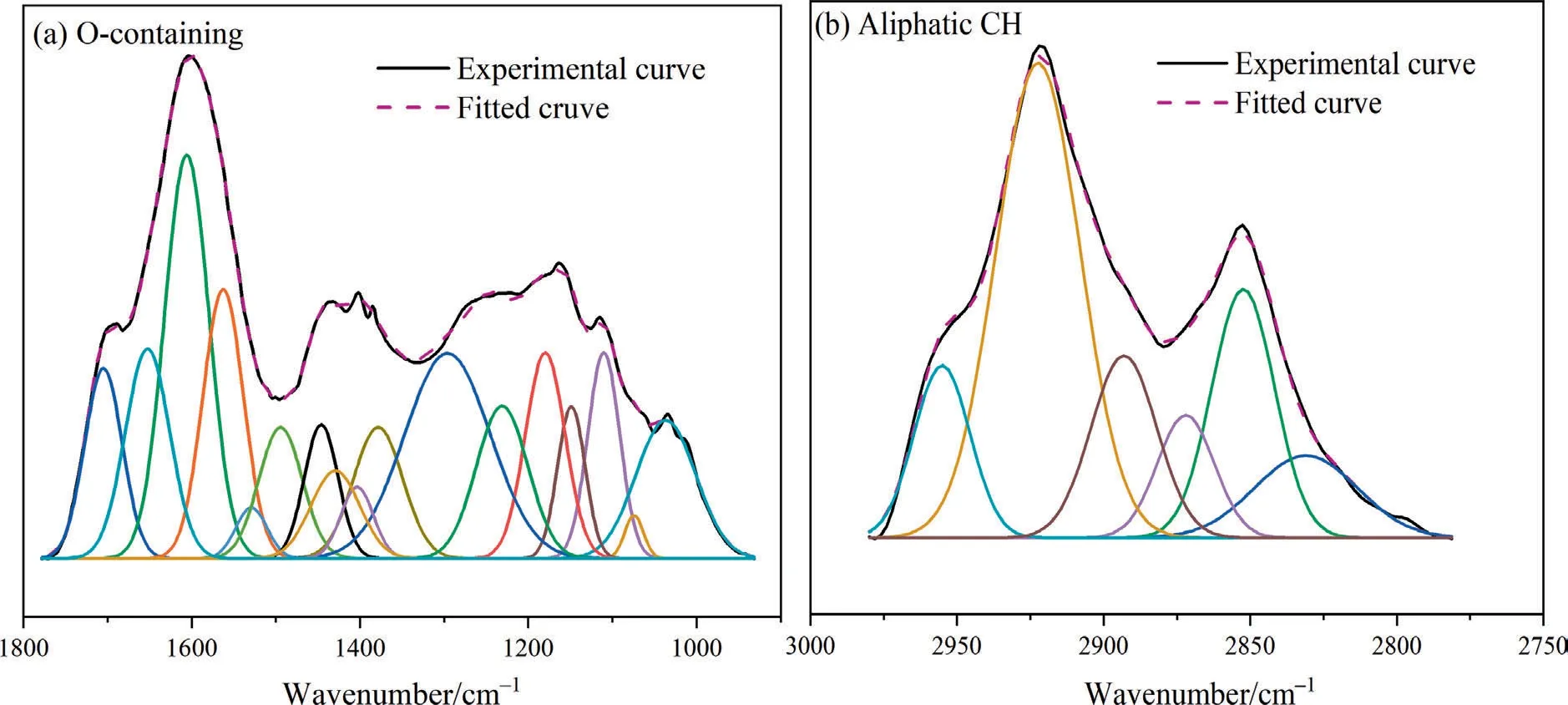
Fig.6.FT-IR fitting diagram of SL lignite:(a) 1000–1800 cm-1 and (b) 2800–3000 cm-1.
3.2.4.13C NMR
The13C NMR spectra of samples were shown in Fig.7(a).It can be seen that the spectrum is mainly divided into three large absorption peaks:aliphatic carbon (0–90),aromatic carbon (90–165),and carbonyl/carboxy carbon(165–220)[39,40].The absorption intensity of the aromatic carbon peak is significantly higher than that of the aliphatic carbon absorption peak,it proved that the content of aromatic carbon is higher than aliphatic carbon.
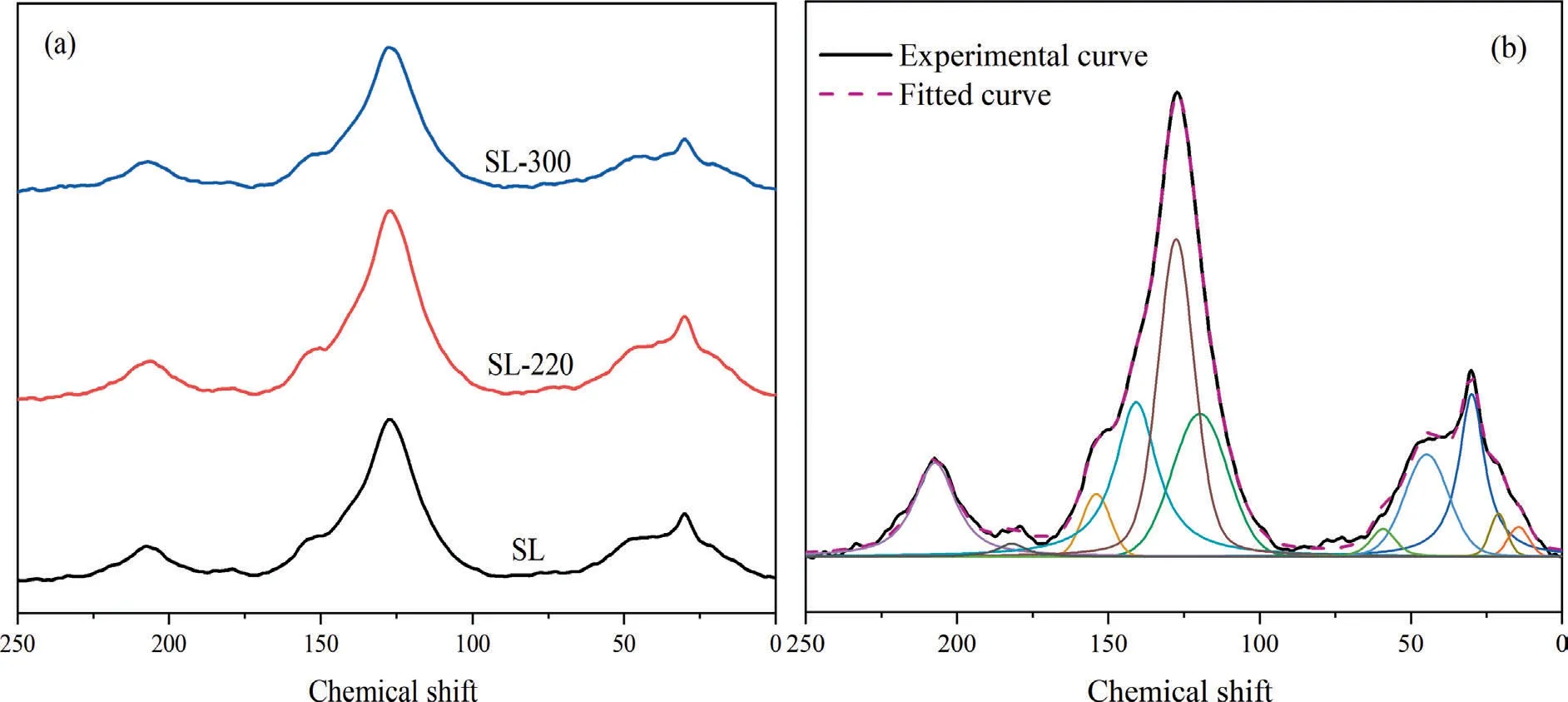
Fig.7.13C NMR spectra (a) and fitting diagram (b) of samples.
The13C NMR spectra were fitted[41–43],as shown in Fig.7(b),R2of the13C NMR fitting curve is shown in Table S3,and four parameters as shown in Table 6 were used to describe the relative content of different carbon groups:fal,fO,andrespectively represent the content of aliphatic carbon,oxygen-linked carbon,carboxyl,and carbonyl groups.CH2/CH3measures the length of aliphatic side chains and bridge bonds.Compared with the raw coal,CH2/CH3of SL-220 slightly increased whilefal,fO,andare reduced.The small change range offalindicates that before 220 °C,the main structures of coal have changed little,and the decomposition of some oxygen-containing functional groups with poor hydrothermal stability mainly occurs,such as carboxyl group and ether bond.Hydrogen in water replaces the oxygen-containing functional groups,thus the atomic ratio of H/C increases and O/C decreases.Thefaldecrease from 27.10% of SL to 26.99% of SL-220,indicating that the aliphatic carbon broke less at 220°C.The reduction offrom 12.25% of SL to 11.61% of SL-220 also proves once again that HTP can effectively remove the carboxylic group and reduce the water content of SL lignite.For SL-300,thefaland CH2/CH3go down.This is due to the further increase of hydrothermal temperature,some longer aliphatic side chains begin to break down into short and release small molecules of hydrocarbons,resulting in a significant decrease in aliphatic carbon content,so the atomic ratio of H/C goes down.Meanwhile,fOandcontinue to decline,indicates that the oxygen-containing functional groups continue to decompose,leading to a further decrease in the atomic ratio of O/C.But the decrease inis smaller(11.61%of SL-220 to 11.36%of SL-300),which may be because,at the same time of carboxyl decomposition,some phenol condensed to form quinone at high temperature [20].The results of13C NMR and FT-IR are in good agreement.
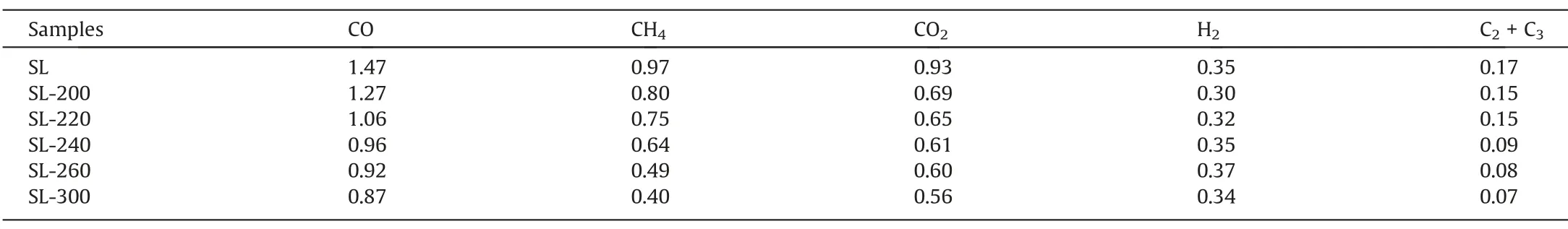
Table 7Pyrolytic gas composition (mmol?g-1,dry ash free basis)
3.3.Influence of HTP on fast pyrolysis of SL lignite
The distribution of pyrolysis products of samples was calculated on the basis of the pretreated coal samples mass and raw coal samples mass,as shown in Fig.8.It can be seen that the two calculation methods show the same trend,the yield of gas and water decreased,while the yield of char decreased,and tar production first increased and then decreased.The distribution and characteristics of pyrolysis products have an important relationship with the change of physicochemical structure of coal by HTP.Therefore,the pyrolysis products are carefully analyzed below.
3.3.1.Gas products
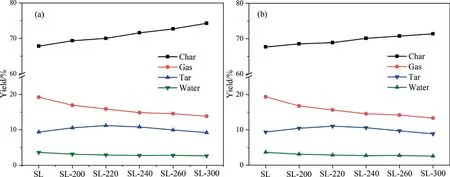
Fig.8.Distribution of pyrolysis products of samples:(a) based on the mass of pretreated coal and (b) based on the mass of raw coal.
The release of pyrolysis gas from coal is mainly related to the fracture of aliphatic compounds,carboxyl,and other oxygencontaining functional groups,HTP effectively reduces the content of these components and releases gas in advance,resulting in the release of pyrolysis gas reducing from 20.89% of SL to 13.47% of SL-300.The pyrolytic gas composition is shown in Table 7.It can be seen that the release of all gases except for H2has decreased because the total pyrolysis gas is reduced.Meanwhile,the removal of metal ions from coal prevents the tar from further cracking into gases.The small change in H2release is due to the addition of some hydrogen radicals to coal in HTP and the reduction of hydrogen radical consumption in the pyrolysis process.Studies [44] shows that the crosslinking reaction degree of lignite at low temperature thermal transformation can be calculated by CO2and water yield,the CO2content in pyrolysis gas after HTP is significantly reduced,which proves that HTP can effectively inhibit the crosslinking reaction in the pyrolysis process of SL coal.The content of C2+C3decreases dramatically after 220 °C,indicating that the aliphatic compounds began to decompose substantially during HTP.
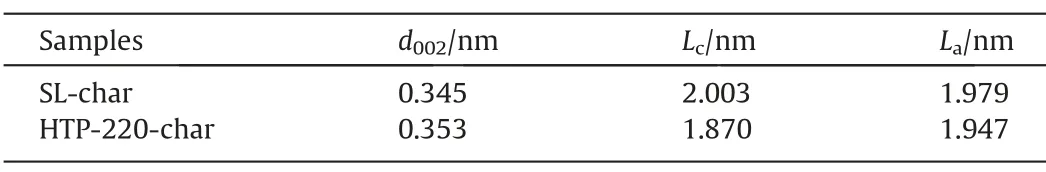
Table 8Microcrystalline parameters of char
3.3.2.Water products
The production of pyrolysis water is mainly due to the decomposition of oxygen-containing functional groups in coal.HTP can effectively remove oxygen-containing functional groups in coal,as shown in the NMR data,the oxygen-linked carbon decrease from 20.30%of SL to 17.54%of SL-300,so the pyrolysis water yield reduced from 3.62% of SL to 2.66% of SL-300.

Fig.9.Composition of tar products.
3.3.3.Tar products
The production of tar consists mainly of the cracking of the aromatic ring and the binding of free radicals.Simultaneously,crosslinking reaction is also a major factor affecting the tar yield:inhibition of crosslinking reaction is conducive to improving pyrolysis tar yield [32,36].During the HTP,part of AAEM is removed,which is beneficial to hinder the occurrence of cross-linking reaction [32,45].Moreover,hydrogen radicals were introduced in HTP and increased the atomic ratio of H/C before 220 °C,which was conducive to the stable of free radical fragments during the pyrolysis process.Consequently,the tar yield increased from 9.36%of SL to 11.21%of SL-220.As the hydrothermal temperature continues to rise,however,the decomposition of the aliphatic side chain and increase of aromaticity results in a decrease in tar yield to 9.22% of SL-300.
Fig.9 shows the composition of tar products by GC–MS analyses(peak area normalization method).It was obvious that the content of oxygen-containing compounds and aliphatic compounds decreased due to the decomposition and fracture of oxygencontaining functional groups and aliphatic side chains during HTP.The relative content increase of phenols may be caused by the increase of phenols content in coal caused by HTP.The content of aromatics (1-ring,2-ring,especially polycyclic aromatic hydrocarbons)was increased,possibly because the addition of hydrogen radical during HTP prevented the secondary condensation of aromatic hydrocarbons in the pyrolysis process,increases aromatics content.The content ofn-hexane soluble substance in tar increased from 43.1% of SL to 50.1% of SL-220,indicating that the content of light tar increased,which was consistent with the above conclusion (then-hexane solubility of tar indicated the amount of light tar).
3.3.4.Char products
HTP will remove some oxygen-containing functional groups and aliphatic compounds in coal and release gas,reducing the vola-tile content of coal and increasing the fixed carbon content.Therefore,the char yield rises from 67.65% of SL to 74.25% of SL-300.
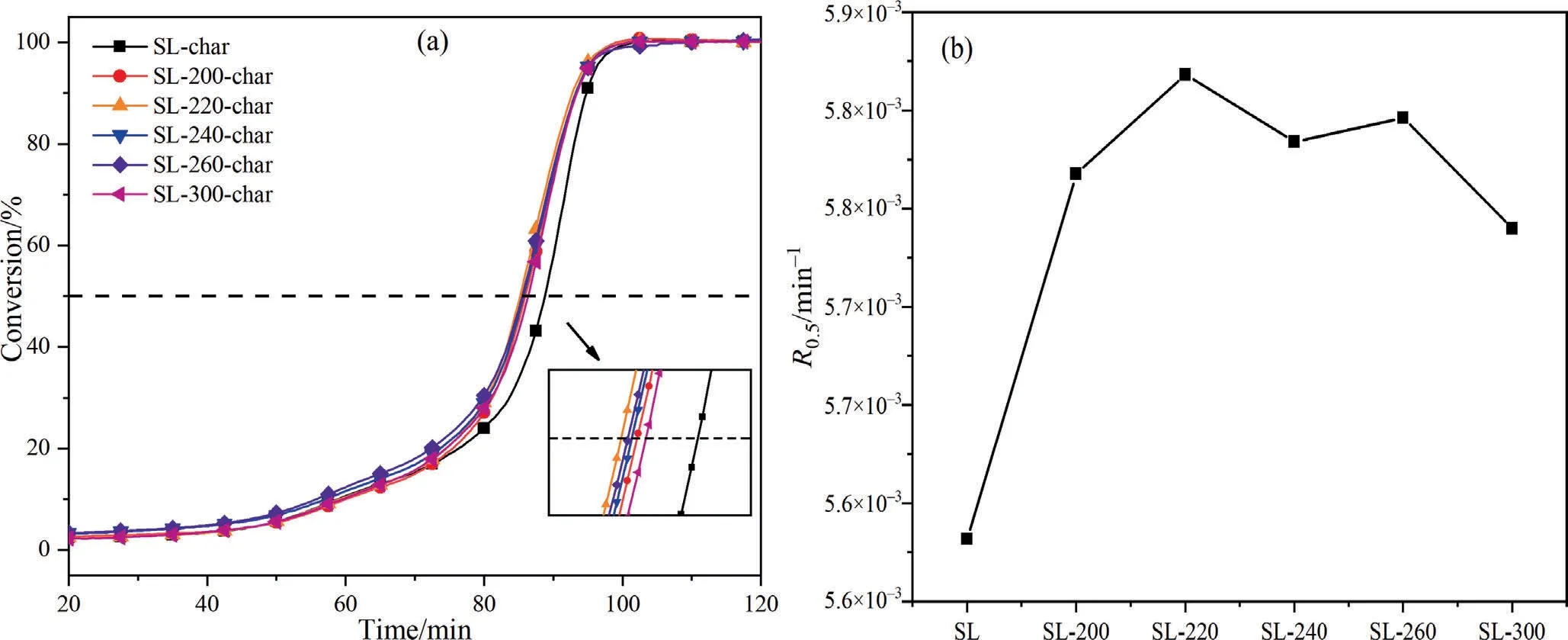
Fig.10.Gasification conversion (a) and R0.5 (b) of char.
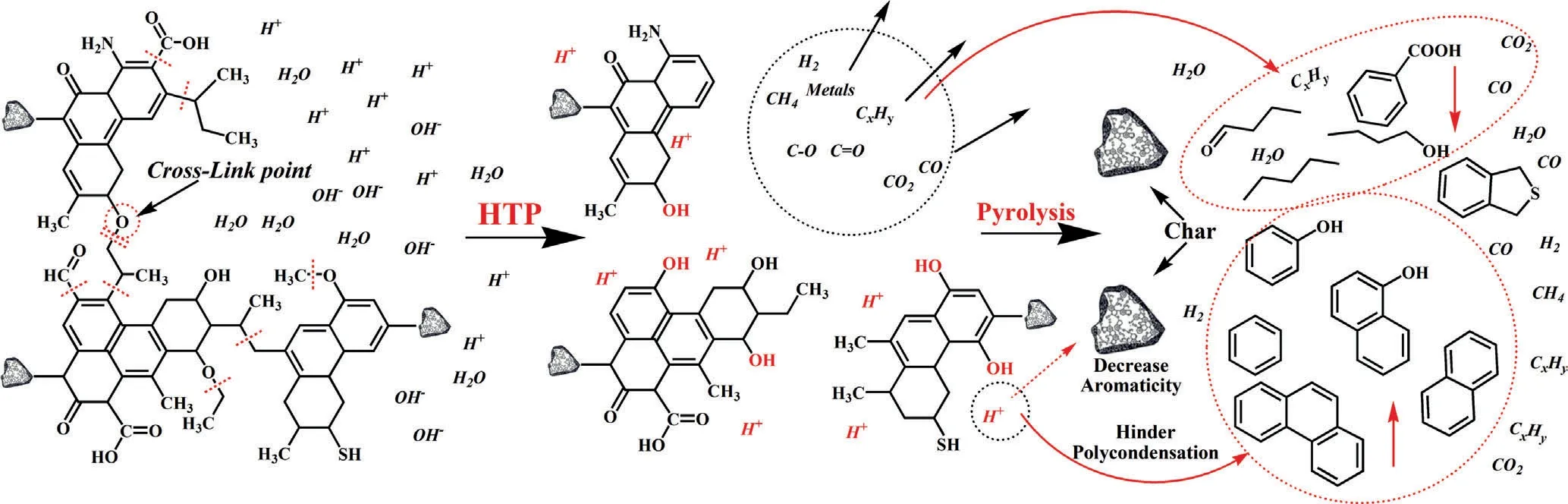
Fig.11.Possible mechanism of HTP effect on SL lignite structure and fast pyrolysis characteristic.
Fig.10 shows the gasification performance of char by CO2.It can be seen that the gasification performance of HTP-char has changed significantly,the gasification curve shifted to low temperature,and theR0.5exhibited a generally increasing trend,which means that the SL-HTP-char has higher gasification activity,and the gasification temperature moves to the low temperature zone.
HTP-220-char shows the best gasification activity and the highestR0.5,so the XRD analysis of SL-char and HTP-220-char was carried out,and the microcrystalline parameters of them are calculated,as shown in Table 8.It can be seen that SL-HTP-char has a smallerLaandLcand a largerd002than SL-char,which proves that hydrogen free radicals added in the HTP hinder the secondary condensation reaction in the pyrolysis,and reduce the aromaticity of SL-HTP-char.
3.4.Possible mechanism of HTP effect on SL lignite fast pyrolysis
Based on the above characterization and theoretical analysis,a possible mechanism of HTP effect on SL lignite structure and fast pyrolysis characteristic was proposed in this work,as shown in Fig.11.It can be seen that aldehyde and carboxyl functional groups were decomposed to form CO and CO2,some aromatic ether bonds were broken and combined with hydrogen radical to form phenolic hydroxyl group,results in the increase of phenol content in pyrolysis tar.Some aliphatic side chains break and release a small amount of C1–C3,results in the decrease of aliphatic compounds in pyrolysis tar and C1–C3in gas.The decomposition of the oxygen-containing functional group and the release of gas reduce the yield of water and pyrolysis gas.At the same time,part of metal ions was removed during the HTP,some crosslinking sites were broken,it is beneficial to increase the yield of pyrolysis tar.Some hydrogen radicals were introduced into the lignite,prevents the secondary condensation during pyrolysis,increases the content of aromatics and light tar,reduces the aromaticity of char,thus improving char’s CO2gasification activity.
4.Conclusions
In this work,HTP was used to change the physical and chemical structure of SL lignite,a series of characterization methods (BET,XRD,FT-IR,and13C NMR) were used to analyze the structure of samples,and the fast pyrolysis behavior of samples was studied by a powder-particle fluidized bed reactor.The following conclusions are obtained:
(1) During HTP,part of oxygen-containing functional groups and metal ions in SL lignite were removed,especially carboxyl and calcium ions,reduce the degree of cross-linking;some holes were collapsed and merged into mesoporous with larger pore size,increasing the average pore size;hydrogen radicals were added to the coal,H/C atomic ratio increase,these reasons lead to an increase in tar yield.When theTHTP>220°C,however,the aliphatic side chain begins to break substantially,and the aromaticity of coal samples increased,so the tar yield decrease with the increase of HTP temperature.Meanwhile,the removal of oxygencontaining functional groups reduces the content of oxygen-containing compounds in pyrolysis gas and tar,and the yield of water decreases.
(2) Based on this work,the structure–activity relationship between SL lignite pyrolysis characteristics and its structure was proposed,it shows that hydrogen radicals were added to the coal by HTP,combine with benzene or phenoxy in the coal,increasing the content of phenolic,leads to the increase of phenolic substance content in pyrolysis tar.At the same time,hydrogen radicals play the role of stabilizing other free radicals and hinders the secondary condensation during pyrolysis,increases the content of aromatics and light tar,reduces the aromaticity of char,and improves its gasification activity.
Declaration of Competing Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work,there is no professional or other personal interest of any nature or kind in any product,service and/or company that could be construed as influencing the position presented in,or the review of,the manuscript entitled.
We declare that we have no conflicts of interest to this work.
Acknowledgements
This work was supported by the National Natural Science Foundation of China(21536009),and Science and Technology Plan Projects of Shaanxi Province (2017ZDCXL-GY-10-03).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.05.006.
 Chinese Journal of Chemical Engineering2021年7期
Chinese Journal of Chemical Engineering2021年7期
- Chinese Journal of Chemical Engineering的其它文章
- Interactions of dynamic supercritical CO2 fluid with different rank moisture-equilibrated coals:Implications for CO2 sequestration in coal seams
- Selective preparation of light aromatic hydrocarbons from catalytic fast pyrolysis vapors of coal tar asphaltene over transition metal ion modified zeolites
- Formation and emission characteristics of VOCs from a coal-fired power plant
- Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers
- Modification of ash flow properties of coal rich in calcium and iron by coal gangue addition
- Kinetics of steam regeneration of SAPO-34 zeolite catalyst in methanol-to-olefins (MTO) process
