Inhibition of Xanthine Oxidase Activity by Gnaphalium Affine Extract
Wei-qing Lin, Jian-xiang Xie, Xiao-mu Wu, Lin Yang, and Hai-dong Wang*
1Nanchang University School of Medicine Graduate School, Nanchang 330006, China
2Department of Integrated Traditional Chinese Medicine and Western Medicine, Jiangxi Provincial People’s Hospital, Nanchang 330006, China
3Department of Integrated Traditional Chinese Medicine and Western Medicine, Jiangxi Provincial Children’s Hospital, Nanchang 330006, China
GOUT is a growing health problem worldwide, especially in developed countries. Epidemiologic data indicate that the incidence of primary gout has increased by 2-fold over the past 20 years.1In recent years, xanthine oxidase (XO) has emerged as an important target not only for gout but also for cardiovascular and metabolic disorders involving hyperuricemia. XO is a key enzyme that catalyses the oxidation of xanthine and hypoxanthine into uric acid, and plays a vital role in the development of hyperuricemia and gout.2Allopurinol is a clinically used XO inhibitor in the treatment of gout, which blocks the terminal step in uric acid biosynthesis, therefore lowering the plasma uric acid concentration. However, due to its adverse effects, such as hepatitis, nephropathy, and allergic reactions, new alternatives with higher therapeutic potency and less adverse effects to replace alllopurinol are desired. Moreover, superoxide anion radicals generated by XO are involved in various pathological states such as hepatitis, inflammation, aging, carcinogenesis, and ischemia- reperfusion.2Thus, the search for novel XO inhibitors would be beneficial not only to the treatment of gout but also to the improvement of various other diseases.
In China, natural herbs have been used for a long time in the treatment of gout and hyperuricemia-related disorders. Gnaphalium affine is the main drug in the empirical prescription “Baiai Tongfengling”, which has proved therapeutic effect in clinical cases. Gnaphalium affine has the effects of drying dampness, resolving phlegm, and purging turbidity. The Gnaphalium affine, also named Cudweed or Qing Ming vegetable in China, is extensively harvested as a wild vegetable around the Qing Ming festival (in early April) and processed into drinks, canned products, and frozen vegetables, etc. Gnaphalium affine is believed to be of high nutritional value since it contains the 8 essential amino acids for human body in an appropriate proportion, a large content of minerals, trace elements, and vitamins. Besides the nutritional value, Gnaphalium affine was reported to exhibit many pharmacological activities.3
Based on a previous study of this research team, which demonstrated that water-boiled Gnaphalium affine extraction can lower blood uric acid in rats significantly,4we conducted this in vitro investigation to identify the active substances, thus clarify potential pharmaceutical basis for the traditional use of Gnaphalium affine in treating gout and other diseases involving hyperuricemia.
MATERIALS AND METHODS
Plant material
The Gnaphalium affine used in this study was collected in May 2009 from near Jing’an County in Jiangxi Province and identified by Professor Qian-feng Gong from School of Pharmaceutical sciences, Jiangxi University of Traditional Chinese Medicine. A voucher specimen was stored at 4°C in the Department of Traditional Chinese Pharmacy of Jiangxi Provincial Children’s Hospital.
Air-dried Gnaphalium affine (7.5 kg) were extracted with 60 L 95% ethanol5at room temperature (three times, once every week), and concentrated in vacuum to yield 910 g of crude extraction, prepared by drug manufacturing department of Jiangxi Provincial Children’s Hospital.
The extract was suspended in H2O and then partitioned with petroleum ether (PE) and ethyl acetate (EA) to produce a PE-soluble fraction and EA-soluble fraction, respectively. The EA fraction (179 g) was subjected to a silica gel column chromatography eluted with a PE/acetone gradient (60:1 to 1:1) to give seven subfractions (fr. EA 1-7). Fr. EA 3 was chromatographed over a silica gel column chromatography (PE/EA, 20:1 to 10:1) to yield compound 1 (200 mg). Fr. EA 4 was subjected to a silica gel column chromatography (PE/EA, 10:1 to 4:1) and Sephadex LH-20 to yield compounds 4 (20 mg) and 5 (32 mg). Fr. EA 6 was subjected to Sephadex LH-20 to isolate compounds 6 (8 mg), 7 (24 mg) and 8 (40 mg). Fr. EA 7 was chromatographed over a reverse phase-18 gel column chromatography (MeOH/H2O, 20:1 to 10:1) and Sephadex LH-20 to yield compounds 2 (22 mg) and 3 (10 mg). The isolated compounds were identified as β-sitosterol (1), rutin (2),6quercetin-3-O-β-D glucopyranoside (3),7eupatilin (4),85-hydroxy-6,7,3’,4’- tetramethoxyflavone (5),8quercetin (6),9luteolin (7)10and apigenin (8)10by comparison of their spectroscopic data with those reported in literature except for compound 1, which was identified by comparison of thin layer chromatography and melting point with those of β-sitosterol sample.
Drugs and chemicals
Allopurinol, xanthine and XO were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents used in this study were of analytical grades and purchased from Aladdin Biomart Company (Shanghai, China).
In vitro inhibition on XO activity
The activity of XO was measured spectrophotometrically under aerobic conditions using xanthine as the substrate by following the increase in absorbance at 292 nm using an absorption coefficient of 9 600 L/(mol·cm).11Allopurinol as well as Gnaphalium affine and its isolated compounds were pre-incubated with xanthine at 25°C for 15 minutes.2,12The reaction was started by adding XO. Kinetic measurements were performed in 4 different inhibitor concentrations and 5 different xanthine concentrations (60, 100, 200, 300, 400 μmol/L).
Gnaphalium affine were used at 5, 10, 20, 40 μg/ml, its isolated compounds at 0.25, 0.5, 1, 2 μg/ml, with allopurinol (0.25, 0.5, 1, 2 μg/ml) as the positive control. Different concentrations of Gnaphalium affine were dissolved in dimethyl sulfoxide (DMSO) to reach a final concentration of DMSO less than 1%. DMSO was used as the negative control.
The reaction mixture for the XO inhibition assay consisted of 300 μL of 200 mmol/L sodium pyrophosphate buffer (pH 7.5), 60-400 μl of 1 mmol/L xanthine, 100 μL of sample solution dissolved in distilled water or 1% DMSO, and 200 μl of XO (0.1 U). A solution in 1% DMSO was used for those samples insoluble in distilled water. The increased UV absorption at 292 nm indicated the formation of uric acid.13All measurements were performed in triplicate.
Statistical analysis
The data collected during these measurements were analysed using Dixon plots, drawn with Excel, to determine Ki values for the tested samples. The Ki values can be directly obtained from the intersection of the straight lines representing constant substrate concentrations. The initial enzymatic rates using different inhibitor concentrations and a fixed xanthine concentration always fell into straight lines. Lineweaver-Burk plots were drawn to reveal the inhibition mode.
RESULTS
The Gnaphalium affine extracts and its compounds had inhibitory effect on XO activity, among which 4 compounds exhibited little or weak inhibition in XO activity, therefore not included in the following analysis. The Ki values of Gnaphalium affine extracts, its compounds, and the standard drug allopurinol are displayed in Table 1.
Lineweaver-Burk plots of reactions in the presence and absence of Gnaphalium affine in a XO reaction mixture is shown in Figure 1. Allopurinol exhibited a competive inhibition. The inhibitory effect of 40 μg/ml Gnaphalium affine extract is stronger than that of 2 μg/ml allopurinol (Fig.1). The mode of XO inhibition by Gnaphalium affine and the 4 compounds are mixed inhibition (Fig. 2).
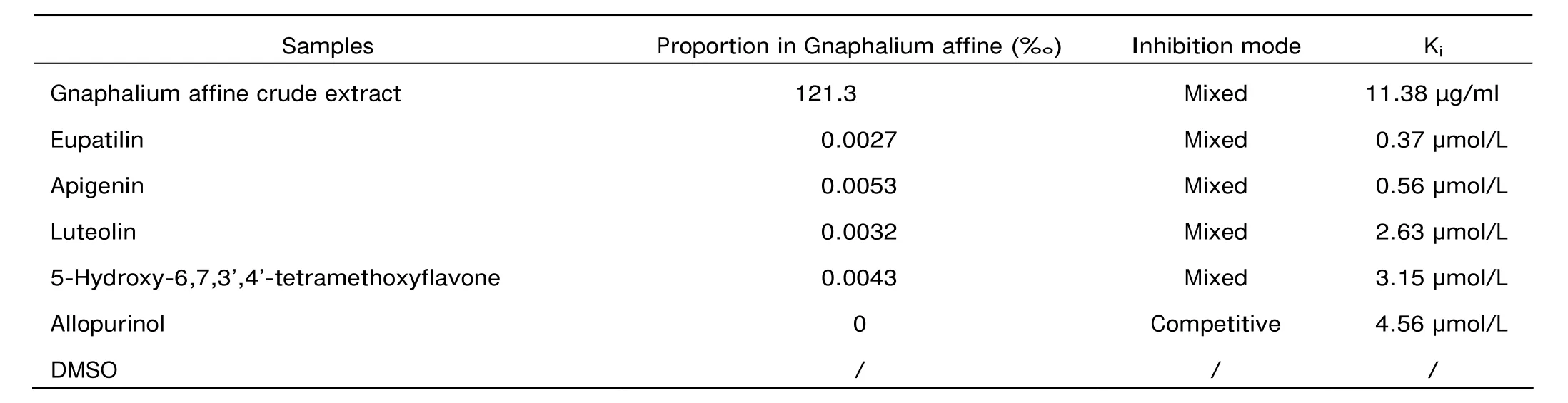
Table 1. Inhibitory effect of Gnaphalium affine extracts and allopurinol on xanthine oxidase activity in vitro
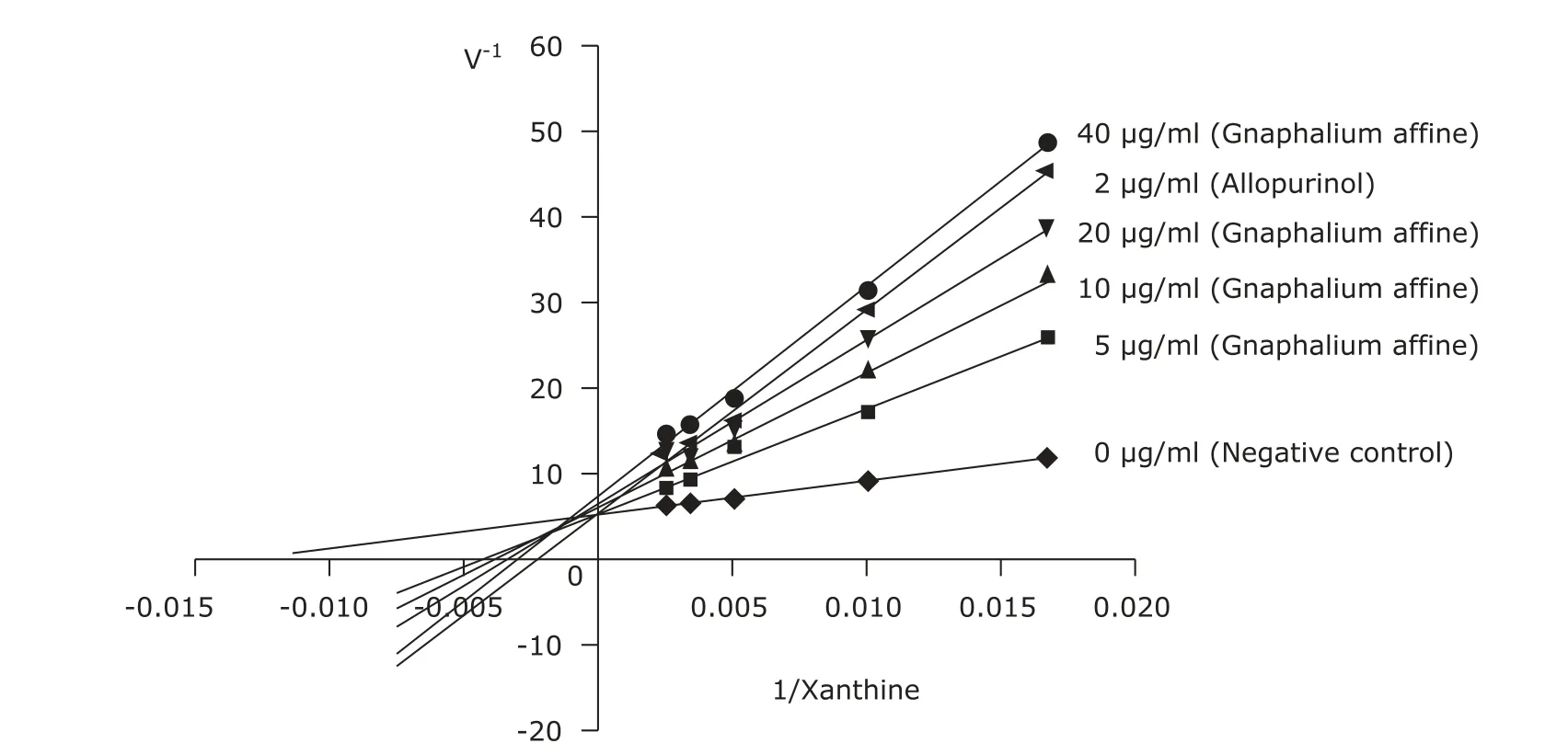
Figure 1. Lineweaver-Burk plot of the inhibition of xanthine oxidase by Gnaphalium affine, allopurinol, and negative control.
As shown in Figure 3A-F, the inhibition of the Gnaphalium affine extracts and its 4 compounds on XO activity was in a dose-dependent manner. From the intersection of these lines, the Ki values were deter- mined as: 11.38 μg/ml for Gnaphalium affine (Fig. 3A), 0.62 μg/ml (4.56 μmol/L) for allopurinol (Fig. 3B), 0.13 μg/ml (0.37 μmol/L) for eupatilin (Fig.3C), 0.15 μg/ml (0.56 μmol/L) for apigenin (Fig.3D), 0.75 μg/ml (2.63 μmol/L) for luteolin (Fig.3E), and 1.13 μg/ml (3.15 μmol/L) for 5-hydroxy-6,7,3’,4’-tetramethoxyflavone (Fig.3F).
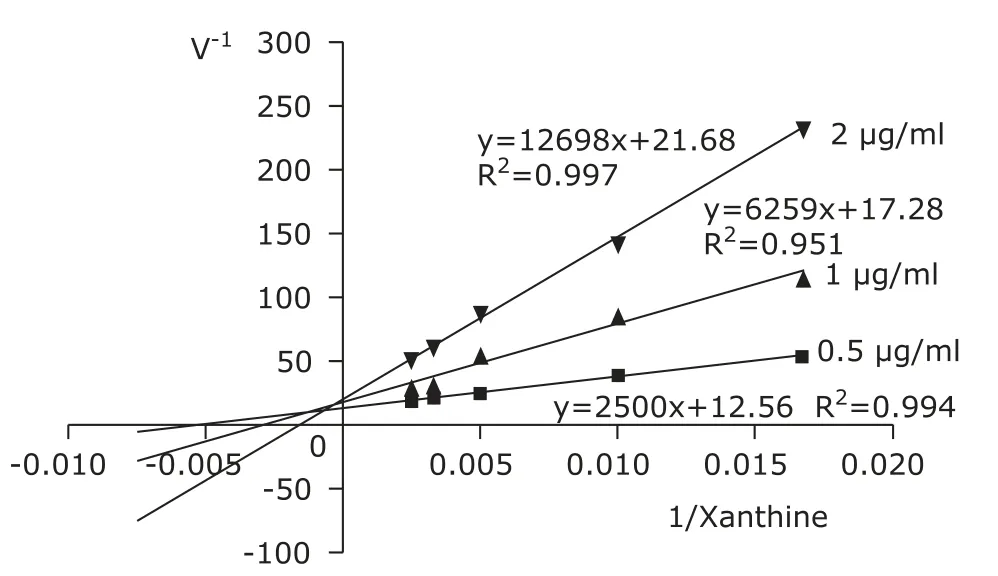
Figure 2. Lineweaver-Burk plot of the inhibition of xanthine oxidase by eupatilin.
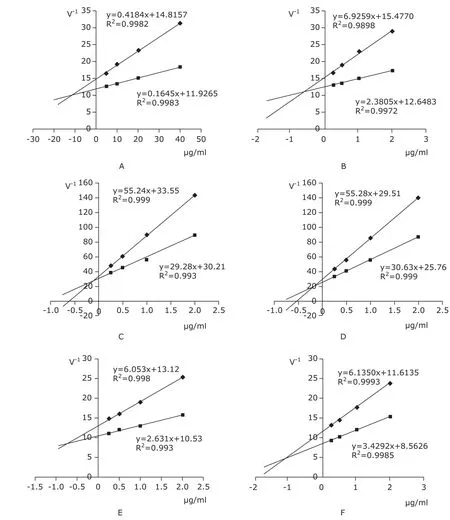
Figure 3. Dixon plot analysis of the inhibitory effect of Gnaphalium affine (A), allopurinol (B), eupatilin (C), apigenin (D), luteolin (E), and 5-hydroxy-6,7,3’,4’-tetramethoxyflavone (F) on xanthine oxidase, with the intersection point of the lines indicating the Ki value of each compound. ◆ xanthine 100 μmol/L ■ xanthine 200 μmol/L.
DISCUSSION
The increased risk of hyperuricemia has been linked with not only gout, but also the development of hypertension, hyperlipidaemia, cancer, diabetes, and obesity.14Recent investigations strongly indicate an important pathophysiological role of XO in various forms of post- ischemic injuries, inflammatory diseases, and chronic heart failure.15
In general, allopurinol is the most common clinical inhibitor of XO. However, about 5% of patients are unable to tolerate its adverse effects, which include gastrointestinal irritation, bone marrow suppression, and hypersensitivity syndromes ranging from simple skin rash to life-threatening conditions in which the patients develop toxic epidermal necrolysis, fever, hepatitis, eosinophilia, and worsening renal function.16-18Thus, new alternatives with higher therapeutic potency and less adverse effects are desired.
Gnaphalium affine is often used by Chinese people to cure musculoskeletal pains, carbuncle ulcers, etc. The in vitro inhibition of XO by Gnaphalium affine and its isolated compounds demonstrated one pharmaceutical effect of them. The ingredients of Gnaphalium affine are rather complicated, some of which exhibit strong inhibitory effect on the activity of XO. Aritomi and Kawasaki19derived a new chalcone and two flavonoids from the methanol extract of fresh Gnaphalium affine flower, and defined them as 2’,4,4’-trihydroxy-6’methoxychalcone, luteolin and luteolin- 4’-D-glucoside by infrared, ultraviolet, MRI, spectrum and chemical analysis. The antifeedant flavonoids, 5-hydroxy- 3,6,7,8,4’-pentamethoxyflavone, 5-hydroxy-3,6,7,8-tetrame- thoxyflavone, 5,6-dihydroxy-3, 7-dimethoxyflavone, and 4,4’,6’-trihydroxy-2’-methoxychalcone, have been isolated from cudweed Gnaphalium affine D. Don using high performance liquid chromatography.20With chromatographic fractionation and spectroscopic methods, Aquino et al21identified some cinnamic acid derivatives and 6 flavonoid constituents from an ethanol extract of the Gnaphalium uniflorum Lan, which showed a significant in vitro antioxidant effect and in vivo photoprotective activity. The flavonoids are rutin, quercetin-3-O-β-D-galactopyrano- side-4’-O-β-D-glucopyranoside, quercetin-3-O-β-D-glu-cop- yranoside, isorhamnetin-3-O-β-D-galactopyranoside, quercetin and kaempferol.
Flavonoids are a group of polyphenolic compounds reported to inhibit XO activity.22Flemmig et al11reported that in comparison to the known synthetic XO inhibitor allopurinol with a Ki of 7.3 μmol/L, the flavone aglycone apigenin exhibited a strong inhibitory effect on XO with a Ki of 0.52 μmol/L, luteolin23had a Ki of 2.9 μmol/L and luteolin-7-O-β-D-glucoside had a Ki value of 15.0 μmol/L. Eupatilin has been reported to act as a novel antioxidant and may play an important role in the formulated ethanol extract DA-9601, which is now commercially available as a hepatoprotective, antioxidative, anti-inflammatory and antibacterial agent24in South Korea, and will be on sale in other Asian countries in the near future.25
In comparable experiments published, XO inhibitors were pre-incubated with the enzyme before addition of xanthine.26In contrast, we applied a more physiological approach, i.e. pre-incubation of the inhibitor with the substrate. Therefore, the presented kinetic data cannot be directly compared to the results obtained under different experimental conditions or using a non-kinetic approach.2In the experimental conditions of this study, the 4 phenolics even demonstrated inhibitory effects exceeding that of allopurinol (Table 1).
In this in vitro investigation, we identified 4 potent XO inhibitors. Among them, the flavone eupatilin exhibited the most potent inhibitory effect on XO with a Ki value of 0.37μmol/L. In comparison, the known synthetic XO inhibitor, allopurinol, showed a Ki of 4.56 μmol/L. Apigenin (Ki of 0.56 μmol/L), luteolin (Ki of 2.63 μmol/L) and 5-hydroxy-6,7,3’,4’-tetramethoxyflavone (Ki of 3.15 μmol/L) also contributed considerably to the inhibitory effect of Gnaphalium affine on XO.
Eupatilin and 5-hydroxy-6,7,3’,4’-tetramethoxyflavone have not been isolated from this plant before. Apigenin and luteolin were found in the Gnaphalium hypoleucum.27We also found 2 componds, rutin and quercetin, which have been reported as weak XO inhibitors.22They might also contribute to the inhibitory effect of Gnaphalium affine on XO.
In conclusion, this study suggests that Gnaphalium affine and its components have potent inhibitory effect on XO, which might be helpful in preventing or slowing the progress of gout. Further in vivo research is being carried out to identify a potential chemical entity for clinical use in the prevention and treatment of gout and related inflammatory disorders.
The authors are thankful to the research council of Nanchang University, Jiangxi Provincial Children’s Hospital and Jiangxi Provincial People’s Hospital for granting permission to carry out this study and publish the data. We also thank Professor Qian-feng Gong from School of Pharmaceutical Sciences, Jiangxi University of Traditional Chinese Medicine for the assistance in this study.
1. Arromdee E, Michet CJ, Crowson CS, et al. Epidemio- logy of gout: is the incidence rising? J Rheumatol 2002; 29: 2403-6.
2. Cos P, Ying L, Calomme M, et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 1998; 61: 71-76.
3. Zeng WC, Zhu RX, Jia LR, et al. Chemical composition, antimicrobial and antioxidant activities of essential oil from Gnaphlium affine. Food Chem Toxicol 2011; 49: 1322-8.
4. Lin WQ, Xie JX, Wang HD. Experimental study of the Gnaphalium affine extraction treat hyperuricemia in rats. Chin J Rheumatol 2005; 9: 509-10.
5. Graziose R, Rojas-Silva P, Rathinasabapathy T, et al. Antiparasitic compounds from Cornus florida L. with activities against Plasmodium falciparum and Leishmania tarentolae. J Ethnopharmacol 2012; 142: 456-61.
6. Zhang J, Li X, Ren L, et al. Chemical constituents from Exochorda racemosa. Zhongguo Zhong Yao Za Zhi 2011; 36: 1198-201.
7. Shao S, Yang MM, Bi SN, et al. Flavonoids of Erigeron canadensis. Zhongguo Zhong Yao Za Zhi 2012; 37: 2902-5.
8. Nakasugi T, Nakashima M, Komai K. Antimutagens in gaiyou (Artemisia argyi levl. et vant.). J Agric Food Chem 2000; 48: 3256-66.
9. Xu X, Ye H, Wang W, et al. Determination of flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by capillary electrophoresis with electrochemical detection. Talanta 2006; 68: 759-64.
10. Lv XL, Mai X, Guo H, et al. Chemical constituents of the roots of Vaccinium bracteatum. Zhong Yao Cai 2012; 35: 917-9.
11. Flemmig J, Kuchta K, Arnhold J, et al. Olea europaea leaf (Ph.Eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine 2011; 18: 561-6.
12. Umamaheswari M, Asokkumar K, Sivashanmugam AT, et al. In vitro xanthine oxidase inhibitory activity of the fractions of Erythrina stricta Roxb. J Ethnopharmacol 2009; 124: 646-8.
13. Kong LD, Cai Y, Huang WW, et al. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol 2000; 73: 199-207.
14. Lin KC, Lin HY, Chou P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J Rheumatol 2000; 27: 1501-5.
15. Eger BT, Okamoto K, Enroth C, et al. Purification, crystallization and preliminary X-ray diffraction studies of xanthine dehydrogenase and xanthine oxidase isolated from bovine milk. Acta Crystallogr D Biol Crystallogr 2000; 56: 1656-8.
16. Yale SH, Yale ES, Mann DS. Fever, rash, and angioedema after a course of allopurinol. Hosp Pract (1995) 1996; 31: 92-4.
17. Umpiérrez A, Cuesta-Herranz J, De Las Heras M, et al. Successful desensitization of a fixed drug eruption caused by allopurinol. J Allergy Clin Immunol 1998; 101: 286-7.
18. Bomalaski JS, Clark MA. Serum uric acid-lowering therapies: where are we heading in management of hyperuricemia and the potential role of uricase. Curr Rheumatol Rep 2004; 6: 240-7.
19. Aritomi M, Kawasaki T. Dehydro-para-asebotin, a new chalcone glucoside in the flowers of Gnaphalium affine D. Don. Chem Pharm Bull (Tokyo) 1974; 22: 1800-5.
20. Morimoto M, Kumeda S, Komai K. Insect antifeedant flavonoids from Gnaphalium affine D. Don. J Agric Food Chem 2000; 48: 1888-91.
21. Aquino R, Morelli S, Tomaino A, et al. Antioxidant and photoprotective activity of a crude extract of Culcitium reflexum H.B.K. leaves and their major flavonoids. J Ethnopharmacol 2002; 79: 183-91.
22. Costantino L, Albasini A, Rastelli G, et al. Activity of polyphenolic crude extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase. Planta Med 1992; 58: 342-4.
23. Pauff JM, Hille R. Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J Nat Prod 2009; 72: 725-31.
24. Min SW, Kim NJ, Baek NI, et al. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol 2009; 125: 497-500.
25. Choi EJ, Oh HM, Na BR, et al. Eupatilin protects gastric epithelial cells from oxidative damage and down- regulates genes responsible for the cellular oxidative stress. Pharm Res 2008; 25: 1355-64.
26. Lespade L, Bercion S. Theoretical study of the mechanism of inhibition of xanthine oxidase by flavonoids and gallic acid derivatives. J Phys Chem B 2010; 114: 921-8.
27. Sun Q, Lu Y, Wu SQ, et al. Study on the chemical constituents from Gnaphalium hypoleucum. Zhong Yao Cai 2012; 35: 566-8.
 Chinese Medical Sciences Journal2014年4期
Chinese Medical Sciences Journal2014年4期
- Chinese Medical Sciences Journal的其它文章
- Evaluation of Risk Factors for Arytenoid Dislocation after Endotracheal Intubation: a Retrospective Case-control Study
- Non-enhanced Low-tube-voltage High-pitch Dual-source Computed Tomography with Sinogram Affirmed Iterative Reconstruction Algorithm of the Abdomen and Pelvis
- Primary Combined Intra-articular and Extra-articular Synovial Osteochondromatosis of Shoulder: a Case Report
- Squamous Cell Carcinoma of Small Intestine: a Case Report△
- BRAF V600E Mutation as a Predictive Factor of Anti-EGFR Monoclonal Antibodies Therapeutic Effects in Metastatic Colorectal Cancer: a Meta-analysis
- Multiple Myeloma Mimicking Spondyloarthritis: a Case Report
