Serum HIF-1α and VEGF Levels Pre-and Post-TACE in Patients with Primary Liver Cancer
Zhong-zhi Jia *,Guo-min Jiang ,and Yao-liang Feng
1Department of Interventional Radiography,Changzhou Second People’s Hospital Affiliated to Nanjing Medical University,Changzhou 213003,China
2Department of Interventional Radiography,First Affiliated Hospital of Nanjing Medical University,Nanjing 210029,China
PRIMARY liver cancer (PLC) is the fifth most common malignancy worldwide and the third-leading cause of cancer mortality in the world.1Approximately 560 000 cases are diagnosed each year and 550 000 deaths due to liver cancer occur.2Surgical resection is recognized as the most effective treatment method for patients with PLC.3Transcatheter arterial chemoembolization (TACE) has been widely accepted as a choice of treatment for PLC patients who are considered to be unsuitable for surgery,however,the rate of post-TACE recurrence and metastasis remains high.Recent studies have shown that tumor angiogenesis may play an important role in the recurrence and metastasis of PLC.4Hypoxia inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF) are the two most important regulators of angiogenesis.Huanget al5found that the levels of HIF-1α and VEGF expressed in tumor tissue were significantly higher than those in normal liver tissue and HIF-1α might play an important role in neovascularization in PLC through regulation of VEGF transcription.To our knowledge,there were few reports on the application of HIF-1α and VEGF in evaluating the efficacy of TACE based on the quantitative analysis of serum HIF-1α and VEGF levels.Therefore,in this prospective study we assessed the expression levels of serum HIF-1α and VEGF pre-and post-TACE in patients with PLC,and aimed to see whether serum HIF-1α and VEGF could be as predictors of the efficacy of TACE and relapse of PLC.
PATIENTS AND METHODS
Patients
Forty consecutive patients (male 34,female 6;age range,36 to 77 years,mean,52.6 years) fulfilling diagnostic criteria for PLC adopted by Barcelona-2000 EASL conference referred to the First Affiliated Hospital of Nanjing Medical University for TACE from March 2008 to May 2009 were included in the study.The clinical,etiological,radiological and cytohistological profiles were analyzed.The average size of tumor was 8.95±3.65 cm,ranging 3.2-18.0 cm.Totally 85.0% (34/40) patients were with Child-Pugh grade A hepatic impairment at admission,and 15.0%(6/40) with grade B.Thirty-one (77.5%) patients at admission were in clinical stage I/II,the remaining nine(22.5%) in stage Ⅲ.Patients were divided into complete response (CR) and partial response (PR),stable disease(SD),progressive disease (PD) groups according to the therapeutic efficacy.CR was defined as serum alpha fetoprotein (AFP) level reduced to 20 μg/L in AFP positive patients and recurrence had not be found by Doppler ultrasound,CT perfusion imaging (CTPI) or digital subtraction angiography (DSA).Patients who did not fulfill the requirements of CR were enrolled into PR+SD+PD group.Twenty healthy control subjects were included in our study∶male 14,female 6,age range,26 to 65 years,mean 48.5 years.There was no significant difference in age and sex distribution between two groups (P>0.05).All patients provided written informed consent.The study was approved by the institutional review board at Nanjing Medical University.
TACE technique
Diagnostic arteriogram of the common,right or left hepatic artery was obtained in all patients pre-TACE.Superselective catheterization of the feeding hepatic artery branch was performed.After a combined chemotherapy with fluorouracil (750-1000 mg),hydroxycamptothecin(20 mg),and mitomycin C (10 mg) was administrated,6-30 mL epirubicin-lipiodol emulsion was used (10 mg epirubicin dissolved in 10 mL lipiodol).The dosage of emulsion was judged by tumor size and achievement of stagnant arterial flow.Make sure the tumor was well filled with the emulsion.For patients whose tumors were with rich blood supply,gelatin sponge particles were used.
Measurement of serum HIF-1α and VEGF
Serum samples were obtained from these patients 1 day prior to,1 day,1 week and 1 month after TACE.Venous blood was drawn and centrifuged at 1571gfor 15 minutes,then stored at -70°C until use.The levels of serum HIF-1α and VEGF were quantitatively measured by ELISA (Human HIF-1α and VEGF ELISA kits,Xitang Biological Technology Co.Ltd.,Shanghai,China).The absorbance was measured at 450 nm.Each measurement was made in duplicate,and the HIF-1α and VEGF levels were determined from a standard curve generated for each set of samples assayed.The sensitivity of the assay was 1 pg/mL.
Follow-up
The patients were regularly followed up at outpatient clinics.AFP,HIF-1α and VEGF assay and liver sonography and CTPI were performed 1 month post-TACE.Liver DSA was performed if deemed necessary.When a tumor was detected with sonography or CTPI or DSA,it was recorded as a recurrence.
Statistical analysis
The values of serum HIF-1α and VEGF levels were recorded as mean±SD.Thet-test was used to compare the differences in serum HIF-1α and VEGF levels.Pearson correlation was used to determine whether there was a significant correlation between serum HIF-1α and VEGF levels and a significant correlation between clinical prognostic indexes of PLC and serum HIF-1α as well as VEGF levels.All statistical analyses were performed using statistical software SPSS (version 11.5).A value ofPless than 0.05 was considered to be statistical significance.
RESULTS
Correlation between serum HIF-1α and VEGF levels and the efficacy of TACE in treating PLC
Before TACE,the serum levels of both HIF-1α and VEGF in the PLC group were significantly higher than those in the control group (allP<0.01).After TACE,both serum HIF-1α and VEGF levels were significantly increased (allP<0.01),and reached the peak values 1 day after TACE(Table 1).
Eleven patients achieved CR.The levels of both HIF-1α(133.96±57.02vs.255.74±123.44 pg/mL) and VEGF(150.96±84.89vs.368.95±161.90 pg/mL) in CR group 1 month post-TACE were significantly lower than those in PR+SD+PD group (allP<0.01).
Correlation between serum HIF-1α and VEGF levels
There was a positive correlation between the levels of serum HIF-1α and VEGF (r=0.42,P<0.001).
Correlations between clinical prognostic factors of PLC and serum HIF-1α as well as VEGF levels before TACE
The serum HIF-1α level was correlated with presence of capsule (r=3.1,P<0.05),portal vein tumor thrombi (PVTT)(r=2.8,P<0.05) and clinical stage (r=2.1,P<0.05),and serum VEGF level was correlated with PVTT (r=3.3,P<0.05)and metastasis (r=3.5,P<0.05,Table 2).
DISCUSSION
HIF-1α,which is extensively expressed in multiple human tumor cells,consists of HIF-1α and HIF-1β subunits,both of which belong to the basic-loop-helix Per-Arnt-Sim (PAS) protein family of transcription factors.HIF-1β is constitutively expressed,while HIF-1α is the functional subunit and is regulated by oxygen.HIF-1α plays critically important roles in maintaining the energy metabolism of tumor cells,tumor angiogenesis,accelerating tumor proliferation,and metastasis.6Rajaganeshanet al7proved that HIF-1α had a significant impact on disease-free survival in colorectal cancers and liver metastases.
VEGF,a hexose-modified multifunctional protein,could exist at least in one of the four forms,namely,VEGF206,VEGF189,VEGF165 and VEGF121.8VEGF acts on vascular endothelial cells,inducing micro-angiogenesis and causing tumor invasion and metastasis.9Sergioet al10proved that the serum VEGF level was increased after TACE and this affected patient’s survival.Schoenleberet al11proved that tissue and serum VEGF levels appeared to have significantly predictive ability for estimating overall survival in hepatocellular carcinoma and may be useful for defining prognosis in hepatocellular carcinoma.
It has been suggested that the expression of VEGF was regulated by HIF-1α in a hypoxic environment,leading to hyperplasia of tumor vessels and eventually causing tumor growth.12Studies have indicated that HIF-1α could regulate VEGF expression at several levels by enhancing VEGF transcriptional activity and increasing VEGF mRNA stability.13Expression of HIF-1α was also positively correlated with VEGF expression,indicating that HIF-1α may be an upstream regulator of VEGF expression.14
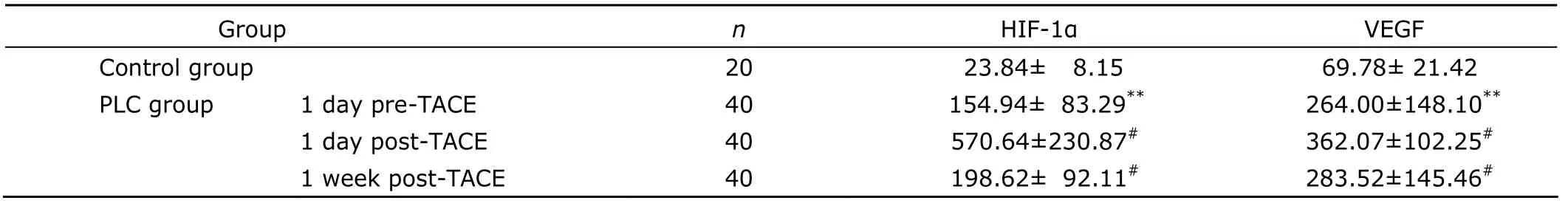
Table 1.Comparison of serum HIF-1α and VEGF levels in control group and PLC group pre-and post-TACE§ (pg/mL)
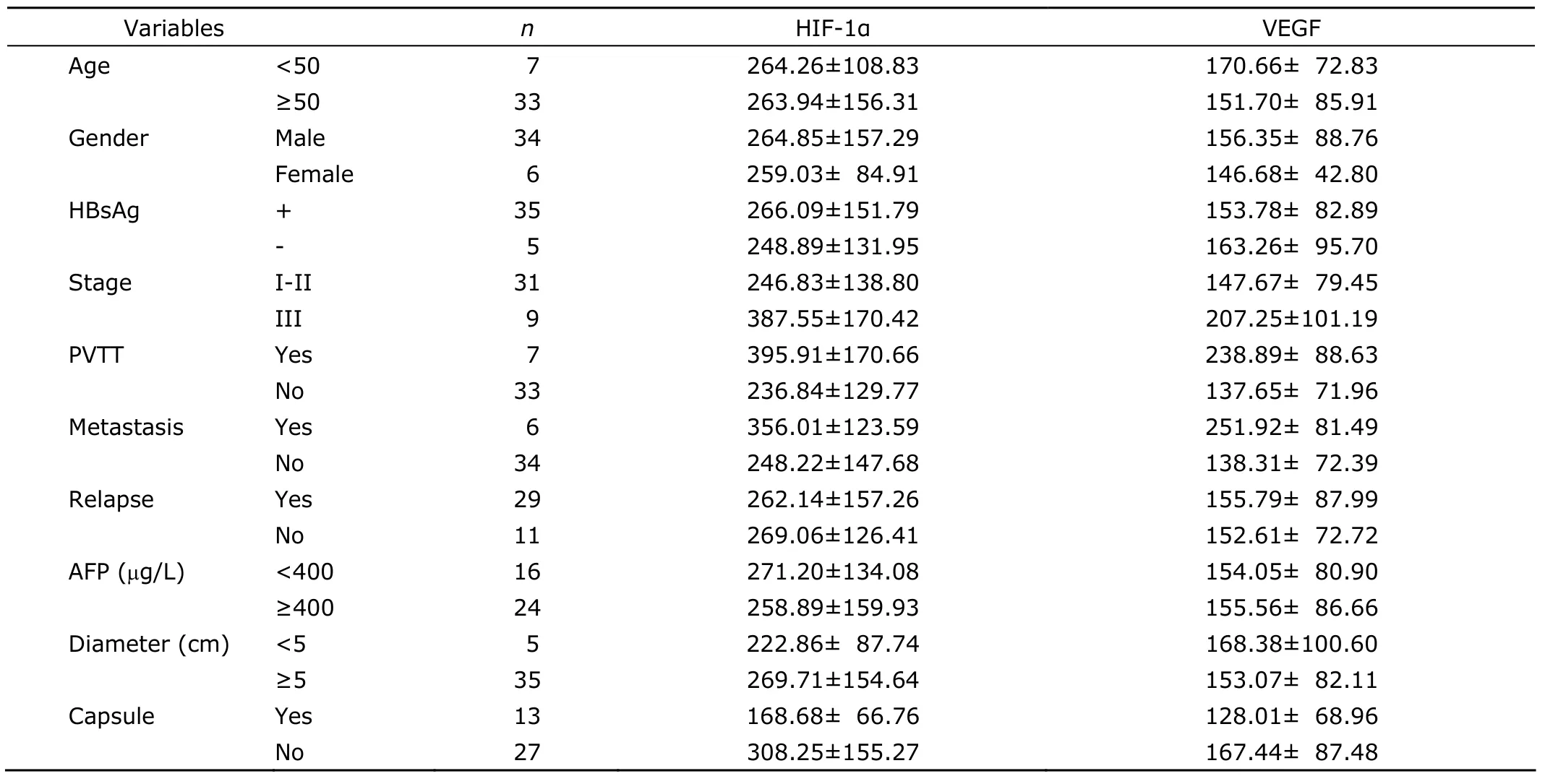
Table 2.Correlation between clinical characteristics of PLC and serum HIF-1α as well as VEGF levels pre-TACE§ (pg/mL)
Notably,our results demonstrated that the levels of HIF-1α and VEGF in serum were significantly higher in PLC patients than those in the control group (P<0.01).It is confirmed that in rapidly growing solid tumors,the tumor tissue grows faster than angiogenesis,resulting in hypoxia of tumor tissue.Hypoxia induces the expression of HIF-1α,which induces expression of VEGF,and subsequently promotes the formation of blood vessels to supply tumors with oxygen and nutrients.Shimet al15proved that the serum VEGF level was significantly higher 1-2 days post-TACE in PLC patients and was associated with distant metastasis and unfavorable outcomes.In our study,the levels of serum HIF-1α and VEGF were significantly higher 1 day post-TACE in PLC patients owing to the fact that ischemia and hypoxia of tumor tissue upregulated the expression and release of HIF-1α and VEGF.The tumor tissue were necrosis because of ischemia and hypoxia and the half-decay period of HIF-1α and VEGF was very short,resulting in the decrease of serum HIF-1α and VEGF 1 week post-TACE.As the tumor tissue was almost necrosis and absorbed,the levels of serum HIF-1α and VEGF were significantly decreased until reached an invariable level 1 month post-TACE in CR group,but the remaining tumor tissue upregulated the levels of serum HIF-1α and VEGF because of ischemia and hypoxia 1 month post-TACE in PR+SD+PD group.The upregulation of serum HIF-1α and VEGF pomoted neovascularization.As a result,the remaining tumor grew faster than before.So the tumor would be almost necrosis if lipiodol was completely accumulated in the tumor and tumor-feeding arteries were completely embolized.Wanget al16proved that there was a significant positive correlation between HIF-1α and VEGF levels and both might play important roles in tumor occurrence and development during rat hepatocarcinogenesis,possibly through promoting tumor angiogenesis,which are consistent with our findings.In our cohort,there was a positive correlation of serum HIF-1α and VEGF levels in PLC patients (r=0.42,P<0.001).
Our results also showed that the expression of serum HIF-1α correlated with presence of the tumor capsule,PVTT,and clinical stage (P<0.05),and serum VEGF correlated with PVTT and metastasis (P<0.05).May be owing to the fact that the clinical stage,capsule,PVTT determine the growth pattern and growth rate of tumor.The more advanced PLCs with PVTT and without capsule grew invasively,resulting in relative retardation of neovascularization and ischemia and hypoxia in tumor.The tumor cells activated by ischemia and hypoxia overexpressed HIF-1α and VEGF,which contributed to the structural and functional abnormalities of the tumor vessels,promoting invasion and metastasis of tumor cells.
In conclusion,this prospective study presented that the levels of serum HIF-1α and VEGF had a positive correlation and both of them were correlated with PVTT and metastasis,suggesting that they might be the useful serum markers for evaluation of efficiency of TACE to treat PLC and recurrence of PLC.
1.Parkin DM,Bray F,Ferlay J,et al.Global cancer statistics,2002.CA Cancer J Clin 2005;55∶74-108.
2.McGlynn KA,London WT.Epidemiology and natural history of hepatocellular carcinoma.Best Pract Res Clin Gastroenterol2005;19∶3-23.
3.Cai J,Hu J,Che X,et al.Prognosis of primary liver carcinoma treated with local resection.Chin Med J(Engl)2003;116∶187-90.
4.Carmeliet P,Jain RK.Angiogenesis in cancer and other diseases.Nature 2000;407∶249-57.
5.Huang GW,Yang LY,Lu WQ.Expression of hypoxiainducible factor 1α and vascular endothelial growth factor in hepatocellular carcinoma∶impact on neovascularization and survival.World J Gastroenterol 2005;11∶1705-8.
6.Rankkin EB,Giaccia AJ.The role of hypoxia-inducible factors in tumorigenesis.Cell Death Differ 2008;15∶678-85.
7.Rajaganeshan R,Prasad R,Guillou PJ,et al.Expression patterns of hypoxic markers at the invasive margin of colorectal cancers and liver metastases.Eur J Surg Oncol 2009;35∶1286-94.
8.Houck KA,Ferrara N,Winer J,et al.The vascular endothelia growth factor family∶identification of a fourth molecular species and characterization of alternative splicing of RNA.Mol Endocrinol 1991;5∶1806-14.
9.Guba M,Von Breitenbuch P,Steinbauer M,et al.Rapamycin inhibits primary and metastatic tumor growth by antiangenesis∶involvement of vascular endothelial growth factor.Nat Med 2002;8∶128-35.
10.Sergio A,Cristofori C,Cardin R,et al.Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC)∶the role of angiogenesis and invasiveness.Am J Gastroenterol 2008;103∶914-21.
11.Schoenleber SJ,Kurtz DM,Talwalkar JA,et al.Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma∶systematic review and meta-analysis.Br J Cancer 2009;100∶1385-92.
12.Liao D,Johnson RS.Hypoxia∶a key regulator of angiogenesis in cancer.Cancer Metastasis Rev 2007;26∶281-90.
13.Pugh CW,Ratcliffe PJ.Regulation of angiogenesis by hypoxia∶role of the HIF system.Nat Med 2003;9∶677-84.
14.Jiang BH,Liu LZ.PI3K/PTEN signaling I tumorigenesis and angiogenesis.Biochim Biophys Acta 2008;1784∶150-8.
15.Shim JH,Park JW,Kim JH,et al.Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients.Cancer Sci 2008;99∶2037-44.
16.Wang W,Xu GL,Jia WD,et al.Expression and correlation of hypoxia inducible factor-1α,vascular endothelial growth factor and microvessel density in experimental rat hepatocarcinogenesis.J Int Med Res 2009;37∶417-25.
 Chinese Medical Sciences Journal2011年3期
Chinese Medical Sciences Journal2011年3期
- Chinese Medical Sciences Journal的其它文章
- Sclerosing Cholangitis after Transcatheter Arterial Chemoembolization:a Case Report
- Sutureless Intestinal Anastomosis with a Novel Device of Magnetic Compression Anastomosis△
- Choroidal Tuberculoma in an ImmunocompetentYoung Patient
- Influence of Deleted in Colorectal Carcinoma Gene on Proliferation of Ovarian Cancer Cell Line SKOV-3 In Vivo and In Vitro
- Cytogenetic and Clinical Analysis of 340 Chinese Patients with Primary Amenorrhea
- Effect of Multiple Coatings of One-step Self-etching Adhesive on Microtensile Bond Strength to Primary Dentin
