Effect of pre-incisional anterior scalp block on intraoperative opioid consumption in adult patients undergoing elective craniotomy to remove tumor: study protocol for a randomized double-blind trial
Pathomporn Pin-on*, Yodying Punjasawaswong
Department of Anesthesiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
INTRODUCTION
The level of noxious stimuli and nociception during craniotomy procedure vary according to the stage of surgery.The nociception and anesthetic requirement are signi ficantly less during brain tissue exposure and exploration compared to those during skin, bone, and dura incision (DeBenedittis et al., 1996; Quiney et al., 1996; Long, 2001; Bruder et al.,2010). Acute severe post-craniotomy pain usually results from inadequate somatic pain relief, underestimation and under-recognition of pain in neurosurgical patients (De-Benedittis et al., 1996; Quiney et al., 1996; Dunbar et al.,1999; Long, 2001; Ortiz-Cardona and Bendo, 2007; Pardey Bracho et al., 2014; Sokhal et al., 2015). There are few qualified evidence-based reports on the discrepant conclusions regarding the optimal perioperative pain management in neurosurgical patients. Most of the patients receive inappropriate care.
The severity of post-craniotomy pain is reportedly moderate to severe depending on the duration of surgery, age,gender, surgical site, and the extensiveness of surgical procedure (Stoneham and Walters, 1995; DeBenedittis et al., 1996; Quiney et al., 1996; Dunbar et al., 1999; Verchere et al., 2002; Rahimi et al., 2006; Ortiz-Cardona and Bendo,2007). Most of neurosurgical patients experience a disabling pain within the first 48 hours after surgery.
The inadequate alleviation of perioperative pain not only leads to chronic pain through central sensitization pathway but also causes complications, such as agitation,sympathetic overactivity, increased intracranial pressure,and consequently intracerebral hemorrhage, particularly in the surgical lesion in neurosurgical patients (Verchere et al., 2002; Ortiz-Cardona and Bendo, 2007).
Opioid administration is the current practice of analgesic therapy during craniotomy procedure. Intraoperative opioid titration is usually guided by the hemodynamic changes from preoperative baseline, which derives from the physiologic objective measurement rather than the method of just asking the patients (the subjective measurement).
It is certainly known that physiological objective parameters, i.e., blood pressure, heart rate, and respiratory rate cannot be consistently translated into the subjective pain perception (Ortiz-Cardona and Bendo, 2007). Currently,there is no consensus on the appropriate dosage of opioid consumed during craniotomy procedure.
Opioid overuse during the surgery brings about delayed emergence from anesthesia and disturbs subsequent postoperative neurological assessment. The importance of suf ficient intraoperative analgesia and the limitation of intraoperative opioid consumption remain poorly understood.Effective regional nerve block plays a signi ficant role in neurosurgical procedures.
Scalp block is a technique of subcutaneous anesthetic administration in the region of the skull and is developed based on classic scalp in filtration. The objective of scalp block is to block afferent input from the nerves that innervate the scalp, subcutaneous tissue, pericranial muscles, and dura mater. All of these are the origin of certain somatic pains (DeBenedittis et al., 1996; Nguyen et al., 2001; Ortiz-Cardona and Bendo, 2007). These nerves primarily originate from the trigeminal and spinal nerves. The trigeminal nerve is the sensory nerve of the head and face. The supraorbital and supratrochlear nerves divide from the ophthalmic division (V1) of the trigeminal nerve and are the sensory nerves that innervate the forehead and anterior scalp, respectively.Infraorbital, zygomaticofacial, and zygomaticotemporal nerves are terminal branches of purely sensory maxillary division (V2) of the trigeminal nerve. They innervate the scalp and the face above the zygomatic cheek prominence.The mandibular division (V3) of the trigeminal nerve innervates the lower lip, mandible, buccal mucosa, and gives off the auriculotemporal cutaneous nerve to innervate the scalp in front of and above the auricle.
The posterior scalp includes the area behind the auricle and is innervated by the greater and the lesser occipital nerves, which emerge from the second and third cervical nerve roots (C2, C3). Therefore, the nerves to be blocked for anterior craniotomy procedures include supraorbital,supratrochlear, auriculotemporal, and zygomaticotemporal nerves. The nerves to be blocked for posterior craniotomy procedures are zygomaticotemporal, greater occipital, and lesser occipital nerves (Ortiz-Cardona and Bendo, 2007;Osborn and Sebeo, 2010).
The addition of epinephrine to local anesthetics has been reported to maximize the duration of blockade, reduce systemic absorption of local anesthetics, and subsequently decrease the incidence of systemic local anesthetic toxicity.It is recommended especially in regional blockade in the highly vascularized area such as the scalp.
Choice and dose of local anesthetics for scalp block have been widely studied. However, the results have not been conclusive. Among various long-acting local anesthetic agents (bupivacaine, mepivacaine, levobupivacaine, or ropivacaine), bupivacaine gains most popularity (Colley and Heavner, 1981; Archer et al., 1988; Costello and Cormack, 2004; Costello et al., 2004; Ortiz-Cardona and Bendo, 2007). The doses of bupivacaine used for scalp block range from 0.25% to 0.5% (Bloom field et al., 1998;Bala et al., 2006; Geze et al., 2009; Mohammadi et al.,2009). The appropriate dose that balances between sufficient perioperative craniotomy pain relief and possible complications is controversial. Although the scalp block technique is safe and can be performed even in children,potential complications have been reported. They include an inadvertent intravascular injection, which causes hypertension, especially in case the epinephrine is added into the local anesthetic. Local anesthetic toxicity has also been reported, albeit uncommon (Christensen et al.,1980; Scott et al., 1989).
There was a case report of subarachnoid space injection of anesthetic during occipital nerve block in a patient undergoing retromastoid craniotomy and leaving an occipital bone defect. Accordingly, patients who have the skull defect in the area of local anesthetic injection are relatively contraindicated to scalp block (Okuda et al., 2001). The anatomical proximity of facial nerve renders another concern of facial nerve paralysis, even though it has not been reported in the medical literature (Osborn and Sebeo, 2010). Infection should be prevented using strictly sterile technique during the blockade. A careful anatomical review prior to procedure and aspiration prior to local anesthetic agent can prevent the complications of facial nerve injury and intravascular injection.
Controversies regarding scalp block in neurosurgical patients are listed as follows:
· The classic scalp block technique has been reported to involve six terminal nerves including supraorbital, supratrochlear, auriculotemporal, zygomaticotemporal, greater occipital, and lesser occipital nerves in eligible patients even though undergoing anterior craniotomy procedure(Hartley et al., 1991; Bala et al., 2006; Mohammadi et al.,2009). There has been no evidence supporting whether blockage of only four nerves (supraorbital, supratrochlear, auriculotemporal, and zygomaticotemporal nerves)is suf ficient for anterior craniotomy procedure or not. If the results show that blockage of the nerves that mainly innervate anterior scalp is adequate, it will be an alternative for neuroanesthesiologists to reduce the dosage of local anesthetics and minimize the subsequent systemic adverse events.
· What is the appropriate concentration of the most commonly used local anesthetic agent, bupivacaine, in scalp block?
· A prospective, double-blinded, randomized, placebocontrolled trial showed that 0.25% bupivacaine scalp in filtration did not have any signi ficant effect on postcraniotomy pain and the postoperative analgesic requirement (Biswas and Bithal, 2003). However, comparisons between 0.25% and 0.5% bupivacaine, two common clinically used concentrations in a scalp block, have not been demonstrated.
· Scalp block has been reported to be a pre-emptive analgesic method and an adjunct technique to provide postoperative transitional analgesia from intraoperative use of remifentanil to other pain relief medications (Ayoub et al., 2006). It plays an important role in the reduction of postoperative analgesic requirement, particularly within the first 48 hours after craniotomy (Nguyen et al., 2001;Bala et al., 2006). However, the intraoperative opioid requirement after pre-incisional scalp block has not been reported.
The primary hypothesis is that there will be a greater number of adult patients scheduled for a craniotomy surgery and given 0.5% bupivacaine for anterior scalp block prior to a skull pin insertion who will experience mild pain than the number of patients in the placebo group. The severity of pain will be assessed objectively based on opioid administration guided by hemodynamic changes greater than 20% from preoperative baseline.
The secondary hypotheses are as follows:
· The patients given the anterior scalp block with 0.5%bupivacaine will have signi ficantly fewer hemodynamic changes after skull pin insertion during a craniotomy surgery than the patients in the placebo group.
· The time duration from the end of the surgery to successful extubation in patients given the anterior scalp block with 0.5% bupivacaine is shorter than that in patients in the placebo group.
METHODS/DESIGN
Study design
Prospective, randomized, double-blind, placebo-controlled trial.
Study setting
Department of Anesthesiology, Faculty of Medicine, Chiang Mai University, Thailand.
Study procedures
Patients with supratentorial tumor scheduled for craniotomy will be screened against our inclusion and exclusion criteria (see below). After providing informed consent and completing a baseline evaluation, 200 eligible patients will be randomly allocated to undergo either anterior scalp block with 0.5% bupivacaine (bupivacaine group, n = 100) or scalp block with normal saline (placebo group, n = 100).
Study participants
We will recruit adult neurosurgical patients (age ≥ 18 years ).
Inclusion criteria
· Patients with supratentorial brain tumor
· Patients scheduled to undergo an elective craniotomy to remove tumor in any surgical position
· Patients generally anesthetized with endotracheal intubation and controlled ventilation
· Provision of informed consent for participation in the trial and for use of their medical record in the research
Exclusion criteria
· Pregnant
· Have a history of local anesthetic allergy and/or anaphylaxis
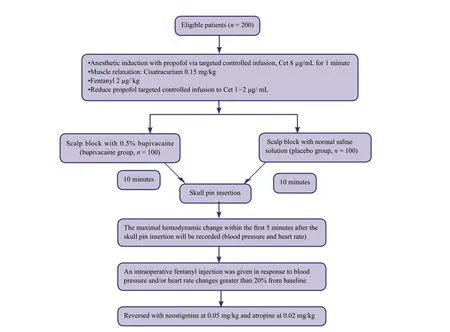
Figure 1: The schematic diagram of patient allocation.
· With persistent skull defects after previous craniotomy
Termination criteria
The study will be terminated if serious adverse events occur because of scalp block with 0.5% bupivacaine.
Randomization and interventions
Doctors, patients, and assessors will be blinded to patient group assignments to avoid measurement bias. Two hundred consecutive patients will be randomly assigned to bupivacaine or placebo groups (n = 100/group) using a random number table method. Patients in the bupivacaine group will undergo anterior scalp block with 20 mL of 0.5%bupivacaine and patients in the placebo group will receive anterior scalp block with 20 mL of normal saline solution.The procedure will be performed by the staff anesthesiologists by injecting the solution into the supratrochlear,supraorbital, preauricular, zygomaticofacial and zygomaticotemporal nerves. The injected solution will be provided by the nurse who is unrelated to the outcome assessment and analysis. In both groups, if the blood pressure and/or heart rate changes greater than 20% from preoperative measures will be controlled with fentanyl (2 μg/kg, intravenously). The schematic diagram of patient allocation is shown in Figure 1.
Sample size
We set 5% as the maximum chance of incorrectly rejecting the null hypothesis if it is really true. Therefore, the level of statistical signi ficance (type I error) or alpha (α) is 0.05.We set a power of 0.90. Thus, a type II error (beta, β) equals 0.10, which means that we are willing to accept a 10%chance of failing to reject the null hypothesis even though it is actually false. To the best of our knowledge, there is no report regarding the difference in the intraoperative opioid consumption between neurosurgical patients who receive a scalp block and those who did not receive. We have no information regarding an effect size to calculate the sample size. We decide to do a pilot study by recruiting approximately 20 subjects (10 subjects per group) to determine the effect size. However, the results from these 20 patients will not be added to the total sample size. The proposed sample size of 200 patients is an approximation.The accurate sample size will be changed according to the effect size obtained from the pilot study.
Recruitment
Patients with supratentorial tumor from the neuro-outpatient wards and clinics will be informed of this trial by their attending physicians. Patients who are interested in participation in the trial can contact the responsible researchers by telling the physicians.
Outcome measures
Primary outcome will be the difference of intraoperative fentanyl consumption between patients who receive anterior scalp block with 0.5% bupivacaine and those who receive normal saline solution administration. Secondary outcomes will be the hemodynamic changes from preoperative baseline at a skull pin insertion and the time from the end of surgery to successful extubation.
Adverse events
An inadvertent intravascular bupivacaine injection and consequent events will be recorded. The position of needle will be re-arranged immediately after detection of intravascular injection. Other complications of local anesthetic injection such as infection and in flammation over the injecting sites will be recorded. However, the procedure will be done under strictly aseptic conditions.
Data collection and management
Demographic data of each patient will be collected, which includes age, gender, body weight (kg), height (cm), and body mass index. The American Society of Anesthesiology(ASA) physical status, surgical position, duration of surgery(minutes), and type of anesthetic technique, i.e., volatile anesthetic maintenance or total intravenous anesthesia will be also recorded. The preoperative hemodynamic pro file(blood pressure, heart rate, and oxygen saturation) is collected from the vital sign record sheet. The intraoperative hemodynamic profile will be recorded throughout the operation. The intraoperative blood pressure will be averaged hourly by the sum of blood pressures measured at 5-minute intervals divided by the number of blood pressure measurements, which is constant at 12 times (60/5 = 12).The intraoperative opioid consumption will be recorded in μg unit. The time from the end of surgery to successful extubation will be recorded.
Statistical analysis
The demographic data of patients were compared between bupivacaine and placebo groups. The intraoperative fentanyl consumption was compared by independent t-test.Confounding factors will be adjusted by the multivariate analysis. The intraoperative hemodynamic changes from preoperative baseline will be analyzed using the repeated measures analysis of variance. The time from the end of the surgery to successful extubation will be compared by chi-square test. We will use the intention-to-treat method in all cases.
Trial quality assurance and control
We will be vigilant for any complications caused by scalp block itself or 0.5% bupivacaine solution.
Auditing
Trial progression will be reported to the ethics committee of Chiang Mai University, Chiang Mai, Thailand every 1 year, and the trial’s status will be updated in the registration database.
Confidentiality
Trial data include paper and electronic forms. Electronic data will be preserved in a dedicated password-protected computer and managed by a data management professional.Data reported on paper will be preserved in a secure, locked place for future viewing.
DISCUSSION
The main purpose of this study is to identify the bene fit of anterior scalp block with 0.5% bupivacaine in adult patients who are scheduled for supratentorial craniotomy.The bene fit is objectively measured from the requirement of intraoperative fentanyl, which is guided by blood pressure and heart rate changes greater than 20% of preoperative values. We propose that the reduction in fentanyl consumption will shorten the time from the end of surgery to successful extubation. Because of a very painful skull pin insertion, the ef ficacy of anterior scalp block can be measured by hemodynamic changes immediately after skull pin insertion.
Trial status
Recruiting is ongoing at the time of submission.
Conflicts of interest
None declared.
Author contributions
PP was the principal investigator, designed the trial protocol,collected the data and wrote the manuscript. YP participated in trial design and conduction. Both of these two authors approved the final version of this manuscript for publication.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Archer DP, McKenna JM, Morin L, Ravussin P (1998) Conscioussedation analgesia during craniotomy for intractable epilepsy: a review of 354 consecutive cases. Can J Anaesth 35:338-344.
Ayoub C, Girard F, Boudreault D, Chouinard P, Ruel M, Moumdjian R (2006) A comparison between scalp nerve block and morphine for transitional analgesia after remifentanil-based anesthesia in neurosurgery. Anesth Analg 103:1237-1240.
Bala I, Gupta B, Bhardwaj N, Ghai B, Khosla VK (2006) Effect of scalp block on postoperative pain relief in craniotomy patients.Anaesth Intensive Care 34:224-227.
Biswas BK, Bithal PK (2003) Preincision 0.25% bupivacaine scalp in filtration and postcraniotomy pain: a randomized double-blind,placebo-controlled study. J Neurosurg Anesthesiol 15:234-239.
Bloom field EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY (1998) The in fluence of scalp in filtration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg 87:579-582.
Bruder N, Ravussin P (2010) Supratentorial masses: Anesthetic Considerations. In: Cottrell and Young’s Neuroanesthesia (Cottrell JE, ed), pp184-202. Philadelphia: Mosby Elsevier.
Christensen KN, Jensen JK, Sogaard I (1980) Blood pressure response to administration of local anaesthetics with noradrenaline in craniotomies. Acta Neurochir (Wien) 51:157-160.
Colley PS, Heavner JE (1981) Blood levels of bupivacaine after injection into the scalp with and without epinephrine. Anesthesiology 54:81-84.
Costello TG, Cormack JR (2004) Anaesthesia for awake craniotomy: a modern approach. J Clin Neurosci 11:16-19.
Costello TG, Cormack JR, Hoy C, Wyss A, Braniff V, Martin K,Murphy M (2004) Plasma ropivacaine levels following scalp block for awake craniotomy. J Neurosurg Anesthesiol 16:147-150.
DeBenedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tibiero F,Villani RM (1996) Postoperative pain in neurosurgery: A pilot study in brain surgery clinical study. Neurosurgery 38:4666-4670.
Dunbar PJ, Visco E, Lam AM (1999) Craniotomy procedures are associated with less analgesic requirements than other surgical procedures. Anesth Analg 88:335-340.
Geze S, Yilmaz AA, Tuzuner F (2009) The effect of scalp block and local in filtration on the haemodynamic and stress response to skull-pin placement for craniotomy. Eur J Anaesthesiol 26:298-303.
Hartley EJ, Bissonnette B, St-Louis P, Rybczynski J, McLeod ME(1991) Scalp in filtration with bupivacaine in pediatric brain surgery. Anesth Analg 73:29-32.
Long DM (2001) Bonica’s Legacy. Bonica’s Management of Pain. J Pain Symptom Manage 21:527-528.
Mohammadi SS, Shahbazian E, Shoeibi G, Almassi F (2009) Effect of scalp in filtration with Bupivacaine on early hemodynamic responses during craniotomy under general anesthesia. Pak J Biol Sci 12:603-606.
Nguyen A, Girard F, Boudreault D, Fugère F, Ruel M, Moumdjian R, Bouthilier A, Caron JL, Bojanowski MW, Girard DC (2001)Scalp nerve blocks decrease the severity of pain after craniotomy.Anesth Analg 93:1272-1276.
Okuda Y, Matsumoto T, Shinohara M, Kitajima T, Kim P (2001)Sudden unconsciousness during a lesser occipital nerve block in a patient with the occipital bone defect. Eur J Anaesthesiol 18:829-832.
Ortiz-Cardona J, Bendo AA (2007) Perioperative pain management in the neurosurgical patient. Anesthesiol Clin 25:655-674.
Osborn I, Sebeo J (2010) "Scalp block" during craniotomy: a classic technique revisited. J Neurosurg Anesthesiol 22:187-194.
Pardey Bracho GF, Pereira de Souza Neto E, Grousson S, Mottolese C, Dailler F (2014) Opioid consumption after levobupivacaine scalp nerve block for craniosynostosis surgery. Acta Anaesthesiol Taiwan 52:64-69.
Quiney N, Cooper R, Stoneham M, Walters F (1996) Pain after craniotomy. A time for reappraisal? Br J Neurosurg 10:295-299.
Rahimi SY, Vender JR, Macomson SD, French A, Smith JR, Alleyne CH, Jr. (2006) Postoperative pain management after craniotomy:evaluation and cost analysis. Neurosurgery 59:852-857.
Scott DB, Lee A, Fagan D, Bowler GM, Bloom field P, Lundh R(1989) Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg 69:563-569.
Sokhal N, Rath GP, Chaturvedi A, Dash HH, Bithal PK, Chandra PS(2015) Anaesthesia for awake craniotomy: A retrospective study of 54 cases. Indian J Anaesth 59:300-305.
Stoneham MD, Walters FJ (1995) Post-operative analgesia for craniotomy patients: current attitudes among neuroanaesthetists. Eur J Anaesthesiol 12:571-575.
Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P (2002)Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol 14:96-101.
 Asia Pacific Journal of Clinical Trials:Nervous System Diseases2016年3期
Asia Pacific Journal of Clinical Trials:Nervous System Diseases2016年3期
- Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Evaluation of cardiac autonomic status using QTc interval in patients with leprosy
- Efficacy of spontaneous laughter in the post-operative treatment of pain and anxiety in children: study protocol for a randomized controlled trial
- Effects of lung protective ventilation on pulmonary function,inflammation, and oxidative stress in patients undergoing craniotomy: study protocol for a multi-center, randomized,parallel, controlled trial
- Effects of cognitive behavioral therapy on white matter fibers of patients with obsessive-compulsive disorder as assessed by diffusion tensor imaging: study protocol for a parallel group,controlled trial
- Migraine prevention by noninvasive electrical fastigial nucleus stimulation: a multi-center, randomized, double-blind,sham-controlled trial
- Scalp acupuncture twisting manipulation for treatment of hemiplegia after acute ischemic stroke in patients: study protocol for a randomized, parallel, controlled, single-blind trial
