Influence of Deleted in Colorectal Carcinoma Gene on Proliferation of Ovarian Cancer Cell Line SKOV-3 In Vivo and In Vitro
Yan Cai ,Chun-jie Hu ,Jia Wang *,and Ze-hua Wang
1Department of Obstetrics and Gynecology,Union Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
2Department of Obstetrics and Gynecology,the Fourth Affiliated Hospital,Harbin Medical University,Harbin 150001,China
3Heilongjiang Province Traditional Chinese Medicine Institute,Harbin 150001,China
OVARIAN cancer is one of the most common gynecological malignancies affecting women.The five-year survival rate was only 20%-30%.Because of poor early diagnosis and ineffective treatment,ovarian cancer is the fourth most common cancer among women,and is still the leading cause of death in women with malignancy in the world.The traditional treatments for ovarian cancer include surgery,chemotherapy and radiotherapy.Despite advances in surgery,radiotherapy,and chemotherapy,the survival of patients with ovarian cancer has not changed significantly.Therefore,an innovative approach to treating ovarian cancer is urgently needed.Gene mutation and aberrant expression play a pivotal role in the pathogenesis and aggressiveness of ovarian cancer.1Gene therapy as part of a combined treatment approach to ovarian cancer has been employed.The deleted in colorectal carcinoma (DCC) gene is a candidate tumor suppressor gene.The altered expression of DCC is closely linked with ovarian cancer differentiation and progression.2In this study,we constructed an exogenous recombinant eukaryotic expression vector pcDNA 3.1(+)-DCC,containing human DCC cDNA coding sequences,and transfected the vector into SKOV-3 cells to investigate its effects on proliferation of ovarian cancer cell line SKOV-3.
MATERIALS AND METHODS
Cell line and cultures
SKOV-3 cell line is the ovarian serous nipple cystadenoma carcinoma cell,which was kindly provided by professor Li-hua Sui in the Department of Gynecology of the 3rd Affiliated Hospital of Harbin Medical University,Harbin,China.SKOV-3 cell line was cultured in RPML1640 medium (Hyclone UK Ltd.,Cramlington,UK) supplemented with 15%fetal calf serum,1000 μg/mL penicillin and 100 μg/mL stre-ptomycinin in a 5% CO2humidified atmosphere at 37°C.
Plasmid transfection
The exogenous recombinant eukaryotic expression vector pcDNA3.1(+)-DCC contained human DCC full length cDNA coding sequences and neomycin resistance gene.The construction process was similar to what has been previously described.3Vector pcDNA3.1(+)-DCC and pcDNA3.1(+) were constructed by Sangon Biotech Co.,Ltd.(Shanghai,China).The constructed recombinant expression vector was identified by real-time reverse transcription-PCR.Twenty μL of each vector were transfected into logarithmically growing SKOV-3 cells by LipofectamineTM2000(Invitrogen,Carlsbad,CA) according to manufacturer's instructions.After 48 hours of transfection,selection was started with 600 μg/mL G418 (Invitrogen).Single G418-resistant cell clones were trypsinized and maintained in 300 μg/mL G418.The pcDNA3.1(+)-DCC transfected SKOV-3 cells (SKOV-3/DCC),pcDNA3.1 (+) transfected cells (SKOV-3/Neo),and SKOV-3 cells were used in the following study.
RT-PCR
The total RNA was extracted using TRIzol solution (Gibco BRL,Paisley,UK).First strand cDNA was transcribed from 2 mg total RNA using oligo-dT and MuLV transcriptase (RNA PCR Core kit;Perkin-Elmer,Foster City,CA,USA).cDNA was amplified (35 cycles) in an Eppendorf thermocycler using DCC-specific primers in the RNA PCR Core kit.The primers’ sequences were as follows:sense 5’-cgacaagcttccgccatggtttttaaatcatc-3’,antisense 5’-ctctgagctctggagcttgggattgcaggc-3’4and synthesized by DNA Technology A/S,Aarhus,Denmark.A 380-bp glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was amplified (35 cycles)from each developmental stage as an internal standard.GAPDH primers were as follows:sense 5’-tggtgatggaggaggtttagtaagt-3’,antisense 5’-aaccaataaaacctactcctcccttaa-3’.The reactions for DCC were carried out in a volume of 20 μL consisting of 600 nmol/L of each primer,0.75 U platinum Taq polymerase (Invitrogen),200 μmol/L of each dNTP,200 nmol/L ROX Dye reference (Invitrogen),10×Taq Master Mix (Mercury,CLP,San Diego),16.6 mmol/L(NH4)2SO4,67 mmol/L Trizma (Sigma),2.5 mmol/L MgCl2,10 mmol/L mercaptoethanol,and 0.1% DMSO.The fragment length of PCR product for DCC was 291 bp.The PCR products were subjected to electrophoresis on a 1.5%agarose gel and a SYBR?Gold nucleic acid gel staining(Molecular Probes,Eugene,OR,USA) was used to visualize cDNAs.
Immunocytochemical analysis
Endogenous peroxidase activity was inhibited by immersing the slides in 3% H2O2for 10 minutes.The cells were incubated with rabbit anti-human DCC monoclonal antibody (Ab-1) (Oncogene Science,NY,USA) at 1 mg/mL for 45 minutes,then incubated with a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase(Gibco BRL) at a 1:10 000 dilution for 30 minutes.Signals were detected with DAB.Positive staining was defined as brown-yellow particles in cells.Interpretation of DCC immunostaining was done by a pathologist without knowledge of the genetic findings.
MTT
SKOV-3,SKOV-3/Neo and SKOV-3/DCC cells were seeded in 96-well plates (1×104cells/well) and cultured at satured humidified atmosphere containing 5% CO2at 37°C for 7 days.The cells proliferation was assayed by MTT for 7 consecutive days.A total of 50 μL 3-(4,4-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (5 g/L;Gibco BRL)in PBS was added to each well and incubated for 4 hours at 37°C.Then 50 μL dimethyl sulfoxide was applied to dissolve the MTT formazan crystals.The absorbance was recorded at 492 nm on a microplate reader (Gibco BRL).Cell proliferation was expressed as the half maximal inhibitory concentration (IC50),which represents the dose at which 50% of the cells in culture died.
Cell cycle analysis
When cells were grown to 80% confluence in medium,they were trypsinized,washed,and resuspended in 0.5 mL PBS at a concentration of 1×106cells/mL.After ethanol fixation,propidium iodide DNA labeling was done for analysis of cell cycle distribution.Briefly,the cells were incubated with 1.5 mL cold 100% ethanol for 10 minutes.After a brief centrifugation,the supernatants were removed,and 0.5 mL propidium iodide-RNase solution (Gibco BRL) was added.The cells were incubated for 40 minutes in the dark and analyzed by flow cytometry (FACSCalibur,BD Biosciences,Gibco BRL).
Colony formation assay
Soft agar consists of 6 g/L bottom agar layer and 4 g/L top agar layer.The number of cells plated on soft agar was 2 000 per clone.Plates were incubated with 5% CO2at 37°C for 2 weeks and the formation of cellular colonies were counted and photographed under a phase contrast microscope (Nikon,Tokyo,Japan).
Transmission electron microscopy
Cells were gently washed with serum free medium and fixed with 25 g/L glutaraldehyde in 0.1 mol/L sodium cacodylate buffer.The cultured cells were harvested and centrifuged for 5 minutes at 10 000g.The cells were fixed with 10 g/L osmium tetroxide.The specimens were stained,dehydrated with a graded series of ethanol,infiltrated with propylene oxide,embedded with EMBED overnight,and cured in a 60°C oven for 48 hours.Silver sections were cut using an Ultracut E microtome (Gibco BRL),placed into a formvar and carbon coated grid,stained with uranyl acetate and Reynold’s lead citrate,and viewed under a JEOL 100 CX electron microscope (Gibco BRL).
Evaluation of tumorigenecity in vivo
A total of 5×106cells were subcutaneously xenografted into 4-6-week-old female BALB/c mice (weighing,20 g).Mice were monitored regularly for tumor growth every week for 12 weeks.The tumor volumes were calculated using the ellipsoid formula.5
Statistical analysis
Statistical analysis was done using the Statistical Package for the Social Sciences software version 10.0.Statistical analysis of cell cycle distributions was carried out by theχ2test or Fisher’s exact test.P<0.05 was considered statistically significant.
RESULTS
DCC expression in SKOV-3 cells
The abundant expression of DCC mRNA and protein in SKOV-3/DCC cells was demonstrated by RT-PCR and immunocytochemical analysis,however,DCC mRNA and protein expression could not be observed in both SKOV-3 and SKOV-3/Neo cells (Figs.1,2),showing that exogenous DCC had been successfully transfected into SKOV-3 cell and obtained stable expression.
Effects of DCC on proliferation of SKOV-3 cells
As shown in Table 1,IC50 of SKOV-3/DCC cells was significantly lower than that of SKOV-3 or SKOV-3/Neo cells(allP<0.05).
Flow cytometry assay revealed that SKOV-3/DCC cells had a significant increase in the G1phase and decrease in the G2+S phase compared with SKOV-3 or SKOV-3/Neo cells (allP<0.05,Table 2).
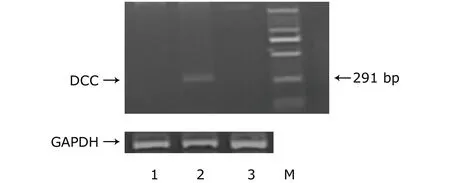
Figure 1.RT-PCR results of the expression levels of deleted in colorectal carcinoma (DCC) mRNA in three kinds of SKOV-3 cells.
A significant decrease in the number of colonies that formed by SKOV-3/DCC cells (104.3±8.9) was observed compared with that formed by SKOV-3/Neo (154.3±10.6,P<0.05) or SKOV-3 cells (145.3±12.6,P<0.05) (Fig.3).
Ultrastructural changes of DCC transfected SKOV-3 cells
Electron microscopic analysis showed SKOV-3/DCC cells displayed typical morphological changes of apoptosis and DNA strand breaks were observed in the cells (Fig.4).
Effect of DCC on tumorigenecity of ovarian cancer
Of the 4 mice xenografted with SKOV-3/DCC cells,2 showed no tumor formation,and the tumors of the other 2 mice took place more slowly (1-2 weeks) than the mice xenografted with SKOV-3/Neo or SKOV-3 cells.The tumor volume of BALB/c mice bearing SKOV-3/DCC cells(3.403 mm3) was smaller than that of SKOV-3 cells(9.206 mm3).
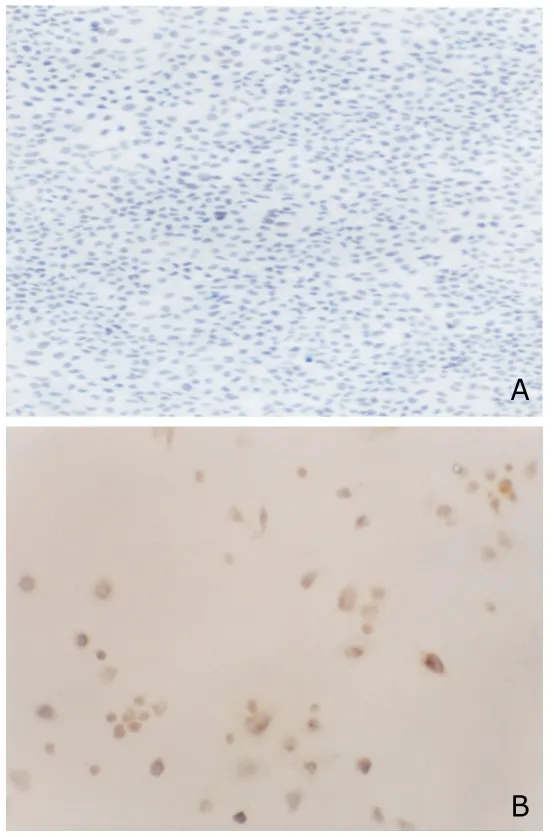
Figure 2.Immunocytochemical results of DCC expression in SKOV-3/DCC cells (A) and SKOV-3/Neo cells (B).SP ×400 The brown-yellow particles could not be seen in SKOV-3/Neo cell,while be seen in SKOV-3/DCC cells.
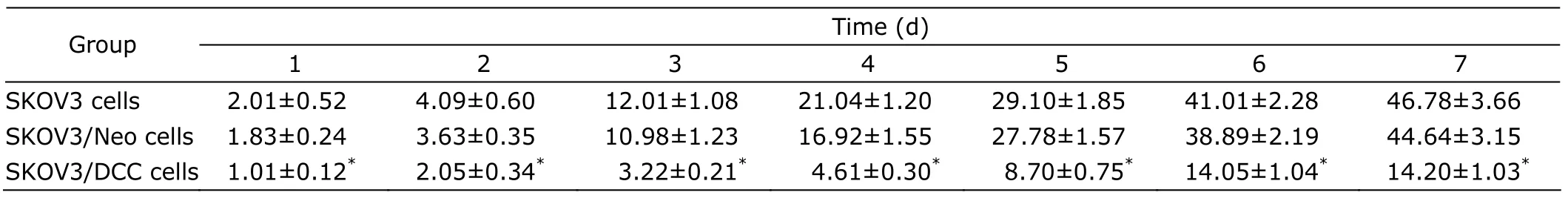
Table 1.Half maximal inhibitory concentration (IC50) of three kinds of SKOV3 cells detected for 7 consecutive days§ (×104/mL)
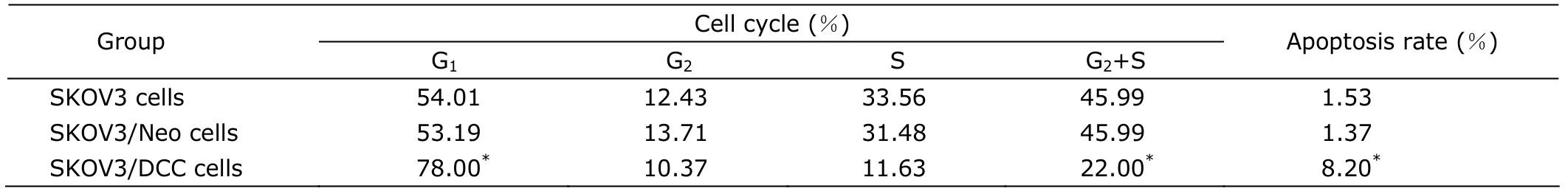
Table 2.Cell cycle distribution of three kinds of SKOV3 cells detected by flow cytometry
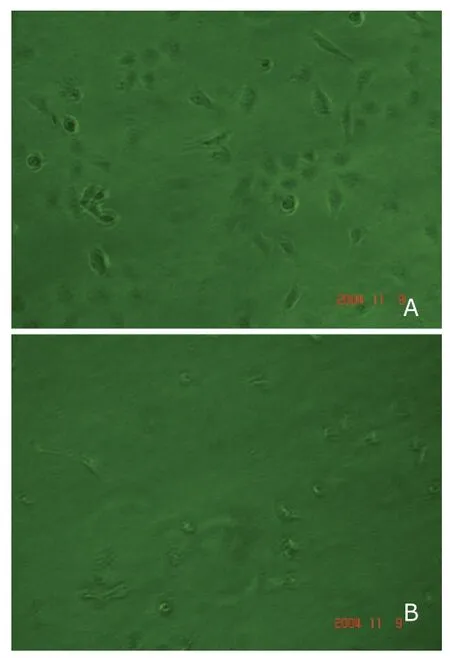
Figure 3.Anchorage-independent growth of SKOV-3/Neo (A)and SKOV-3/DCC cells (B) in soft agar.×40

Figure 4.Morphology of SKOV-3/DCC cells under electron microscopy. ×10 000
DISCUSSION
Clinically,the ovarian epithelial carcinoma is one of the most important tumors of the female reproductive system.Since approximately 75% of patients are diagnosed at an advanced stage,ovarian cancer is a common cause of cancer death.Despite the advances in the clinical management of this disease,prognosis remains dismal.Furthermore,the molecular changes associated with acquisition of tumorigenesis in ovarian cancer are poorly understood.More important is the observation that loss of 18q12.3-q23 is the most frequent event in human ovary cancer.6,7Similarly,significant incidence of loss of heterozygosity on chromosome 18q and decreased or absent expression of the DCC gene (located at chromosome 18q21)comparing with the normal counterparts are revealed in a variety of other human malignancies of epithelial origin as well as gastric,pancreatic,breast and prostate cancers.8-14DCC encodes for a membrane-bound protein with immunoglobulin like and fibronectin type III domains and a unique cytoplasmic domain,15which is a candidate tumor suppressor gene.Recently,DCC came back to the spotlight as a better understanding of its function and relationship with its ligand (netrin-1) had shown that DCC may act as a conditional tumor suppressor gene,3,17,18which was also lowly expressed or lost in esophageal cancer,endometrial cancer,ovary cancer and neuroblastoma as further studies showed.16,17In addition,colonic cancer patients with liver metastasis expressed significantly lower levels of DCC than those without,suggesting the prognostic value of DCC expression.18
According to our previous observation,significantly decreased levels of overall DCC values in carcinomas compared with benign and low malignant potential lesions were revealed by RT-PCR assay.Similar findings were also noted when subdivision was into serous and mucinous categories.In carcinomas,reduction or loss of DCC expression was significantly related to the serous phenotype,a high histological grade and a more advanced stage.4DCC gene expression is lost in 20% benign ovarian tumor,in 56% malignant tumor,and the difference has the statistical significance.As expected,DCC protein was scarcely detectable in human cancer cell lines,except for some haematopoietic lines.18The parental SKOV3 cell line did not have the gene expression,showing that DCC presence is a negative constraint for tumor development.So the recombinant eukaryotic expression vector was transfected into the cell line in order to study the gene’s function further.In addition,we previously demonstrated that normal human ovary tissue expressed high levels of DCC protein,and the majority of the poorly differentiated ovary tissue specimens showed significantly decreased expression of DCC compared with well-differentiated,moderately differentiated,or benign ovary tissue,suggesting that DCC expression levels are downregulated in poorly differentiated ovary cancer.Taken together,DCC had an inverse correlation tendency with the malignant degree of ovary cancer.These results are very similar to Saegusaet al’s observasion.1In their large series of ovarian carcinomas,the reduction or loss of the DCC immunoreactivity was closely related to a higher histological grade and a more advanced clinical stage of carcinomas,indicative of a linkage between DCC and malignant potential.Enomotoet al19also described similar findings using 22 ovarian carcinomas.
SKOV-3 cells were established from ascitic fluid collected from patient with ovary cancer and could form malignant tumors in nude mice.20In DCC overexpressed cells(SKOV-3/DCC),the cell proliferation and the soft agar colony formation ability were decreased compared with SKOV-3/Neo or parental SKOV-3 cells.A balance between proliferation and apoptosis of tumor cells is important for tumor growth.The introduction of DCCcDNA into colon cancer cells was sufficient to decrease cell proliferation or suppress tumorigenesis.21,22However,Fabreet al23observed no effects on cell proliferation or differentiation phenotype after introducing full-length DCC cDNA into undifferentiated HT-29 cells.In addition,we found that DCC inhibited ovary cancer cells proliferation by altering cell cycle progression and promoting apoptosis.DCC exerts its cytotoxic action by the induction of apoptosis as evidenced by electron microscopy.This finding is in accordance with that of Katoet al,20who reported that DCC could induce DCC–deficient endometrial carcinoma cells to apoptosis.DCC induces apoptosis in the absence of netrin-1 ligand,but blocks apoptosis when engaged by netrin-1.24The data suggest that DCC functions as a tumor suppressor protein by inducing apoptosis in settings in which ligand is unavailable.
A high level of DCC protein expression results in the inhibition of transformed cell propertiesin vitroin ovary carcinoma cells,whereas no inhibitory effect was shown in parental or control SKOV-3 cells lacking expression of the endogenous DCC protein.Similar results were observed in endometrial carcinoma cells.20
This study showed the percentage of phase G1cells increased and that of G2and M cells decreased after transfection of the DCC gene,while the parental SKOV-3 and SKOV-3/Neo cell line did not show the above change.G1arrest can inhibit the growth of cell,indicating malignant character might be inhibited by the exogenous DCC gene.25
The exogenous gene DCC was transfected into the cell line and expressed successfully.The growing speed of the DCC gene repairing cell line was much slower than that of the control cell.The decreased number of clones formed by the SKOV-3/DCC cells manifested that the SKOV-3 cells have been repaired by the DCC gene.Therefore,the apoptosis phenomenon of the DCC gene repairing cell line influenced the proliferation and carcinogenic character.
According to the recent study,the mechanism of DCC gene inducing apoptosis is different from the classical way,and which values the further study.26Klingelhutzet al25also demonstrated a direct role of DCC in tumour suppression of nitrosomethylurea-transformed tumorigenic human papillomavirus (HPV)-immortalized human epithelial cells (1811-NMU-T1 cells).This observation supports the function of DCC as a tumor suppressor.Another study also showed that DCC overexpression in immortalized human kerationcytes caused poor growthin vitroand reduced tumor growthin vivo.However,DCC-deficient transgenic mice showed no increased incidence of colorectal cancers.27The role of DCC gene in tumorigenesis is yet controversial.3Therefore,the role of DCC in suppression of the malignant phenotype still remains to be elucidated.
ACKNOWLEDGEMENTS
We thank Wei Liu for her technical assistance.
1.Saegusa M,Machida D,Okayasu I,et al.Loss of DCC gene expression during ovarian tumorigenesis:relation to tumor differentiation and progression.Br J Cancer 2000;82:571-8.
2.Yao HP.Diagnosis and treatment of recurrent ovarian cancer.Zhonghua Fu Chan Ke Za Zhi 2003;38:659-60.
3.Carvalho AL,Chuang A,Jiang WW,et al.Deleted in colorectal cancer is a putative conditional tumor-suppressor gene inactivated by promoter hypermethylation in head and neck squamous cell carcinoma.Cancer Res 2006;66:9401-7.
4.Li PL,Liu MM,Ni J.Study on the expression of the gene deleted in colorectal carcinoma in ovarian carcinoma.Zhonghua Fu Chan Ke Za Zhi 2003;38:207-2.
5.Yao D,He X,Yang RL,et al.Sonographic measurement of thyroid volumes in healthy chinese infants aged 0 to 12 months.J Ultrasound Med 2011;30:895-8.
6.Sato T,Satio H,Morita R,et al.Allelotype of human ovarian cancer.Cancer Res 1991;51:5118-22.
7.Cliby W,Ritland S,Hartmann L,et al.Human epithelial ovarian cancer allelotype.Cancer Res 1993;53:2393-8.
8.Brewster SF,Gingell JC,Browne S,et al.Loss of heterozygosity on chromosome 18q is associated with muscle-invasive transitional cell carcinoma of the bladder.Br J Cancer 1994;70:697-700.
9.Devilee P,van Vlietl M,Kuipers-Dijkshoorn N,et al.Somatic genetic changes on chromosome 18 in breast carcinomas:is the DCC gene involved? Oncogene 1991;6:311-5.
10.Gao X,Honn KV,Grignon D,et al.Frequent loss of expression and loss of heterozygosity of the putative tumor suppressor gene DCC in prostatic carcinoma.Cancer Res 1993;55:2723-7.
11.Hoene MW,Halatsch M-E,Kabl GF,et al.Frequent loss of expression of the potential tumor suppressor gene DCC in ductal pancreatic adenocarcinoma.Cancer Res 1992;52:2616-9.
12.Miyake K,Inokuchi K,Dan K,et al.Alterations in the deleted in colorectal carcinoma gene in human primary leukemia.Blood 1993,82:3927-30.
13.Neuman WL,Wasylyshyn ML,Jacoby R,et al.Evidence for a common molecular pathogenesis in colorectal,gastric,and pancreatic cancer.Genes Chromosomes Cancer 1991;3:468-73.
14.Scheck AC,Coons SW.Expression of the tumor suppressor gene DCC in human gliomas.Cancer Res 1993;53:5605-9.
15.Fearon ER,Cho KR,Nigro JM,et al.Identification of a chromosome 18q gene that is altered in colorectal cancers.Science 1990;247:49-56.
16.Mazelin L,Bernet A,Bonod-Bidaud C,et al.Netrin-1 controls colorectal tumorigenesis by regulating apoptosis.Nature 2004;431:80-4.
17.Arakawa H.Netrin-1 and its receptors in tumorigenesis.Nat Rev Cancer 2004;4:978-87.
18.Goi T,Yamaguchi A,Nakagawara G,et al.Reduced expression of deleted colorectal carcinoma (DCC) protein in established colon cancer.Br J Cancer 1998;77:466-71.
19.Enomoto T,Fujita M,Cheng C,et al.Loss of expression and loss of heterozygosity in the DCC gene in neoplasms of the human female reproductive tract.Br J Cancer 1995;71:462-7.
20.Kato H,Zhou Y,Asanoma K,et al.Suppressed tumorigenicity of human endometrial cancer cells by the restored expression of the DCC gene.Br J Cancer 2000;82:459-66.
21.Tanaka K,Oshimura M,Kikuchi R,et al.Suppression of tumorigenicity in human colon carcinoma cells by introduction of normal chromosome 5 or 18.Nature 1991;349:340-2.
22.Velcich A,Corner G,Palumbo L,et al.Altered phenotype of HT29 colonic adenocarcinoma cells following expression of the DCC gene.Oncogene 1999;18:2599-606.
23.Fabre M,Martin M,Ulloa F,et al.In vitroanalysis of the role of DCC in mucus-secreting intestinal differentiation.Int J Cancer 1999;81:799-807.
24.Mehlen P,Rabizadeh S,Smipeas SJ,et al.The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis.Nature 1998;395:801-4.
25.Klingelhutz AJ,Hedrick L,Cho KR,et al.The DCC gene suppresses the malignant phenotype of transformed human epithelial cells.Oncogene 1995;10:1581-6.
26.Forcet C,Ye X,Granger L,et al.The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation.Proc Natl Acad Sci U S A 2001;98:3416-21.
27.Fazeli A,Dickinson SL,Hermiston ML,et al.Phenotype of mice lacking functional deleted in colorectal cancer (DCC)gene.Nature 1997;386:796-804.
 Chinese Medical Sciences Journal2011年3期
Chinese Medical Sciences Journal2011年3期
- Chinese Medical Sciences Journal的其它文章
- Sclerosing Cholangitis after Transcatheter Arterial Chemoembolization:a Case Report
- Sutureless Intestinal Anastomosis with a Novel Device of Magnetic Compression Anastomosis△
- Choroidal Tuberculoma in an ImmunocompetentYoung Patient
- Cytogenetic and Clinical Analysis of 340 Chinese Patients with Primary Amenorrhea
- Serum HIF-1α and VEGF Levels Pre-and Post-TACE in Patients with Primary Liver Cancer
- Effect of Multiple Coatings of One-step Self-etching Adhesive on Microtensile Bond Strength to Primary Dentin
