Five-year major clinical outcomes between first-generation and second- generation drug-eluting stents in acute myocardial infarction patients underwent percutaneous coronary intervention
Yong Hoon Kim, Ae-Young Her,Seung-Woon Rha, Byoung Geol Choi, Se Yeon Choi, Jae Kyeong Byun, Ju Yeol Baek, Woong Gil Choi, Tae Soo Kang, Ji Hoon Ahn, Sang-Ho Park, Ahmed Mashaly, Jin Oh Na, Cheol Ung Choi, Hong Euy Lim, Eung Ju Kim, Chang Gyu Park, Hong Seog Seo, and Dong Joo Oh
?
Five-year major clinical outcomes between first-generation and second- generation drug-eluting stents in acute myocardial infarction patients underwent percutaneous coronary intervention
Yong Hoon Kim1*, Ae-Young Her1*,Seung-Woon Rha2,3, Byoung Geol Choi2, Se Yeon Choi3, Jae Kyeong Byun3, Ju Yeol Baek4, Woong Gil Choi5, Tae Soo Kang6, Ji Hoon Ahn7, Sang-Ho Park8, Ahmed Mashaly2, Jin Oh Na2, Cheol Ung Choi2, Hong Euy Lim2, Eung Ju Kim2, Chang Gyu Park2, Hong Seog Seo2, and Dong Joo Oh2
1Division of Cardiology, Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, South Korea2Cardiovascular Center, Korea University, Guro Hospital, Seoul, South Korea3Department of Medicine, Korea University Graduate School, Seoul, South Korea4Cardiovascular Center, Cheong Ju St. Mary hospital, Cheong Ju, Chung Buk, South Korea5Cardiology Department, Konkuk University Chungju Hospital, Chungju, South Korea6Department of Internal Medicine, Cardiovascular Division, Dankook University Hospital, Cheonan, South Korea7Department of Cardiology, Soonchunhyang University Gumi Hospital, Gumi, South Korea8Cardiology Department, Soonchunhyang University Cheonan Hospital, Cheonan, South Korea
There were limited data comparing the major clinical outcomes between first-generation (1G)-drug eluting stents (DES) and second-generation (2G)-DES in patients with acute myocardial infarction (AMI) after percutaneous coronary intervention (PCI) during very long follow-up periods. We thought to investigate the comparative efficacy and safety of 2G-DES compared with 1G-DES in AMI patients during 5-year follow-up periods.A total of 1016 eligible AMI patients who underwent PCI with 1G-DES [paclitaxel-, sirolimus-, 1G-zotarolimus-eluting stent (endeavor?or endeavor sprint?),= 554] or 2G-DES [2G-zotarolimus (endeavor resolute?)- or everolimus-eluting stent,= 462] were enrolled. The primary endpoint was the occurrence of major adverse cardiac events (MACE) defined as total death, non-fatal myocardial infarction (MI), target lesion revascularization (TLR), target vessel revascularization (TVR), non-target vessel revascularization (Non-TVR) and the secondary endpoint was stent thrombosis (ST) at 5 years.Two propensity score-ma-tched (PSM) groups (232 pairs,= 464, C-statistic = 0.802) were generated. During the 5-year follow-up period, the cumulative incidence of TLR [hazard ratio (HR): 3.133; 95% confidence interval (CI): 1.539–6.376;= 0.002], TVR (HR: 3.144; 95% CI: 1.596–6.192;= 0.001) and total revascularization rate (HR: 1.874; 95% CI: 1.086–3.140;= 0.023) were significantly higher in 1G-DES compared with 2G-DES after PSM. However, the incidence of total death, non-fatal MI and ST were similar between the two groups.In this single-center and all-comers registry, 2G-DES’s superiorities for TLR, TVR and total revascularization in AMI patients suggested during 5-year clinical follow-up periods.
J Geriatr Cardiol 2018; 15: 523?533. doi:10.11909/j.issn.1671-5411.2018.08.006
Acute myocardial infarction; Clinical outcomes; Drug-eluting stent
1 Introduction
At present, second-generation (2G)-drug-eluting stents (DES) have nearly replaced first-generation (1G)-DES during percutaneous coronary intervention (PCI) in our routine daily clinical practice. Although acute myocardial infarction (AMI) milieu tends to higher thrombotic condition compared to stable coronary artery disease, DES implantation during primary PCI or staged PCI commonly done from the beginning of DES era up to now. During the last few years, several studies demonstrated that 1G-DES such as sirolimus-eluting stent (SES, Cypher?, Cordis Corp., Miami Lakes, Florida)[1]or paclitaxel-eluting stent (PES, Taxus?, Boston Scientific, Natick, Massachusetts)[2]were associated with reductions in angiographic target vessel revascularization (TVR) and major adverse cardiac events (MACE) compared with bare-metal stents (BMS). Chen,.[3]reported everolimus-eluting stent (EES, Xience V?, Abbott Vascular, Santa Clara, CA) in the setting of AMI appears to be superior to PESs in reducing target lesion failure, and stent thrombosis (ST). Kang,.[4]also reported Zotarolimus-eluting stent (ZES, Resolute?, Medtronic Inc, Santa Rosa, California) showed similar rates of MACE, cardiac death and recurrent myocardial infarction (MI) compared with SES and PES at 12 months and 18 months of follow-up periods in patients with ST-segment elevation MI (STEMI) who undergoing primary PCI. Most 2G-DES were showed non-inferior clinical outcomes compared with 1G-DES.[5,6]However, there is limited very long-term clinical outcome data comparing the safety and efficacy between 1G-DES and 2G-DES in patients with AMI who underwent successful PCI. Recently one all-comer, randomized, multicenter AMI trials[7]showed that cardiac death (EES: 2.5%. SES: 2.7%,= 0.86) and ST (EES: 2.3%. SES: 3.2%,= 0.60) rates were comparable in both groups during 3-year follow-up periods. The aim of this study was to compare the efficacy and safety of 2G-DES with 1G-DES in AMI patients during long-term clinical follow-up periods.
2 Methods
This study is a single-center, prospective, all-comers registry designed to reflect the “real world” practice since 2004. Data were collected by a trained study-coordinator with a standardized case report form. This study examined and approved by the institutional review committee and the subjects gave informed written consent. This study performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki. Informed consent was obtained from all individual participants included in the study prior to enrollment.
2.1 Study design and population
A total 1161 AMI patients were underwent coronary angiography (CAG) from January 2004 to October 2012. Among them, these patients were excluded if they had: (1) BMS (= 26), (2) other types of DES [except for SES, PES, 1G-ZES (endeavor, endeavor sprint), 2G-ZES (endeavor resolute) and EES,= 40] implantation, (3) stent size required to treat lesion > 3.5 mm (maximum diameter of SES,= 61), (4) not participated or follow-up loss (= 18). Finally, a total 1016 eligible AMI patients who treated with 1G-DES (SES, PES, or 1G-ZES, total= 554) or 2G-DES (2G-ZES, or EES, total= 462) were enrolled. After a pro-pensity score matched (PSM) analysis, two propensity-ma-tched groups (232 pairs,= 464) were generated (Figure 1).
2.2 PCI procedure and medical treatment
A diagnostic CAG and PCI done through either trans-femoral or trans-radial approaches after an administration of unfractionated heparin (70–100 IU/kg). Patients’ activated clotting time maintained above 250 seconds during the procedure. All patients received a loading dose of 200 to 300 mg aspirin and 300 to 600 mg of clopidogrel as the dual antiplatelet therapy (DAPT) and maintained with 100 mg of aspirin and 75 mg of clopidogrel. The use of cilostazol (Pletaal?, Otsuka Pharmaceutical Co., Tokyo, Japan) or platelet glycoprotein IIb/IIIa receptor blockers was left to the discretion of the individual operators. After stent implantation, DAPT (100 mg daily aspirin and 75 mg daily clopidogrel) prescribed at least 12 months. During hospi- talization, enrolled patients had taken cardiovascular beneficial medications, including beta-blockers (BB), angiotensin converting enzyme inhibitors (ACEI), or angiotensin receptor blockers (ARB), calcium channel blockers (CCB), and lipid lowering agents. After discharge, the patients were encouraged to stay on the same medications they received during hospitalization.
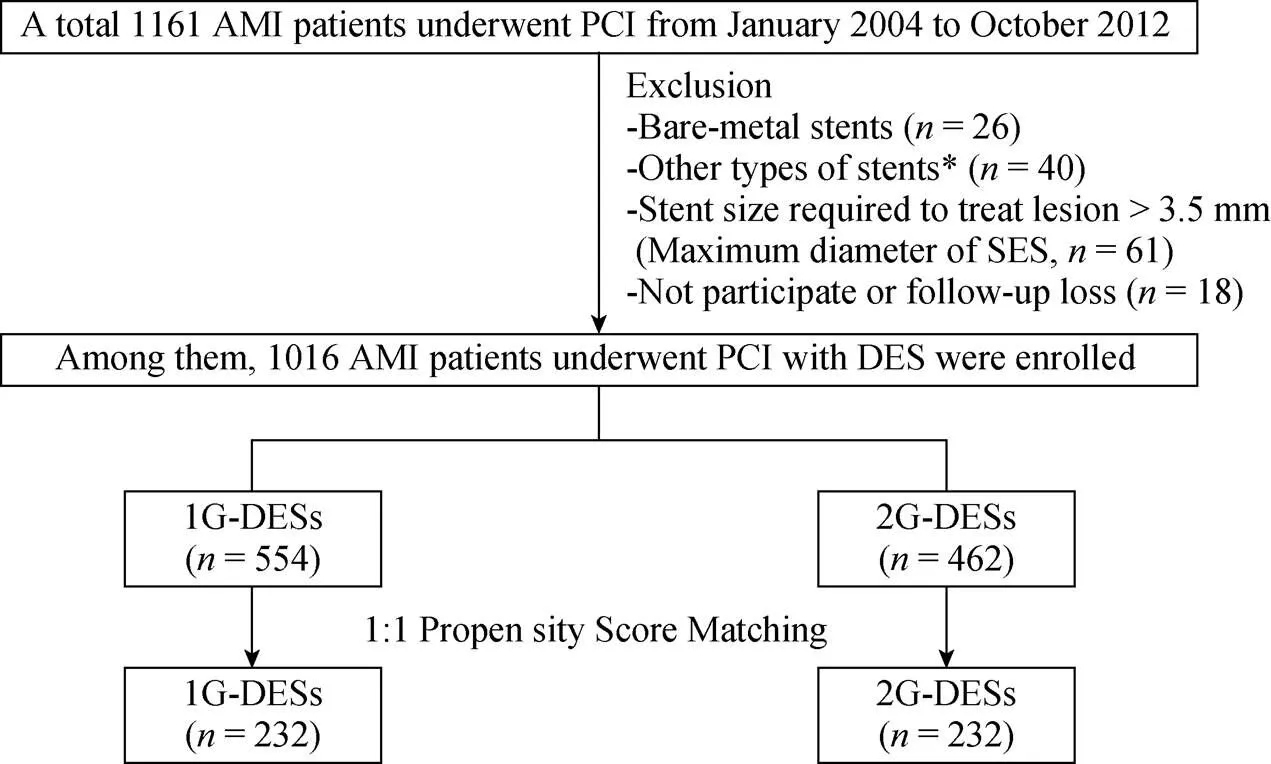
Figure 1. Flow chart of study number of patients. AMI: acute myocardial infarction; DES: durg eluting balloon; PCI: percutaneous coronary intervention; SES: sirolimus-eluting stent; 1G: first-generation; 2G: second-generation.
2.3 Study de?nitions and clinical follow-up
The recording of cardiovascular risk factors and past medical histories based on patients’ self-report. The primary endpoint was the occurrence of MACE defined as total death, recurrent non-fatal MI, target lesion revascularization (TLR), TVR, Non-TVR. The secondary endpoint was ST. All deaths classified as cardiac or non-cardiac death. Re-AMI was de?ned as the presence of clinical symptoms, electrocardiographic changes, or abnormal imaging findings of MI, combined with an increase in the creatine kinase myocardial band fraction above the upper normal limits or an increase in troponin-T/troponin-I to greater than the 99thpercentile of the upper normal limit. TLR was de?ned as a revascularization of the target lesion due to restenosis or re-occlusion within the stent or 5 mm in and adjacent of the distal or proximal segment. TVR was defined as a revascularization of the target vessel or any segment of the coronary artery containing the target lesion. Non-TVR defined as a revascularization of any segment of the non-target coronary artery. Multi-vessel disease was defined as the presence of a lesion with > 50% diameter stenosis in a non–infarct related coronary artery by visual estimation. ST defined as acute (0–24 h), subacute (24 h–30 d), late (30 d–1 year) and very late (> 1 year) according to the onset time of stent thrombosis.[8]The participants were required to visit the outpatient department of cardiology at the end of the ?rst month and then every 3 to 6 months after the index PCI procedure and we could follow up on the clinical data of all enrolled patients through face-to-face interviews at regular outpatient clinic, medical chart reviews, and telephone contacts.
2.4 Statistical analysis
For continuous variables, differences between the two groups evaluated with the unpaired-test or Mann-Whitney rank test. Data expressed as mean ± SD. For discrete variables, differences expressed as counts and percentages and analyzed with2or Fisher’s exact test between the groups as appropriate. To adjust for any potential confounders, PSM analysis performed using the logistic regression model. We tested all available variables which could be of potential relevance; gender (men), age, left ventricular ejection fraction (LVEF), STEMI, known cardiovascular diseases (CVD) risk factors, chronic kidney disease, routine angiographic follow-up (RAF), laboratory findings and post-PCI medications (aspirin, clopidogrel, cilostazol, BB, CCB, ACEI, ARB, diuretics, lipid lowering agents). Angiographic and procedural characteristics also considered as covariate. The propensity score (PS) was estimated with the use of C-sta-tistic for the logistic regression model and the C-statistics for the two groups was 0.802. Subjects matched with a caliper width equal to 0.01. Various clinical outcomes estimated with the Kaplan-Meier method, and differences between the two groups compared with the log-rank test. Proportional hazard models used to assess the hazard ratio of the 1G-DESs compared with 2G-DESs adjusted PS. For all analyses, a 2-sided< 0.05 considered statistically significant. All data processed with SPSS (version 20.0, SPSS-PC, Inc. Chicago, Illinois).
3 Results
3.1 Baseline clinical and angiographic characteristics
Baseline clinical and angiographic characteristics shown in Table 1. Before PSM adjustment, the mean age of the 1G-DES group was 61.9 ± 11.8 years and 2G-DES was 63.0 ± 12.9 years (= 0.183). Gender distribution was also similar between the two groups (69.3%. 74.0%,= 0.098). The LVEFs were significantly higher in the 2G-DES compared with 1G-DES (49.9 ± 11.2 %. 45.7 ± 10.5%,< 0.001). The histories of previous CVA, MI and PCI were significantly frequent in the 1G-DES. Routine follow-up angiography was more frequently done in the 1G-DES (67.9%. 41.3%,< 0.001). In the aspect of angiographic and procedural characteristics, American College of Cardiology/American Heart Association (ACC/AHA) type B2 and C lesion were more common in 2G-DES and the use of intra-aortic balloon pump (IABP) was more common in 1G-DES. Mean total stent length (25.6 ± 6.4. 23.1 ± 6.4 mm,< 0.001) and total procedure time (minutes, 48.4 ± 35.5. 36.8 ± 24.1,< 0.001) were much longer in 1G-DES compared with 2G-DES. However, all of these differences were disappeared after PSM analysis.
3.2 Post-percutaneous coronary intervention medications
Table 2 shows post-PCI medications between the two groups. Before PSM analysis 1G-DES group was more likely to have received BB, CCB and ACEI after PCI than 2G-DES. But these differences were also disappeared after PSM analysis. The prescription rates of other kinds of medications including aspirin, clopidogrel, cilostazole (Ple-taal?, Otsuka Pharmaceutical Co., Tokyo, Japan), ARB, diuretics and lipid lowering agents were similar between two groups before and after PSM analysis.
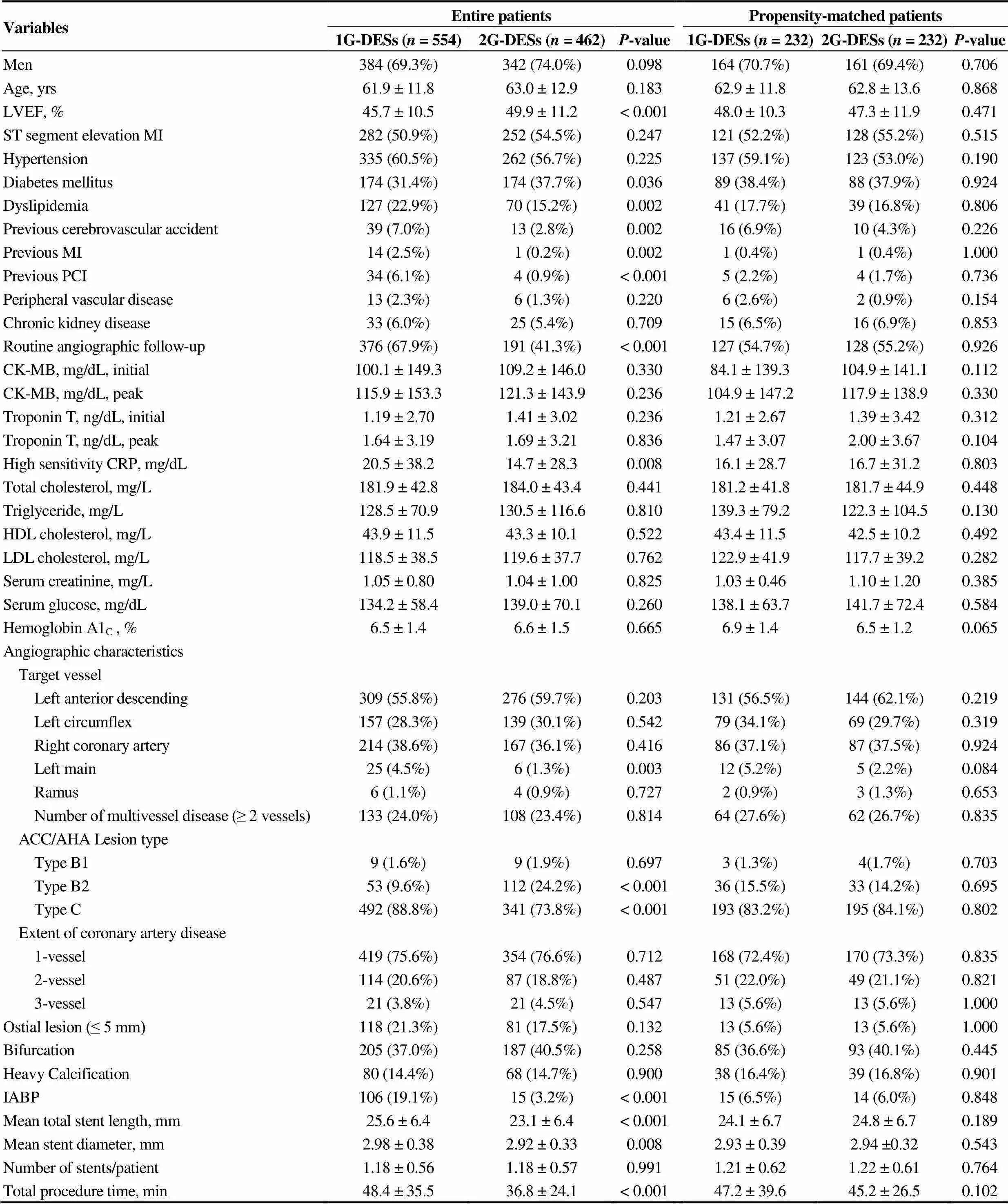
Table 1. Baseline and angiographic characteristics.
Values are mean ± SD or(%). Thevalue for continuous data from analysis of variance; thevalue for categorical data from chi-square test. ACC/AHA: American college of cardiology/American heart association; CK-MB: creatine kinase-muscle and brain; CRP: c-reactive protein; DESs: drug-eluting stents; HDL: high-density lipoprotein; Hemoglobin A1C: glycated hemoglobin; IABP: intra-aortic balloon pump; LDL: low-density lipoprotein; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; 1G: first-generation; 2G: second-generation.
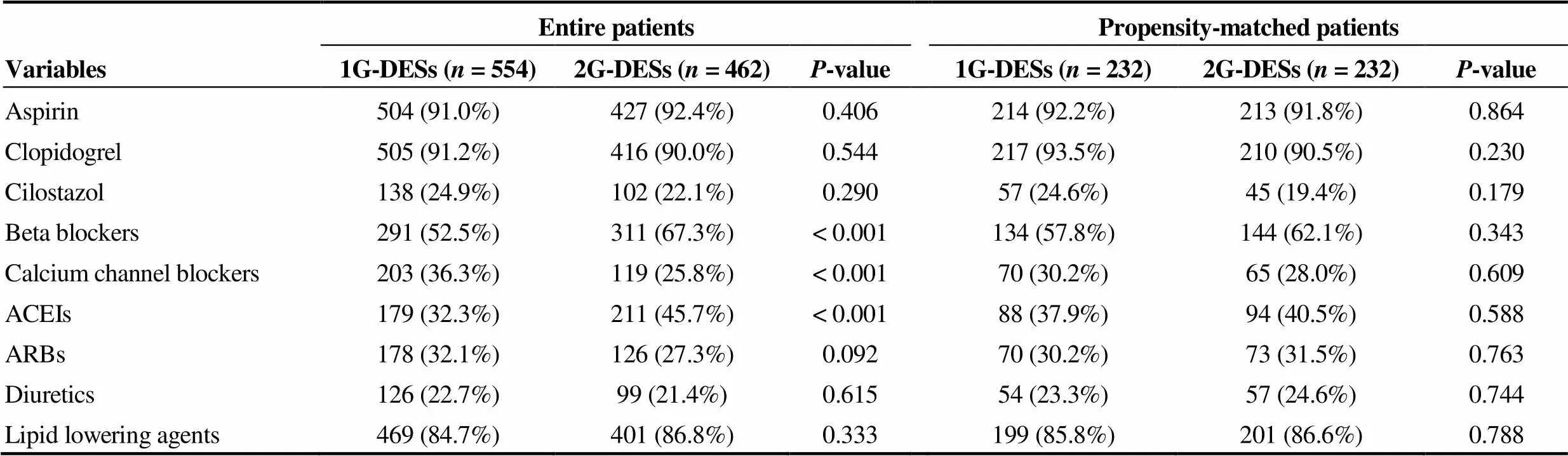
Table 2. Post-percutaneous coronary intervention medications.
Values are mean ± SD or(%). Thevalue for categorical data from chi-square test. ACEIs: angiotensin converting enzyme inhibitors; ARBs: angiotensin receptor blockers; DESs: drug-eluting stents; 1G: first-generation; 2G: second-generation.
3.3 Clinical outcomes
Clinical outcomes at 30 d, one year, 3 years and 5 years shown in Table 3. During one month, the incidence of MACE was not significantly different between the two groups. At 1 year after the index PCI, and the incidence of TLR and TVR was significantly higher in the 1G-DESs group compared with the 2G-DESs group before PSM (TLR: 8.8%. 3.2%,< 0.001; TVR: 10.1%. 3.7%,< 0.001) and after PSM analysis (TLR: 9.1%. 3.4%,0.020; TVR: 9.5%. 3.9%,0.024). At 5 years, the cumulative incidences of TLR [hazard ratio (HR): 3.133; 95% confidence intervals (CI): 1.596–6.376;0.002] and TVR (HR: 3.144; 95% CI: 1.596–6.192;0.001) were significantly higher in the 1G-DESs group compared with the 2G-DESs group after PSM analysis during 5-year follow-up period (Table 4). Figure 2 shows Kaplan-Meier curved analysis of MACE Free survival, TLR, TVR and ST at 5 years according to the generation of DESs (1G. 2G) and the types of DESs (SES. PES. 1G-ZES. 2G-ZES. EES). Figure 3 shows subgroup analysis for MACE and ST up to 5 years. Although, in cases of male, STEMI, non-small vessel disease (≥ 2.5 mm), the choice of 2G-DESs may be prefer rather than 1G-DESs to reduce MACE during PCI for AMI patients.
4 Discussion
The main finding of this “real-world” PSM analysis was that the cumulative incidence of TLR, TVR and total revascularization rates were significantly higher in the 1G-DES group compared with 2G-DES group in AMI vessels after PSM analysis during 5-year follow-up periods. However, the incidence of total death, non-fatal MI and ST were similar between the two groups.
1G-DES (SES) could significantly reduce revasculariza-tion rates compared with BMS among patients with STEMI undergoing primary PCI.[9]However, 1G-DES can cause late ST due to delayed re-endothelization and poor strut coverage.[10]To overcome these limitations stent platforms and polymers have rapidly evolved during a short period. Newer antiproliferative drugs and more biocompatible polymers have been adapted in reducing the rate of late ST in stable coronary artery disease.[11]
4.1 Comparison with randomized controlled study
Until recently, five randomized control trials (RCTs) com-paring clinical outcomes of the 1G-DES versus 2G-DES in patients with AMI were conducted.[4,12–15]However, their follow-up periods were less than 5 years (e.g., 7 months, 12 months, 18 months and 3 years). Among these 5 RCTs, 3 trials had compared ZES versus 1G-DES (two for ZES. SES. PES[4,12]); one for ZES. SES[14]) and other 2 trials compared EES and SES.[13,15]Four trials had evaluated only STEMI patients and one trial included 96% subjects with STEMI and 4% with non-ST segment elevation MI (NSTEMI)[13]and the number of included patients of these 5 RCTs ranged from 35 patients to 625 patients. Compared with these 5 RCTs, our study included relatively large number of NSTEMI patients (= 482), and the total number of STEMI patients were up to 534. This means that our study might have included meaningful number of STEMI vessels. A meta-analysis report of above 5 RCTs[16]showed that 2G-DES could not showed a significant advantage over the 1G-DES in lowering the incidence of TLR, MACE, or all-cause death, only EES seemed to lower the occurrence of MACE than the 1G-DES. Although above 5 RCTs demonstrated useful clinical outcomes between 1G-DES and 2G-DES, more large scaled, randomized long-term follow-up studies are needed due to their relatively small study populations and short-term follow-up periods. In our study, 5-year cumulative incidence of MACE-free survival was significantly lower in the 1G-DES compared with 2G-DES in the entire patients (81.9%. 72.0%, Log-Rank:< 0.001, Figure 2A). The main causes of this differences were caused by the difference between SES and 2G-ZES (Log-Rank:= 0.007), SES and EES (Log-Rank:= 0.001), PES and 2G-ZES (Log-Rank:= 0.020), and PES and EES (Log-Rank:= 0.002). Although the total cumulative incidence of MACE-free survival was not significantly different in PSM patients, EES's beneficial effect for MACE-free survival was sustained only between PES and EES (Log-Rank:= 0.033, Figure 2B).
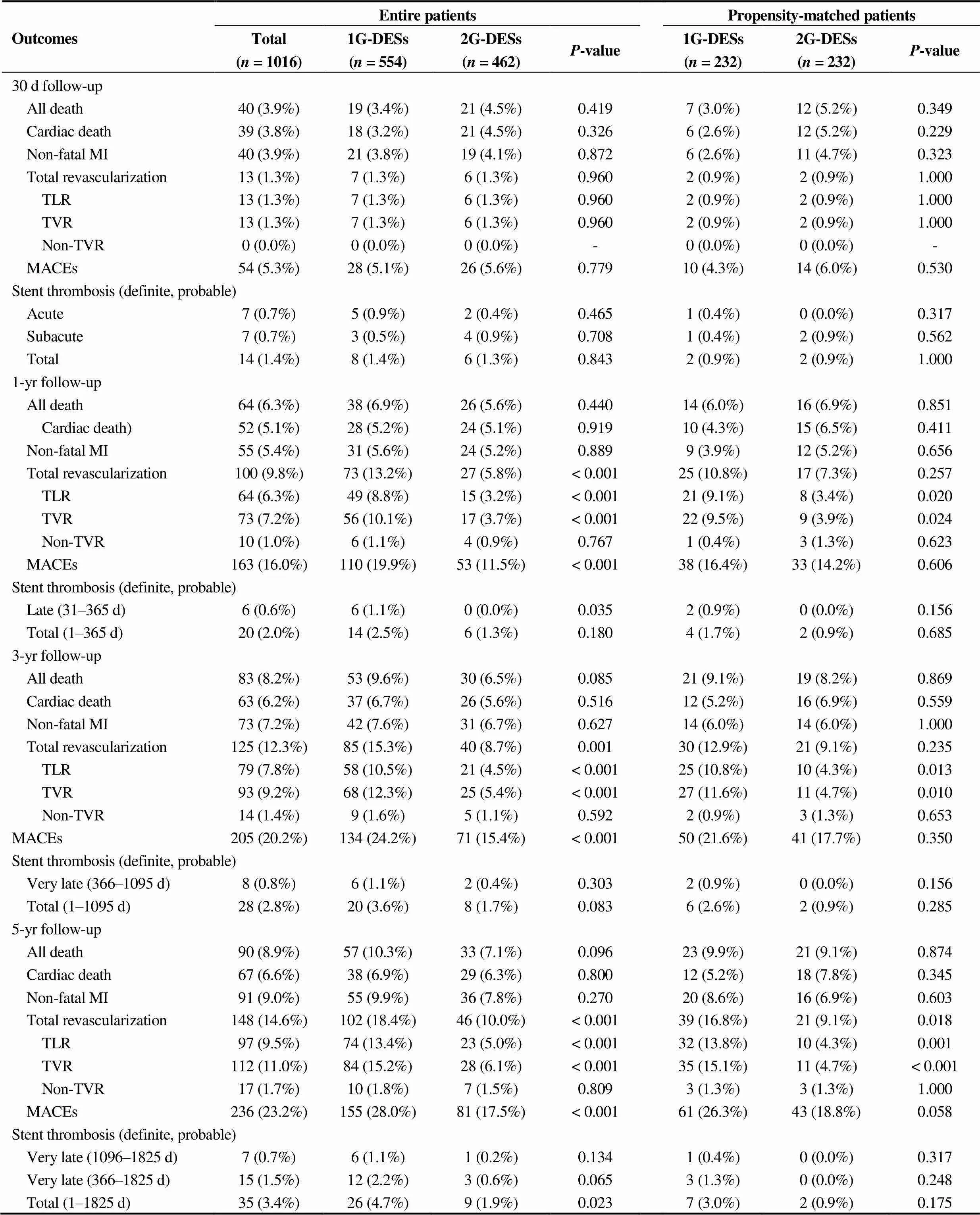
Table 3. Clinical outcomes at 30 days, 1 year, 3 years and 5 years.
Values are presented as(%). Thevalue for categorical data from chi-square test. DESs: drug-eluting stents; MI: myocardial infarction; MACE: major adverse cardiac events; TLR: target lesion revascularization; TVR: target vessel revascularization; 1G: first-generation; 2G: second-generation.
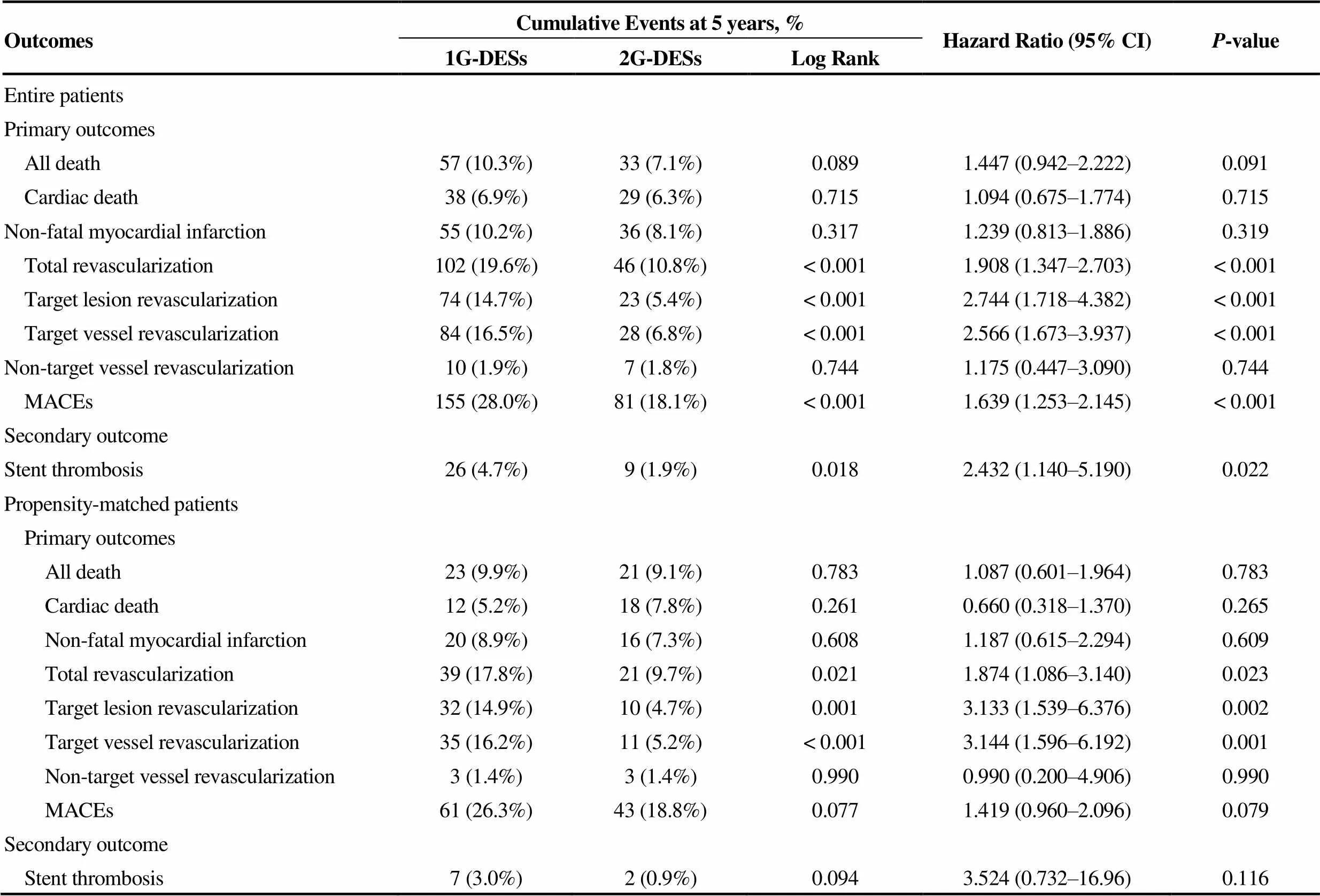
Table 4. Five-year clinical outcomes by Kaplan-Meier curved analysis and cox-proportional hazard ratio model analysis.
Values are presented as(%). DESs: drug-eluting stents; MACEs: major adverse cardiac events;1G: first-generation; 2G: second-generation.
4.2 Comparison with non-randomized controlled study
In non-randomized studies, Chen,.[3]analyzed 2911 AMI patients receiving PESs (= 1210) or EESs (= 1701). In his multicenter registry (Korea Acute Myocardial Infarction Registry, KAMIR) data, EESs group had significantly lower incidence of Re-MI (2.8%. 1.4%,= 0.002), TLR (3.1%. 1.8%,< 0.001) and probable or definite ST (1.8%. 0.3%,< 0.001) than the PESs group during 1 year follow-up period. In our study, as shown in Table 3, Figure 2C and Figure 2D, the incidences of TLR were significantly higher in 1G-DES than 2G-DES after PSM (9.1%. 3.4%,= 0.020) but Re-MI (3.9%. 5.2%,= 0.656) and ST were 1.7%. 0.9%,= 0.685) not significantly different during 1 year follow-up period. At 5 years, the cumulative incidence of TLR was significantly different between PES and EES (19.1%. 6.6%, Log-Rank:= 0.013), PES and 2G-ZES (19.1%. 3.1%, Log-Rank:= 0.001), SES and 2G-ZES (14.4%. 3.1%, Log-Rank:= 0.014), and 1G-ZES and 2G-ZES (14.4%. 3.1%,= 0.009). The 5-year cumulative incidence of TVR was significantly higher in 1G-DES than 2G-DES in entire patients (16.5%. 6.8%, Log-Rank:< 0.001, Table 4) and PSM patients (16.2%. 5.2%, Log-Rank:= 0.001, Table 4, Figures 2E and 2F). In this study, the comparison between the 2G-DESs (2G-ZES and EES) showed that 2G-ZES was non-inferior to EES in 5-year long-term clinical outcomes (MACE-free survival, ST, TLR, TVR, Figures 2A to 2G) and as similar as previous studies.[6,17–20]By contrast, there were several other different results on this comparison. Chen,.[21]also had reported another study comparing ZES and EES. They analyzed 3309 AMI patients treated with ZES (= 1608) or EES (= 1701) from KAMIR. After PSM analysis, EES significantly lowered the incidence of target lesion failure (TLF, 6.5%. 8.7%,= 0.029) and probable or definite ST (0.3%. 1.6%,< 0.001) compared with ZES. We think one of important factors determine this different result is the difference in the follow-up period and Chen,. compared the EES with mainly with original ZES, not the 2G-ZES.
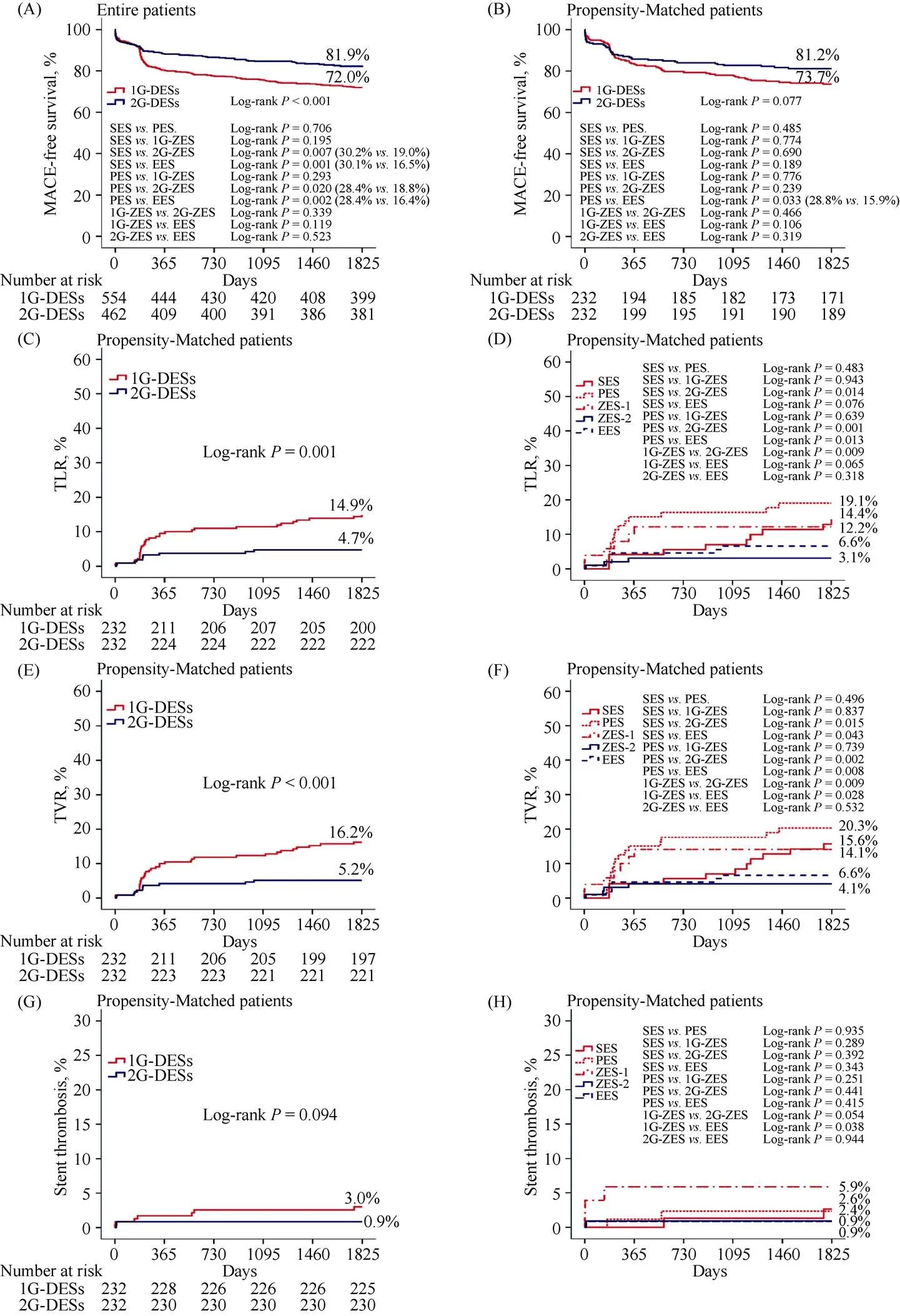
Figure 2. Kaplan-Meier curved analysis of MACEs-free survival (A, B), TLR (C, D), TVR (E, F) and stent thrombosis (G, H) at 5-year according to the generation of DES (1G. 2G) and types of DES (SES. PES. 1G-ZES. 2G-ZES. EES). DES: drug eluting balloon; EES: everolimus-eluting stent; MACEs: major adverse cardiac events; PES: paclitaxel-eluting stent; SES: sirolimus-eluting stent; TLR: target lesion revascularization; TVR: target vessel revascularization; ZES: zotarolimus-eluting stent; 1G: first-generation; 2G: second-generation.
Figure 3. Subgroup analyses for MACE. ACC/AHA: American college of cardiology/American heart association; DESs: drug-eluting stents; LVEF: left ventricular ejection fraction; MACE: major adverse cardiac events; RAF: routine angiographic; STEMI: ST-segment elevation myocardial infarction; 1G: first-generation; 2G: second-generation.
4.3 Comparison between 1G-ZES and 2G-ZES
Characteristically ZES group divided separately as 1G-ZES and 2G-ZES and enrolled as 1G-DESs group or 2G-DESs group in this study. We think that even though endeavor?or endeavor sprint?(early-generation ZES) have relatively thinner stent strut and biocompatible polymer compared with SES or PES, in real-world practice they may be considered as 1G-DES and later-generation ZES (endeavor resolute?, resolute integrity?and resolute onyx?) are really considered as 2G-DESs. There were also limited comparisons of long-term clinical outcomes in patients with AMI after PCI between 1G-ZES and 2G-ZES. The total MACE was not significantly different between these two stents (Log-Rank:= 0.039 in entire patents, Figure 2A and Log-Rank:= 0.466 in PSM patients, Figure 2B) during 5-year follow-up period in our study. However, in addition to increased TLR rate in 1G-ZES compared with 2G-ZES (as shown previously), TVR rate was also higher in 1G-ZES group compared with 2G-ZES (14.1%. 4.1%, Log-Rank:= 0.009, Figure 2F). However, the rate of stent thrombosis was not significantly different between the two groups (5.9%. 0.9%, Log-Rank:= 0.054). Consid-ering these several clinical outcomes, 1G-ZES showed similar clinical outcome patterns of other 1G-DESs (SES and PES).
4.4 Stent thrombosis
Stent thrombosis is another debatable issue in the DES era. Several meta-analyses comparing SESs with PESs demonstrated no significant difference in ST between these two types of stents.[22,23]But Sch?mig,.[24]showed that SESs was better than PESs in terms of reducing ST in his meta-analysis report. Previously mentioned 5 RCTs studies demonstrated that 2G-DES showed decreased incidence of definite or probable stent thrombosis compared with 1G-DES (RR: 0.53; 95% CI: 0.25–1.13;= 0.10).[16]Hofma,[7]reported the 3-year results of their all-comer, randomized, multicenter AMI trial (XAMI trial, Xience V stent. Cypher stent in Primary PCI for AMI trial). According to this report, the incidence of definite/probable ST was similar between the two groups (EES: 2.3%. SES: 3.2%,= 0.60). In our study, the total and individual cumulative incidence of ST were not significantly different between 1G-DES and 2G-DES during 5-year follow-up periods in PSM patients (Figure 2G and Figure 2H). If the sample size were fully larger than our study, there may be statistically significant differences may be exist between 1G-ZES and 2G-ZES or EES.
4.5 Others
The RAF can increase revascularization due to possible 'oculo-stenotic reflex', a term describing revascularization with PCI according to anatomic lesion severity regardless of clinical or physiologic evidence of ischemia.[25]In our study, RAF was considered as an important bias and included covariate of PSM. After PSM this bias was abolished.
Until today, very long-term clinical follow-up data comparing 1G-DES. 2G-DES are rare and debatable especially in patients with AMI. The purpose of this study was to investigate the efficacy and safety of 2G-DES over 1G-DES regardless of specific types of DES in AMI patients during very long-term clinical follow-up periods. Most of the previous studies reported comparative results of only two-types or three-types of DESs, not considered each generation group as a whole.[3–5,8,11–15,22–24]In addition to comparative analysis of 1G-DES. 2G-DES as a whole, we could obtain comparative subgroup results between different types of DESs through Kaplan-Meier curved analysis or Cox-regression analysis. During 5-year follow-up periods, we suggested 2G-DES's superiority for reducing TLR, TVR and total revascularization rates in AMI patients. However, this result maybe more precisely be defined by any other large and long-term follow-up randomized and controlled trials in the future. Under the circumstances where very long-term major clinical outcomes between 1G-DES and 2G-DES are still debatable, our results can be provide useful clinical outcome information and trends between 1G-DES and 2G-DES to some extent during very long-term follow-up periods in the DES era.
This study has some limitations. First, because it is a non-randomized registry design and single center study, several confounding factors such as under-reporting and/or missing value and selection bias may have affect the end results. Second, although PSM analysis and subgroup analysis done, the proportions of each stents in both groups were not evenly distributed. Third, in our study AMI patients were consisted of STEMI and NSTEMI. This heterogeneity can affect each other and may act as bias. Fourth, the strategy of antiplatelet therapy [e.g., DAPT or triple antiplatelet therapy (TAPT)] was left to the physician’s discretion, which may have influenced the major clinical outcomes. Lastly, several important factors that can determine the end results such as total ischemia time could not be precisely obtained in our study patients.
In conclusion, in our single-center, all-comer registry, 2G-DES's superiority in reducing TLR, TVR and total revascularization rate over 1G-DES in AMI patients suggested during 5-year follow-up periods. Special cautions with careful clinical follow-up would be necessary for the AMI patients who are treating with 1G DESs in this aspect. However, long-term follow-up data from large scale, randomized studies are necessary to confirm these results.
Acknowledgements
The authors declare that they have no conflict of interest.
1 Lemos PA, Saia F, Hofma SH,. Short- and long-term clinical benefit of sirolimus-eluting stents compared to conventional bare stents for patients with acute myocardial infarction.2004; 43: 704–748.
2 Dudek D, Mehran R, Dziewierz A,. Impact of advanced age on the safety and effectiveness of paclitaxel-eluting stent implantation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: the HORIZONS-AMI trial.2013; 82: 869–877.
3 Chen KY, Rha SW, Li YJ,. Comparisons of everolimus and Paclitaxel-eluting stents in patients with acute myocardial infarction.2015; 28: 147–156.
4 Kang WC, Ahn T, Lee K,. Comparison of zotarolimus- eluting stents versus sirolimus-eluting stents versus pacli-taxel-eluting stents for primary percutaneous coronary inter-vention in patients with ST-elevation myocardial infarction: results from the Korean Multicentre Endeavor (KOMER) acute myocardial infarction (AMI) trial.2011; 7: 936–943.
5 Kedhi E, Joesoef KS, McFadden E,. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial.2010; 375: 201–209.
6 Serruys PW, Silber S, Garg S,. Comparison of zotaro-limus-eluting and everolimus-eluting coronary stents.2010; 363: 136–146.
7 Hofma SH, Smits PC, Brouwer J,. Long-term follow-up of second-generation everolimus-eluting stents versus first-ge-neration sirolimus-eluting stents in acute myocardial infarc-tion: three-year results of the XAMI trial.2015; 10: 1280–1283.
8 Bundhun PK, Wu ZJ, Chen MH,. Is there any significant difference in stent thrombosis between sirolimus and paclitaxel eluting stents?: A systematic review and meta-analysis of randomized controlled trials.2016; 95: e2651.
9 De Luca G, Dirksen MT, Spaulding C,. Drug-eluting stent in primary angioplasty (DESERT) cooperation. Drug- eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials.2012; 172: 611–621.
10 Daemen J, Wenaweser P, Tsuchida K,. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel- eluting stents in routine clinical practice: data from a large two-institutional cohort study.2017; 369: 667–678.
11 Stone GW, Rizvi A, Newman W,. SPIRIT IV Investigators. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease.2010; 362: 1663–1674.
12 Lee CW, Park DW, Lee SH,. ZEST-AMI Investigators. Comparison of the efficacy and safety of zotarolimus-, siroli-mus-, and paclitaxel-eluting stents in patients with ST-ele-va-tion myocardial infarction.2009; 104: 1370–1376.
13 Hofma SH, Brouwer J, Velders MA,. Second-generation everolimus-eluting stents versus first-generation sirolimus- eluting stents in acute myocardial infarction. 1-year results of the randomized XAMI (XienceV Stent. Cypher Stent in Primary PCI for Acute Myocardial Infarction) trial.2012; 60: 381–387.
14 Chung WY, Kang J, Cho YS,. A randomized, prospective, two-center comparison of sirolimus-eluting stent and zotarolimus-eluting stent in acute ST-elevation myocardial infarction: the SEZE trial.2012; 125: 3373–3381.
15 Sawada T, Shinke T, Otake H,. Comparisons of detailed arterial healing response at seven months following implantation of an everolimus- or sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction.2013; 168: 960–966.
16 Wu G, Sun G, Zhao R,. Clinical outcomes of second- versus first-generation drug-eluting stents in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials.2014; 10: 643–650.
17 Hannan EL, Zhong Y, Wu C,. Everolimus-eluting stents and zotarolimus-eluting stents for percutaneous coronary interventions: two-year outcomes in New York State.2013; 81: 1097–1105.
18 Stefanini GG, Serruys PW, Silber S,. The impact of pa-tient and lesion complexity on clinical and angiographic out-comes after revascularization with zotarolimus- and ever-olimus-eluting stents: a substudy of the RESOLUTE All Comers Trial (a randomized comparison of a zotarolimus- eluting stent with an everolimus-eluting stent for percutane-ous coronary intervention).2011; 57: 2221–2232.
19 Silber S, Windecker S, Vranckx P,. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial.2011; 377: 1241–1247.
20 von Birgelen C, Basalus MW, Tandjung K,. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial.2012; 59: 1350–1361.
21 Chen KY, Rha SW, Wang L,. Unrestricted use of 2 new-generation drug-eluting stents in patients with acute myocardial infarction: a propensity score-matched analysis.2012; 5: 936–945.
22 Kufner S, de Waha A, Tomai F,. A meta-analysis of specifically designed randomized trials of sirolimus-eluting versus paclitaxel-eluting stents in diabetic patients with coronary artery disease.2011; 162: 740–747.
23 Zhang X, Xie J, Li G,. Head-to-head comparison of sirolimus-eluting stents versus paclitaxel-eluting stents in patients undergoing percutaneous coronary intervention: a meta-analysis of 76 studies.2014; 9: e97934.
24 Sch?mig A, Dibra A, Windecker S,. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease.2007; 50: 1373–1380.
25 Uchida T, Popma J, Stone GW,. The clinical impact of routine angiographic follow-up in randomized trials of drug-eluting stents: a critical assessment of "oculostenotic" reintervention in patients with intermediate lesions.2010; 3: 403–411.
*The first two authors contributed equally to this manuscript
Seung-Woon Rha, Cardiovascular Center, Korea University Guro Hospital, Gurodong-ro148, Guro-gu, Seoul 08308, South Korea. E-mail: swrha617@yahoo.co.kr
Telephone: +82-2-2626-3020
Fax: +82-2-864-3062
June 24, 2018
June 28, 2018
June 28, 2018
August 28, 2018
 Journal of Geriatric Cardiology2018年8期
Journal of Geriatric Cardiology2018年8期
- Journal of Geriatric Cardiology的其它文章
- Rare combination of dilated cardiomyopathy and ankylosing spondylitis in a family
- Patent foramen ovale in an old patient with ischemic stroke following carotid surgery
- Normalization of plasma growth hormone alleviated malignant ventricular tachycardia in acromegaly
- Natriuretic peptide family as diagnostic/prognostic biomarker and treatment modality in management of adult and geriatric patients with heart failure: remaining issues and challenges
- Subclinical hypothyroidism is associated with lipid-rich plaques in patients with coronary artery disease as assessed by optical coherence tomography
- Correction of hypovitaminosis D improved global longitudinal strain earlier than left ventricular ejection fraction in cardiovascular older adults after orthopaedic surgery
