Subclinical hypothyroidism is associated with lipid-rich plaques in patients with coronary artery disease as assessed by optical coherence tomography
Xiao-Qing CAI, Feng TIAN, Tian-Wen HAN, Dong-Kai SHAN, Yang LIU,3, Wei-Jun YIN,4, Jing Jing,Qiang Xu, Yun-Dai CHEN
?
Subclinical hypothyroidism is associated with lipid-rich plaques in patients with coronary artery disease as assessed by optical coherence tomography
Xiao-Qing CAI1,2,*, Feng TIAN1,*, Tian-Wen HAN1, Dong-Kai SHAN1, Yang LIU1,3, Wei-Jun YIN1,4, Jing Jing1,Qiang Xu1, Yun-Dai CHEN1
1Department of Cardiology, Chinese PLA General Hospital, Beijing, China2Department of Cardiology, Chinese PLA Lanzhou General Hospital, Lanzhou, China3Department of Cardiology, Chinese PLA 305 Hospital, Beijing, China4School of Medicine, Nankai University, Tianjin, China
Subclinical hypothyroidism (SCH) has recently been acknowledged as an unconventional risk factor for coronary artery disease (CAD) and characterized by poor prognosis, which may be due to atherosclerotic plaque characteristics. We conducted this study to observe coronary plaque characteristics in coronary artery disease patients with concomitant SCH.Patients with coronary artery disease were enrolled in the study and divided into an SCH group (patients,= 26; plaques,= 35) and a non-SCH group (patients,= 52; plaques,= 66). They were divided 1: 2 according to propensity-matched analysis including age, diabetes mellitus, gender, CAD severity and culprit vessel. Optical coherence tomography (OCT) imaging was performed on all patients, and images were analyzed by two independent investigators. Lipid-rich plaques (LRP), the precursor of vulnerable plaques, were defined as having more than one quadrant occupied with lipid pool. Maximum lipid arcs were simultaneously recorded. Fibrotic plaques and calcific plaques were also identified. The presence of coronary dissection, plaque erosion, thrombus, macrophage, calcific nodule, thin-cap fibroatheroma and micro channel were all noted.The ratio of LRP in SCH group was significantly higher than that in non-SCH group (54%30.3%,= 0.037). That was the case as well for the maximum lipid arcs value (181.5°± 61.6°142.1°± 35.9°,= 0.046). While thin-cap fibroatheroma (TCFA) was detected, no difference was identified between the two groups in either TCFA ratio (20%16.7%,= 0.579) or fibrous cap thickness (57.5 ± 14.063.5 ± 10.7 μm,= 0.319). Other OCT characteristics such as dissection, plaque erosion, thrombus, macrophage shadow and calcific nodule were also similar.Higher ratio of LRP with greater lipid arc in SCH patients may be related to the plaque instability and poor prognosis in CAD patients with SCH.
J Geriatr Cardiol 2018; 15: 534?539. doi:10.11909/j.issn.1671-5411.2018.08.007
Coronary artery disease; Optical coherence tomography; Plaque characteristics; Subclinical hypothyroidism
1 Introduction
There are existing comprehensive interventions of clas-sical risk factors including dyslipidemia, hypertension, dia-betes mellitus and tobacco use. In addition, secondary pre-vention strategies are also well established and can be com-bined with such interventions and implemented in clinical practices. Coronary artery disease (CAD) remains a world-wide leading cause of death.[1,2]The role of other unconven-tional risks in initiating and aggravating CAD is increas-ingly acknowledged. Thyroxin is a vital circulated hormone that maintains cardiovascular homeostasis. A subtle de-crease of thyroid hormones is accompanied by elevation of thyroid stimulating hormone (TSH) without clinical mani-festations. This is defined as subclinical hypothyroidism (SCH) and is prevalent but often neglected in study popula-tions.[3]There is a convincing epidemiologic association found between SCH and subsequent coronary heart dis-ease.[4]Some observational studies have shown that minor changes of thyroxin in SCH are associated with a 20%-80% increase in cardiovascular morbidity and mortality.[5,6]This indicates a poorer prognosis of CAD patients with con-comitant SCH. Unfortunately, as an unconventional cardio-vascular risk, the role of SCH is still underestimated and has had few high-quality clinical trials.
Plaque characteristics are proved to be closely associated with the plaque progression and even subsequent coronary events.[7]Plaque characteristics in SCH patients such as intima media thickening, atherosclerotic plaque ulceration and hypercoagulable state have been scarcely reported in carotid artery of SCH patients by computed tomography.[8,9]Other plaque characteristics in coronary artery are still unknown. Optical coherence tomography (OCT) is a modality with unrivaled spatial resolution in recognizing intravascular characteristics and assessing composition of coronary plaque. The aim of our study was to assess plaque characteristics of coronary artery in CAD patients with concomitant SCH by using OCT.
2 Methods
2.1 Study population
From September 2014 to March 2017, 406 CAD patients receiving OCT imaging in our hospital were retrospectively identified and enrolled (Figure 1). Exclusion criteria were settled as follows: (1) chronic total occlusion; (2) left main coronary artery disease; (3) cardiogenic shock; (4) extremely calcified or tortuous vessels with difficulty in advancing OCT catheter; (5) large vessels with diameter beyond the penetration limit of OCT; (6) congestive heart failure with left ventricular ejection fraction (LVEF) < 40%; (7) liver dysfunction with a serum alanine transaminase and glutamic oxalacetic transaminase > 2 times the upper normal limits; (8) renal insufficiency with baseline serum creatinine > 2.0 mg/dL; and (9) acute ST-elevation myocardial infarction (STEMI). Thereafter, 12 patients were excluded (2 in SCH, 10 in non-SCH). Given the huge difficulty in recognizing plaque characteristics in poor quality OCT image due to insufficient blood clearance or heavy thrombus burden, we further excluded 11 patients with poor OCT image quality (1 in SCH, 10 in non-SCH). Finally, a total of 26 SCH patients, who were defined as having thyroid hormones within the normal range and elevated TSH level ( > 4.5 mmol/L) were enrolled in our study and compared 1: 2 with patients without SCH based on a propensity-matched score. Matching criteria included conventional risk factors (in order) as age, diabetes mellitus, gender, CAD severity and culprit vessel. The study was approved by our institutional ethics committee, and all patients gave written informed consent before enrollment.
2.2 OCT data acquisition and medication
Our study included patients with stable angina, non-ST- elevation acute coronary syndrome including unstable an-gina and non-ST-elevation myocardial infarction. Patients with STEMI were excluded due to culprit vessels being always occluded and having a heavy burden of thrombus, which resulted in difficulty in performing OCT without balloon predilations. Dual-antiplatelet drugs including aspirin (100 mg/day) and clopidogrel (75 mg/day) or ticagrelor (90 mg bid, namely, 180 mg/day) were routinely taken before the procedure. Intravenous heparin was given at an initial dose of 2000 U/kg before coronary angiography and then added to 100 U/kg for OCT examination.[10]Coronary imaging was acquired after administering 200 μg of intracoronary nitroglycerin. Based on coronary angiography results, target vessel was determined if the diameter stenosis exceeded 30% quantified by quantitative coronary angiography to conduct frequency-domain OCT examination (C7-XR, OCT Intravascular Imaging System; St. Jude Medical, St Paul, MN). Accordingly, a 2.7 Fr OCT imaging catheter (Dragonfly; LightLab Imaging, Inc.) was advanced distally to the target vessel lesion via a standard working wire, and then, automated pullback of catheter was manually triggered by injecting contrast medium into coronary artery for blood clearance. Pullback speed was 25 mm/s, and the whole scan had a length of 54 mm. Image acquisition was terminated when the region of interest was wholly scanned or the catheter entered the guide wire.[11]
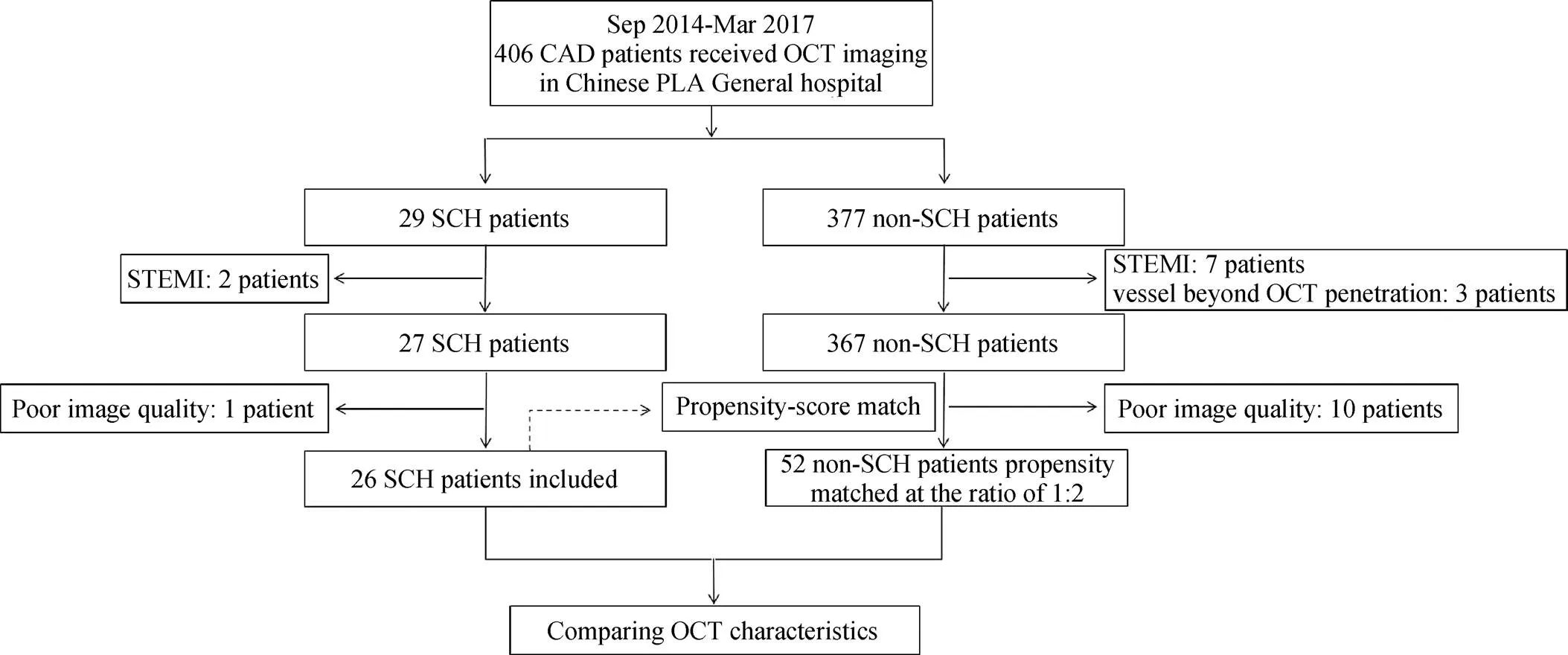
Figure 1. Study flow chart.CAD: coronary artery disease; OCT: optical coherence tomography; SCH: subclinical hypothyroidism; STEMI: ST-elevation myocardial infarction.
2.3 OCT data analysis
OCT images were stored and analyzed offline by using software supplied by LightLab imaging corporation. In one target vessel, two plaques were defined if the distance between the two lesions exceeded 10 mm. A single still frame of OCT image was chosen for every 1 mm segment throughout the entire scan of each lesion. Images were analyzed by two experienced investigators independently using previously established OCT analysis criteria.[12]When discordance occurred, a consensus reading was acquired by a third independent investigator. Specifically, lipid-rich pla-ques (LRP) were characterized as a low-signal region with diffuse border and occupying more than one quadrant, whereas calcific plaque was identified as having a sharp border. Thin-cap fibroatheroma (TCFA) was related to histopathologic definition, which is defined as a lipid-rich plaque with a lipid arc greater than 90°and a fibrous cap < 65 μm at the thinnest point.[13]Meanwhile, presence of dissection, plaque erosion, thrombus, macrophage, calcific no-dule and micro channel were all noted and labeled (Figure 2).
2.4 Statistical analysis
Categorical variables were expressed as absolute number and percentage while continuous variables were recorded as the mean ± SD or as median (25th, 75thpercentile) according to the data distribution. Categorical variables were compared by Chi-squared test or Fisher’s exact test if necessary, while the continuous variables were compared by student’s-tests (if normally distributed) or Mann-Whitney U-test (if not normally distributed). A-value < 0.05 was considered statistically significant. All these data statistics were performed by SPSS, version 20.0 (SPSS Inc., IBM, USA).
3 Results
3.1 Baseline characteristics
A total of 78 patients comprising 26 SCH patients (two with hormone replacement therapy) and 52 non-SCH patients were enrolled in our study. TSH level in SCH patients was significantly higher than that in non-SCH patients (5.96 ± 2.251.77 ± 0.91 mIU/L,< 0.0001). Ratio of patients with noncompliant LDL-C was also significantly higher in the SCH group (80.8%57.7%,= 0.043). Other base- line characteristics including free T3 and free T4 were comparable between the two groups (Table 1). There was no difference in levels of high sensitivity C reactive protein (hs-CRP) (0.56 ± 0.23 mg/dL0.49 ± 0.17 mg/dL,= 0.71), which represented inflammation severity.

Figure 2. Representative images of optical coherence tomography of coronary characteristics.(A): Fibrotic plaque; (B) lipid-rich plaque with lipid pool occupying more than one quadrant; (C) calcific plaque; (D) lumen dissection; (E) plaque erosion with attached white thrombus; (F) thrombus; (G) macrophage; (H) calcific nodule; (I) vasa vasorum presented as micro channels; and (J) thin-cap fibroatheroma.
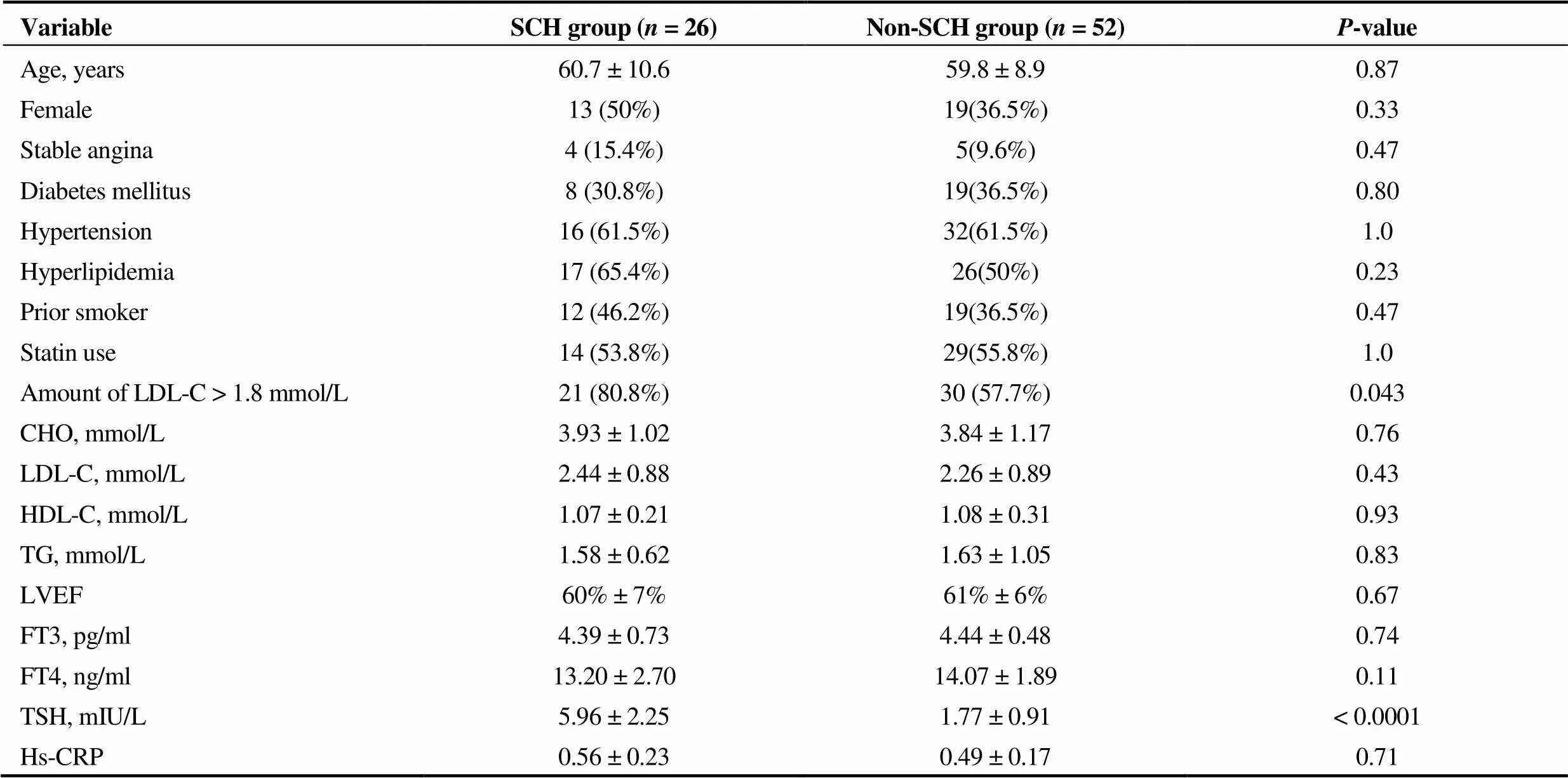
Table 1. Baseline characteristics.
n (%). CHO: cholesterol; HDL-C: high density lipoprotein cholesterol; Hs-CRP: high sensitivity C-reactive protein; FT3: free triiodothyronine; FT4: free thyroxine; LDL-C: low density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; TG: triglyceride; TSH: thyroid stimulating hormone.
3.2 Coronary plaque characteristics
There were a total of 29 vessels in the SCH group and 61 vessels in the non-SCH group. Two plaques in the same vessel were imaged in six SCH vessels, and the same situation was identified in five non-SCH vessels. As depicted in Table 2, coronary plaque characteristics were compared between two groups. LRP in the SCH group were significantly greater than those in the non-SCH (54%30.3%,= 0.037), while more fibrous plaques were identified in the non-SCH group, yet without statistical significance (37.1%56.1%,= 0.059). The mean value of maximum lipid arc was also significantly greater in the SCH group (181.5 ± 61.6°142.1 ± 35.9°,= 0.046). Meanwhile, there were no differences found between the two groups in rates of calcific plaques (28.6%30.3%,= 0.856) or in values of maximum calcific arc (166.2 ± 77.4°157.4 ± 75.6°,= 0.766). Minimum fibrous cap thickness was not significantly different either (252.5 ± 78.9225.6 ± 82.2 μm,= 0.116). According to the definition of TCFA previously described, neither TCFA ratio difference (20%16.7%,= 0.579) nor fibrous cap thickness (57.5 ± 14.063.5 ± 10.7 μm,= 0.319) between the two groups was identified. Other OCT characteristics such as dissection, plaque erosion, thrombus, macrophage shadow and calcific nodule were also similar between the two groups. In a comparison of micro channels representing the vasa vasorum, no statistical significance was found between the two groups (17.1%34.8%,= 0.061).
4 Discussion
In this retrospective study, we assessed coronary plaque characteristics by performing OCT imaging in CAD patients with SCH for the first time, to the best of our knowledge. We found higher frequency of lipid-rich plaque with greater maximum lipid arc in SCH patients than that in non-SCH patients, though the rates of vulnerable plaques were similar between the two groups.
LRP, usually regarded as the precursor of vulnerable plaque and subsequent cardiovascular event, is unstable and prone to rupture.[14]According to a prospective study that performed OCT to observe coronary plaque progression from baseline to follow-up, the frequency of lipid pool was significantly higher in coronary plaques that displayed progression than those that did not. The study concluded that the lipid pool odds ratio (OR) value was 2.16, suggesting the risk factor profile of LRP in predicting plaque progression.[10]Furthermore, another recently published study showed that patients with LRP in non-culprit regions had higher incidence of future cardiac events than those without LRP.[15]In our study, the higher frequency of LRP in SCH patients over non-SCH patients also indicates the instability and the progression of coronary plaques in SCH patients with CAD. Meanwhile, LRP identified in 33.6% of the non-SCH patients is consistent with the rate reported in a previous study.[15]Considering the underlying mechanism, the relationship between SCH and hyperlipidemia was conflicting in population-based studies.[16-18]However, our study showed that the baseline lipid profile was similar in the two groups; therefore, we inferred that high frequency LRP may be due to subtle disturbance of lipid metabolism in SCH.[19]Interestingly, after choosing 1.8 mmol/L (the target value of lipid control in CAD patients) as the cutoff value of LDL-C, we found that there was a greater number of noncompliant patients in the SCH group than that of the non-SCH group. This may indicate the difficulty in lipid control and could be relevant to the prognosis.
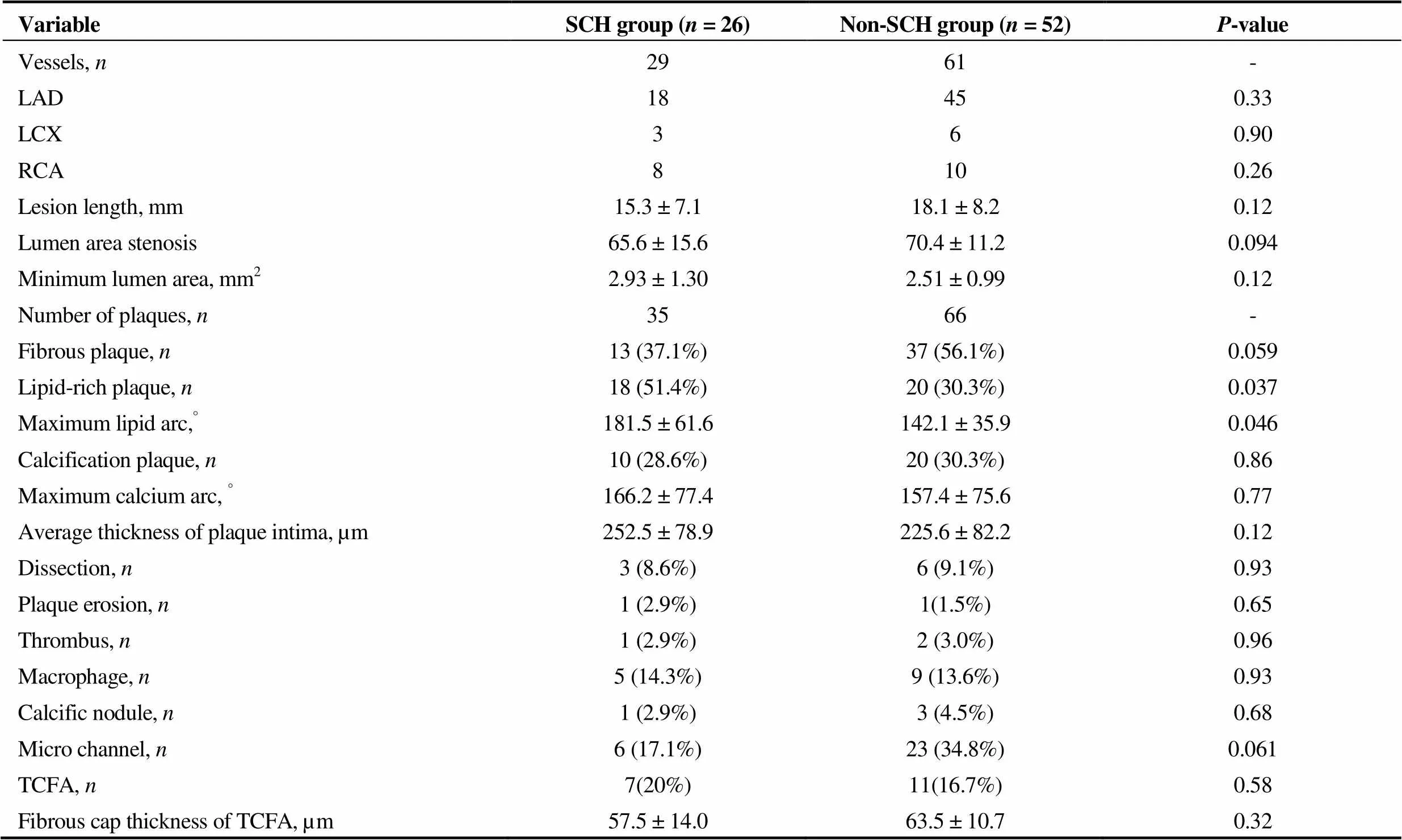
Table 2. Coronary plaque characteristics.
LAD: left anterior descending branch; LCX: left circumflex branch; RCA: right coronary artery; TCFA: thin-cap fibroatheroma.
Angiogenesis, a double-edged sword for responding to CAD, forms collateral arteries. On one hand, it alleviates ischemia, whereas on the other hand, it generates neovascularization, named vasa vasorum, to supply blood cells and inflammatory mediators, resulting in the progression and destabilization of coronary plaques. It has been reported that thyroid hormone induced angiogenesis through nongenomic effects as activation of ERK 1/2 and HIF 1α, while lack of thyroxin caused decreased angiogenesis.[9,19]In our study, we found a trend of reduced micro channel, representing vasa vasorum, in SCH patients identified by OCT cross- sectional images. However, its clinical significance is still to be further elucidated.
Inflammation plays a role in the development and progression of atherosclerosis. hs-CRP is recognized as an inflammatory marker in predicting the instability of coronary plaques.[20,21]It has been reported that hs-CRP levels were elevated in subclinical hypothyroidism patients.[22]In our study, there were no difference in hs-CRP levels between SCH patients with CAD and non-SCH ones, though elevations of hs-CRP occurred in both groups. Results indicated that hs-CRP greater increase caused by CAD possibly covered the hs-CRP lower elevation due to SCH.
There were some limitations to our study. First, this was a single-center retrospective study with enrollment of a small sample with possible selection bias. Second, the limited penetration of OCT image may negatively influence fully detecting deep components of coronary structures or accurately reflecting the “intact” and “true” coronary plaque characteristics in patients suffering CAD and SCH. Lastly, some plaques were excluded due to low adequacy in implementing OCT imaging on patients such as STEMI patients. This inevitably caused some bias by influencing accuracy of presentation and analysis.
In our study, greater lipid-rich plaques and larger lipid arcs were identified in CAD patients with concomitant SCH via, performing OCT imaging. This may indicate instability and vulnerability of coronary plaques in such patients, and influence clinical prognosis. Further studies with larger patient pools and prospective-design studies are warranted to confirm these results.
Acknowledgements
This study was supported by grant from the National Key R&D Program of China (2016YFC1300304). The authors had no conflicts of interests to disclose.
1 Chen WW, Gao RL, Liu LS,China cardiovascular diseases report 2015: a summary.2017; 14: 1–10.
2 Benjamin EJ, Blaha MJ, Chiuve SE,Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association.2017; 135: e146–e603.
3 Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29: 76–131.
4 Rodondi N, den Elzen WP, Bauer DC,Subclinical hypothyroidism and the risk of coronary heart disease and mortality.2010; 304: 1365–1374.
5 Cappola AR, Fried LP, Arnold AM,Thyroid status, cardiovascular risk, and mortality in older adults.2006; 295: 1033–1041.
6 Hak AE, Pols HA, Visser TJ,Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study.2000; 132: 270–278.
7 Ahmadi A, Leipsic J, Blankstein R,Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression.2015; 117: 99–104.
8 Sevuk U, Bahadir MV, Altindag R,Relationship between thyroid function and carotid artery plaque ulceration.2015; 115: 581–587.
9 Jabbar A, Pingitore A, Pearce SH,Thyroid hormones and cardiovascular disease.2017; 14: 39–55.
10 Uemura S, Ishigami K, Soeda T,Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques.2012; 33: 78–85.
11 Feng T, Yundai C, Lian C,Assessment of coronary plaque characteristics by optical coherence tomography in patients with diabetes mellitus complicated with unstable angina pectoris.2010; 213: 482–485.
12 Tearney GJ, Regar E, Akasaka T,Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation.2012; 59: 1058–1072.
13 Virmani R, Burke AP, Farb A,Pathology of the vulnerable plaque.2006; 47: C13–C18.
14 Fernandez-Ortiz A, Badimon JJ, Falk E,Characterization of the relative thrombogenicity of atherosclerotic plaque components: implications for consequences of plaque rupture.1994; 23: 1562–1569.
15 Xing L, Higuma T, Wang Z,Clinical Significance of Lipid-Rich Plaque Detected by Optical Coherence Tomography: A 4-Year Follow-Up Study.2017; 69: 2502–2513.
16 Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia.2004; 2: 351–355.
17 Ineck BA, Ng TM. Effects of subclinical hypothyroidism and its treatment on serum lipids.2003; 37: 725–730.
18 Jayasingh IA, Puthuran P. Subclinical hypothyroidism and the risk of hypercholesterolemia.2016; 5: 809–816.
19 Ichiki T. Thyroid Hormone and Vascular Remodeling.2016; 23: 266–275.
20 Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus.2007; 49: 2129–2138.
21 Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients.2006; 47: C19–C31.
22 Christ-Crain M, Meier C, Guglielmetti M,Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial.2003; 166: 379–386.
*The first two authors contributed equally to this manuscript
Yun-Dai CHEN, Department of Cardiology, Chinese PLA General Hospital, 28 Fuxing Road, Beijing, China. E-mail: cyundai@vip.163.com
August 31, 2017
October 6, 2017
December 6, 2017
August 28, 2018
 Journal of Geriatric Cardiology2018年8期
Journal of Geriatric Cardiology2018年8期
- Journal of Geriatric Cardiology的其它文章
- Rare combination of dilated cardiomyopathy and ankylosing spondylitis in a family
- Patent foramen ovale in an old patient with ischemic stroke following carotid surgery
- Normalization of plasma growth hormone alleviated malignant ventricular tachycardia in acromegaly
- Natriuretic peptide family as diagnostic/prognostic biomarker and treatment modality in management of adult and geriatric patients with heart failure: remaining issues and challenges
- Five-year major clinical outcomes between first-generation and second- generation drug-eluting stents in acute myocardial infarction patients underwent percutaneous coronary intervention
- Correction of hypovitaminosis D improved global longitudinal strain earlier than left ventricular ejection fraction in cardiovascular older adults after orthopaedic surgery
