Patent foramen ovale in an old patient with ischemic stroke following carotid surgery
Elpidio Santillo, Monica Migale, Luciano Marini, Raffaele Antonelli-Incalzi
?
Patent foramen ovale in an old patient with ischemic stroke following carotid surgery
Elpidio Santillo1,*, Monica Migale1, Luciano Marini1, Raffaele Antonelli-Incalzi2
1Geriatric-Rehabilitative Department, Italian National Research Center on Aging IRCCS-INRCA, Fermo, Italy2Chair of Geriatrics, Biomedic Campus University, Via àlvaro del Portillo 21, Rome, Italy
J Geriatr Cardiol 2018; 15: 551?553. doi:10.11909/j.issn.1671-5411.2018.08.001
Carotid surgery; Ischemic stroke; Patent foramen ovale; The elderly
Post-procedural strokes have been observed in 0.5–2.7% of patients after carotid endarterectomy (CEA). They are frequently due to carotid embolism or thrombosis of the operated artery.[1,2]Intracerebral haemorrhages and technical difficulties are less common underlying factors.[3]In cases with bi-hemispheric participation differential diagnosis includes contralateral occlusion of the internal carotid artery and the post-operative hyperperfusion syndrome.[1]Unfortunately, studies investigating the causal role of patent foramen ovale (PFO) in strokes which occur after carotid surgery are lacking. PFO has been implicated in strokes’ pathogenesis via paradoxical embolism. Its autopsy incidence has been estimated to be approximately 27%.[4]The frequency of PFO seems to be lower in older subjects.[5]However, it cannot be excluded that PFO could be involved in stroke pathogenesis also in elderly patients.[6]Transthoracic echocardiography with saline agitated contrast is a simple and inexpensive diagnostic tool to unveil PFO.[7]
A 79 years old man was referred to our Geriatric-Re-habilitative Institute as consequence of an ischemic stroke. Two weeks before the admission, the patient underwent left CEA for an atherosclerotic stenosis > 80%. During the intervention, blood pressure and electrocardiographic monitoring excluded intraoperative hypotension and the onset of arrhythmias. The early post-operative period passed without complications. However, three days after the surgical procedure, the patient developed sudden non-fluent aphasia and double emiparesis, so he was recovered in a neurologic ward. His blood pressure was 150/80 mmHg, heart rate was 90/min with normal sinus rhythm on electrocardiogram (ECG). First brain computed tomography (CT) was negative for both acute ischemic signs and intracranial bleedings. Brain CT after 48 h from symptoms’ manifestation showed two thalamic ischemic lesions (1.4 cm in left thalamus and 1.3 cm in right thalamus) (Figure 1). Angio-CT of epiaortic and intracranial arteries excluded significant stenoses.
At admission at our institute, the patient was re-evaluated in order to optimize the etiology’s definition of the stroke, before starting the rehabilitative program. Hypertension, diabetes and smoking were not reported in patient’s medical history. Except for mild dyslipidemia, he had no other cardiovascular risk factors and in his family history there were no cardiovascular and hereditary diseases. He was taking acetylsalicylic acid 100 mg once daily and sertraline 25 mg once daily for depression.
On general examination, he was well nourished [body mass index (BMI): 22 kg/m2], without cyanosis, pallor and dyspnea at rest. His heart rate was about 80 beats/min. Blood pressure was 136/70 mmHg and body temperature 36.8 °C. Heart, lung and abdominal examination revealed no significant pathological mark. Lymphadenopathies and oedema were not present. On neurological examination double emiparesis and non-fluent aphasia were confirmed. Patient’s orientation to time and space was poor (NIH stroke scale score: 25).
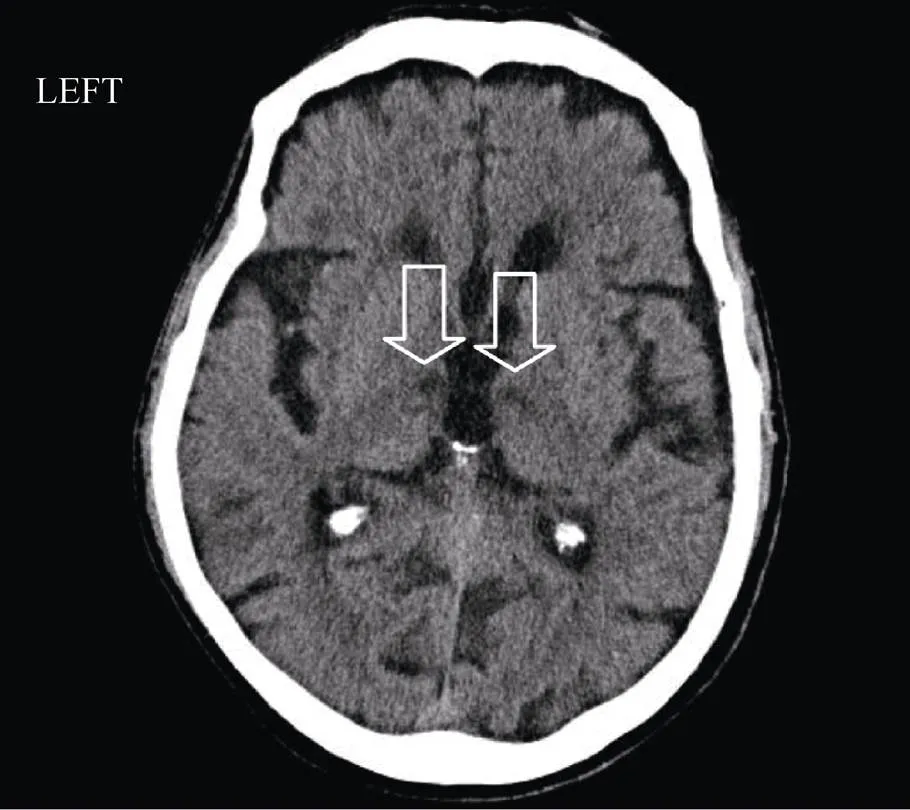
Figure 1. Brain computed tomography (CT) at 48 hours after symptoms’ exordium. At thalamic level two hypodense images were detected bilaterally (arrows). Their extension was estimated to be 1.4 cm and 1.3 cm, respectively in left thalamus and in right thalamus.
Laboratory analyses showed a slight cholesterol elevation (238 mg/dL), while other analytes were normal. In particular, thrombophilic and autoimmune markers were negative. ECG evidenced sinus rhythm without anomalies of conduction and repolarization. Furthermore, in 24 h Holter ECG neither major arrhythmias nor pathologic pauses, were observed.
The patient underwent echocardiogram which displayed normal dimension and ejection fraction of left ventriculum (LV), mild thickening of diastolic interventricular septum (13 mm) and no sign of obstruction of LV outflow tract. Diameters and function of right chambers were normal. Moreover, ultrasound study of the heart excluded images suggestive of intra-cardiac thrombi, neoplasms and endocarditis. Hemodynamically significant valvular diseases were not found.
Of note, PFO with a small right to left shunt, was detected through agitated saline bubble contrast (less than 20 microbubbles passed across interatrial septum within three heart beats after right atrium opacification) (Figure 2). Left bulging of interatrial septum was also evident, but criteria for septal aneurysm were not reached. Chiari network and Eustachium valve were not visible. Because the patient had the stroke while he was assuming acetylsalicylate, we opted for substituting it with clopidogrel 75 mg once daily. Moreover, simvastatin 20 mg once daily was prescribed for dyslipidemia’s treatment. Then, the patient started a rehabilitative program aimed to ameliorate his functional status. Unfortunately, patient’s recovery at discharge was not brilliant. In fact, despite of a mild improvement of limbs’ strength and cognitive status, he remained partially dependent in basal activities of daily living.
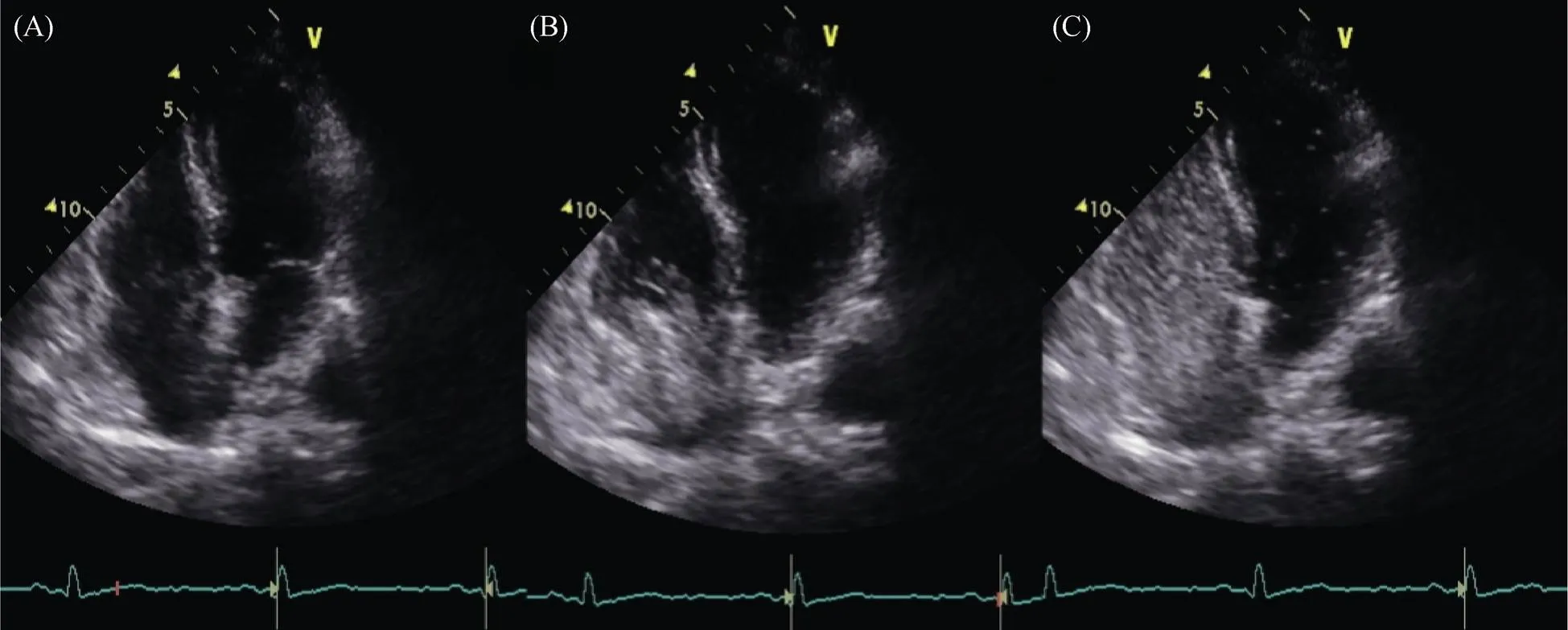
Figure 2. Transthoracic echcocardiography with saline agitated contrast. (A): Transthoracic 2-D echocardiography 4-chambers view at baseline; (B): Right atrium opacification during saline agitated infusion; (C): Appearance of few microbubbles (less than 20) in left atrium and left ventriculum within three heart beats after right atrium opacification, compatible with patent foramen ovale.
Present case poses an intriguing question, whether PFO could have played a possible etiologic role in the stroke occurred after CEA. We argued that PFO was not merely an incidental finding and could have contributed in the development of the stroke. Indeed, brain’s ischemic lesions of the patient were not clearly related to the operated carotid (, the left one), since right brain hemisphere was also involved in the stroke. Moreover, during CEA and in post-operative period the patient remained hemodynamically stable.
So we hypothesized that the stroke could have been caused by a bi-hemispheric embolization originated from PFO. It could be theorized that PFO-related embolism was favoured by both the patient’s reduction of mobility and the pro-inflammatory status, which are usually associated with major surgery interventions.
Interestingly, the case offers further food for thought. As regard to antiplatelet regimen, we decided to start clopidogrel basing our decision on recent evidences displaying the benefits of switching antiplatelet drugs after an ischemic stroke which occurred while patient was on therapy with acetylsalicylate.[8]
The option of PFO closure was also evaluated but excluded for several reasons. First, recent guideline discourages routine percutaneous PFO closure to patients who had cryptogenetic ischemic stroke. Only subjects with recurrent strokes despite optimal medical therapy should undergo PFO closure.[9]Second, echocardiographic characteristics of PFO were suggestive of low embolic risk. In fact, the shunt was not large, in absence of interatrial septum aneurysmChiari network or prominent Eustachium valve, all of which have been proposed as anatomical features favouring embolism.[4,10–12]Lastly, patient’s clinical and functional conditions were poor. Interestingly, in 2017, three large multicenter trials (CLOSE, REDUCE and RESPECT) have shown significant benefit in stroke reduction from PFO closure compared to medical therapy in patients with previous embolic stroke and PFO.[13–15]However, these studies have not enrolled elderly patients, so their conclusions are not applicable to geriatric patients.
In conclusion, the present case report showed that PFO should be searched as stroke cause even when other etiologic factors seem more probable, as well as in old patients who underwent recent carotid surgery. Future prospective studies are needed to verify if PFO is associated with a higher stoke rate in geriatric patients and if PFO closure is an effective strategy for lowering ischemic stroke recurrence also in the elderly. At present, decision to refer old stroke patients to PFO closure should be taken after carefully weighing benefits and risks of single cases. Whether carotid surgery could facilitate paradoxycal embolism in subjects with PFO remains also to be clarified.
1 de Borst GJ, Moll FL, van de Pavoordt HD,. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate?2001; 21: 484–489.
2 Pini R, Faggioli G, Longhi M,. The detrimental impact of silent cerebral infarcts on asymptomatic carotid endarterectomy outcome.2016; 64: 15–24.
3 Huibers A, Calvet D, Kennedy F,. Mechanism of procedural stroke following carotid endarterectomy or carotid artery stenting within the International Carotid Stenting Study (ICSS) randomised trial.2015; 50: 281–288.
4 Kerut EK, Norfleet WT, Plotnick GD,.Patent foramen ovale: a review of associated conditions and the impact of physiological size2001; 38: 613–623.
5 Hagen PT, Scholz DG, Edwards WD,. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts.1984; 59: 17–20.
6 Rigatelli G, Dell’Avvocata F. Patent foramen ovale in the elderly: what to do?.2007; 4: 254–256.
7 Silvestry FE, Cohen MS, Armsby LB,. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American Society of Echocardiography and Society for Cardiac Angiography and Interventions.2015; 28: 910–958.
8 Lee M, Saver JL, Hong KS,. Antiplatelet regimen for patients with breakthrough strokes while on aspirin: a systematic review and meta-analysis.2017; 48: 2610–2613.
9 Messé SR, Gronseth G, Kent DM,. Practice advisory: recurrent stroke with patent foramen ovale (update of practice parameter): report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology.2016; 87: 815–821.
10 Stone DA, Godard J, Corretti MC,. Patent foramen ovale: association between the degree of shunt by contrast transesophageal echocardiography and the risk of future ischemic neurologic events.1996; 131: 158–161.
11 Schuchlenz HW, Saurer G, Weihs W,Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events.2004; 17: 231–233.
12 Schneider B, Hofmann T, Justen MH,. Chiari’s network: normal anatomic variant or risk factor for arterial embolic events?1995; 26: 203–210.
13 Mas JL, Derumeaux G, Guillon B,. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke.2017; 377: 1011–1021.
14 S?ndergaard L, Kasner SE, Rhodes JF,. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke.2017; 377: 1033–1042.
15 Saver JL, Carroll JD, Thaler DE,. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke.2017; 377: 1022–1032.
Correspondence to: elpisant@tin.it
 Journal of Geriatric Cardiology2018年8期
Journal of Geriatric Cardiology2018年8期
- Journal of Geriatric Cardiology的其它文章
- Rare combination of dilated cardiomyopathy and ankylosing spondylitis in a family
- Normalization of plasma growth hormone alleviated malignant ventricular tachycardia in acromegaly
- Natriuretic peptide family as diagnostic/prognostic biomarker and treatment modality in management of adult and geriatric patients with heart failure: remaining issues and challenges
- Subclinical hypothyroidism is associated with lipid-rich plaques in patients with coronary artery disease as assessed by optical coherence tomography
- Five-year major clinical outcomes between first-generation and second- generation drug-eluting stents in acute myocardial infarction patients underwent percutaneous coronary intervention
- Correction of hypovitaminosis D improved global longitudinal strain earlier than left ventricular ejection fraction in cardiovascular older adults after orthopaedic surgery
