Immunomolecular response of CD4+, CD8+, TNF-α and IFN-γ in Myxobolus-infected koi (Cyprinus carpio) treated with probiotics
Uun Ynuhr, Nio Rhmn Cesr, Nur Skinh Junirhm, Rhmt Noer Soelistyodi
a Department of Aquatic Resources Management, Faculty of Fisheries and Marine Sciences, Brawijaya University, Jalan Veteran, Malang, East Java, 65145, Indonesia
b Doctoral Program of Environment Science, Post Graduate Program, Brawijaya University, Jalan Veteran, Malang, East Java, 65145, Indonesia
c Fish Quarantine Center for Quality Control and Safety of Fishery Products Surabaya I, Ministry of Marine Affairs and Fisheries, Surabaya, East Java, 60115, Indonesia
Keywords:Cyprinus carpio Flowcytometry Immune responses Myxobolus koi Probiotic
A B S T R A C T A poor environment increases fish’s susceptibility to myxosporean infection that can cause the death of larval fish, especially for koi fish (Cyprinus carpio).This study aimed to determine the effect of probiotics, local antiparasitic drugs (kutuklin), and the chemical compound diflubenzuron treatments on the koi immune response.This study used PCR with specific primer 18S SSU rDNA and DNA sequencing to detect Myxobulus phylogenetic.The treatments were divided into 5 groups: Treatment (A) (healthy koi without treatment), (B) (infected koi without treatment), (C) (infected koi with 0.55 mL/30 L probiotics), (D) (infected koi with 1 μL/g of feed kutuklin), and (E) (infected koi with 0.02 mg/5 L dimilin).Myxospore has observed with Scanning Electron Microscopy (SEM) and 4′,6-diamidino-2-phenylindole (DAPI) fluorescence staining.The histological analysis using semi-quantitative scoring methods, and flow cytometry was conducted to analyse the immune response of Cluster of differentiation 4 (CD4+), Cluster of differentiation 8 (CD8+), Tumor Necrosis Factor-alpha (TNF-α),Interferon gamma (IFN-γ) cells in the gills.Results show that the histological analysis indicated edema, hyperplasia, lamella fusion, congestion, and hypertrophy lesions in infected koi.Treatment with probiotics shows the lowest damage (30.6%).The immune responses of CD4+ and CD8+ cells to dimilin treatment were 10.54% and 16.86%, respectively.The largest TNF-α and IFN-γ response were for the kutuklin treatment (29.26%) and probiotics treatment (8.23%).
1.Introduction
The development of the ornamental fish sector in the global aquaculture industry has increased over the last decade.Indonesia has always been in the top 5 ornamental fish exporters from 2010 to 2018.Since 2012–2019 the export of ornamental fish has increased significantly from USD 21 million to USD 33,11 million and in first quarter of 2020 reached USD 6.41 million (Arinanto, 2020)..A popular type of ornamental fish is koi, a strain of goldfish (Cyprinus carpio)(Safari & Sarkheil, 2018).Millions of fish are moved around the world every year,supported by developments in global connectivity and shorter transportation times.The increase in the volume of trade in ornamental fish leads to increases in production and the risk of pathogen infection(Swaminathan et al., 2016).In some cases, fish seeds and fish traded under less strict quarantine conditions suffer from myxosporean parasites.These myxosporean parasites move to new territories, causing pathological changes and fish mortality (Yuan et al., 2015).In addition,poor handling of water quality management triggers the emergence of myxosporean parasites (Yanuhar et al., 2020a, 2020b; Yanuhar et al.,2019b).Myxosporean is a type of cnidarian endoparasite with a worldwide distribution.Myxosporean increases koi mortality, especially in those travellng from Asia to Britain or the United States, and almost 20% of farmed goldfish die annually caused by this disease in China (Liu et al., 2019a; Mathews et al., 2018).
The myxosporean that attacks freshwater ornamental fish, especially koi, isMyxobolus koi, also known asMyxobolusis.Myxozoa has a pyriform myxospore structure with an elongated anterior end and two subequal pyriform polar capsules, parallel to each other and without intercapsular processes.Myxobolus spores are oval or round and possess sporoplasm produced by the myxosporean.The difference between the genus Myxobolus and Thelohanellus is the number of polar capsules.Myxobolusspp.have two polar capsules, andThelohanellusspp.have a polar capsule (Gupta & Kaur, 2020; Saha & Bandyopadhyay, 2018).Myxobolus disease is a lethal threat for koi and several other ornamentalfish.It causes mortality of up to 30%, with a prevalence of up to 90%,especially in the juvenile phase (Liu, Wei, Wang, Yang, & Wang, 2019b;Yanuhar et al., 2020a).Infected fish show anorexia, lethargy and sluggish swimming, inflammation of the pharynx, and protruding eyes due to mechanical pressure from the parasite mass.In some cases, fish continue to swim in a circular motion (Neto et al., 2016; Yanuhar et al.,2019a,2019b)) and have difficulty breathing due to nodules or cysts on the gill filaments (Yanuhar et al., 2019a).Several myxosporean species cause significant pathological problems such as reduced gill epithelial area, cardiac myocarditis, deformation, displacement, retraction and compression of gill lamellae capillaries, large skin nodules, thickening of the external tunica of the bladder with granulomatous reactions, perivascular edema in the interlamellar region, and stretching the epithelium of the cornea and bladder (Mathews et al., 2020).
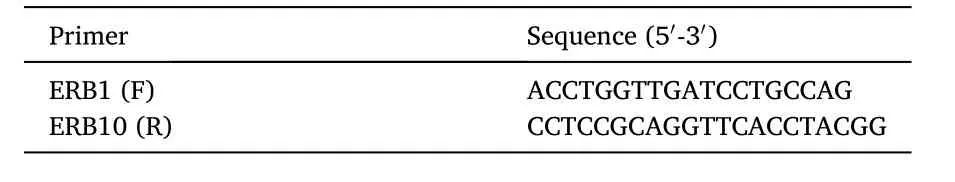
Table 1 The primer pair used to detect Myxobolus sp.parasite.
Fish diseases are difficult to control and cure, especially as fish disease is often handled late.Therefore, it is necessary to determine the health level of fish.Diagnosis can be observed based on physical symptoms include changes in behavior, body lesions, morphological changes, and fish anatomy.The need to understand fish immunity and disease is crucial for the aquaculture industry to detect the disease early(Mohammadian et al., 2018).However, clinical parameters and zootechnical indices alone are insufficient to monitor fish health at the onset of infection.Therefore, a rapid and accurate diagnosis is needed in handling disease diagnosis problems.The molecular analysis can also be used to find out more about the health status of fish.The immune response to parasites varies depending on the attacking myxozoan species, the target tissue, and the host species.Lymphocytes in blood cells introduce the myxosporean parasite antigen.Lymphocytes recognize and respond to external antigens and subsequently act as cellular and humoral immune mediators.Functionally different lymphocytes are distinguished by the expression of mutually exclusive CD4+or CD8+co-receptors (Jung et al., 2020).Immune cells expression of CD4+and CD8+can increase in fish cells due to antigen’s exposure (Yanuhar,2011; Ashfaq et al., 2019).CD4+cells coordinate specific immune responses by releasing different types of cytokines depending on the invading antigen (Piazzon et al., 2018).The CD4 molecule involved in the development and activation of T cells is a significant cell surface marker and is used to identify a subset of T-helper cells.The characterization of the CD4+cell population and function has been determined due to suitable available markers for T-lymphocytes in fish (Ashfaq,Soliman, Saleh, & El-Matbouli, 2019; Jung et al., 2020; Yanuhar et al.,2012).Meanwhile, CD8+cell directly kill cells by cross-linking death receptors or releasing cytolytic effector molecules such as perforin or granzyme (Piazzon et al., 2018).The mechanism of leukocyte differentiation depends on IFN (Interferon), which can be activated by the inflammatory response from the innate response to infection.IFN-induced inflammation has a critical impact on the activation of adaptive immunity and hematopoiesis and encourages pathology through several activated signalling pathways, including cytokines and proinflammatories NF-κB, proteasome, apoptosis, and ubiquitination(Langevin et al., 2019; Li et al., 2019).Tumor necrosis factor-alpha(TNFα) are cytokines involved in a broad spectrum of cellular and organismal responses.Its main function (as a powerful proinflammatories mediator against pathogenic invasion and antimicrobial defense) is mediated through lymphocytes, leukocyte activation, cell proliferation, differentiation, and apoptosis (Ronza et al., 2015).
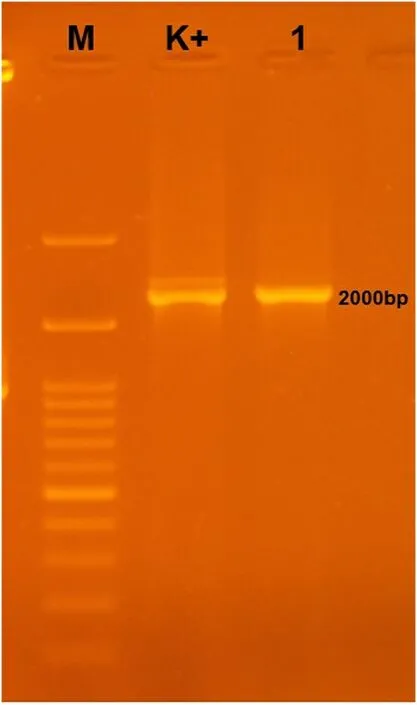
Fig.2.The confirmation result of Myxobolus identification by using PCR.The electrophoresis amplification visualization result of the 18S SSU rDNA gene in the gill organs of Myxobolus infected koi.M (Marker); K + (Positive Control).

Fig.1.Clinical symptoms of infected fish by Myxobolus koi.Observation showed comparison between infected fish before and after treatment.a) before treatment,the koi gill operculum cannot be completely closed due to nodules, b) after treatment, the operculum appears to return to normal, and the body length increases.
Table 2 Sequence results of Myxobolus koi using 18S SSU rDNA marker.
?
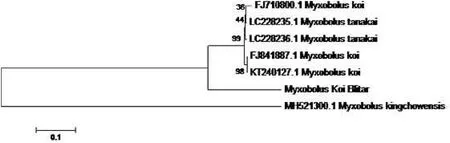
Fig.3. Myxobolus phylogenetic map.The taxa evolutionary relationship through the phylogenetic tree sample of Myxobolus koi compared with other isolates using the Neighbor-Joining method.
In general, antibiotics and chemicals are used to treat diseases in aquatic animals.The adoption of drugs, such as formaldehyde, florfenicol, sulfamerazine, chloramine-T, peracetic acid, diflubenzuron, and hydrogen peroxide in aquaculture seems to be profit-oriented and unsustainable.It increases pathogen drug resistance in fish, causes environmental pollution, and accumulates chemical residues potentially harmful to health (Abe et al., 2019; Lieke et al., 2020; Saha et al., 2020).Kutuklin is a type of practical medicine mainly consisting of deltamethrin that often used to treat parasites by local koi fish farmers,especially in the District of Nglegok, Blitar, East Java, Indonesia.The administration is by mixing it with the feed.The absorption rate of deltamethrin orally is up to 75% and can be rapidly excreted in the urine and feces (Standing Committee on Biocidal Products, 2011).The use of deltamethrin compounds in fish with a maximum residue limit according to Committee for Veterinary Medicinal Products (EMEA,2000) based on Annex III Council Regulation (EEC) No.2377/90 is 10 μg/kg.Dimilin? (Chemtura Industria Quimica Ltda, Brazil), contains the diflubenzuron compound.The effect of diflubenzuron on some fish species causes 50% mortality (LC50 96h) at doses higher than 100 mg/L(Dantzger et al., 2018).An alternative treatment for koi farmers is the use of probiotics (Yanuhar et al., 2019b).Probiotics promote growth,stimulate immune system function, and increase fish resistance to infectious diseases (Jahangiri & Esteban, 2018).Generally, the main constituents of probiotics in aquaculture are lactic acid bacteria(Mohammadian et al., 2018; Singh et al., 2020; Wang et al., 2017).Various factors such as source, dosage, method of administration, and duration of probiotic supplementation can affect the immunomodulatory activity of probiotics (Hai, 2015).Therefore, it is necessary to determine the appropriate treatment to increase immune activity in myxosporean-infected koi fish.This study aimed to compare the response of CD4+, CD8+, TNF-α, and IFN-γ as potential biomarkers to evaluate the health level of koi under treatments with probiotics, local anti-parasitic drugs (kutuklin), and diflubenzuron (dimilin).
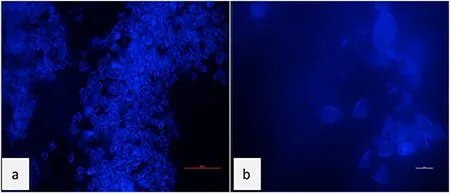
Fig.4.Fluorescent test results of Myxobolus infected gill organs.(a) 40x (50 μm); and (b) 100x (10 μm) magnification.
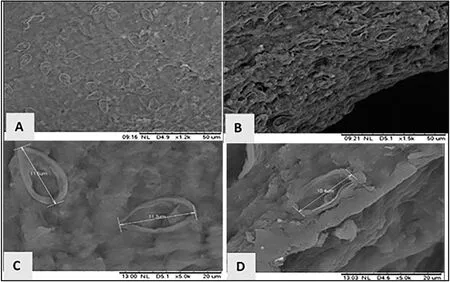
Fig.5. Myxobolus koi spores infecting koi gills process observed using Scanning Electron Microscopy.(a) 1200x (scale bar =50 μm); (b) 1500x (scale bar =50 μm);(c) 5000x (Myxobolus Spore size 11.5 μm and 11.3 μm; scale bar = 20 μm); (d) 5000x (Myxobolus Spore size 10.4 μm; scale bar = 20 μm) magnification.
2.Materials and methods
2.1. Sample collection
Koi (Cyprinus carpio) samples were collected from Nglegok Village,Nglegok District, Blitar Regency, East Java, Indonesia.The koi samples,5–7 cm in length, consisted of 12 healthy fish as a control group and 48 fish infected with Myxobolus based on observations of clinical symptoms morphologically.Healthy fish obtained directly from the rearing pond.Infected fish with Myxobolus were obtained from the quarantine pond.The samples divided into five treatments.Each treatment container contains of 12 fish samples.The fish was first stunned and then stabbed in the medulla oblongata for further dissection.The gill of koi fish samples was dissected from the most severely infected fish in each treatment based on clinical symptoms observation.
The preparation of gill organs refers to (Riisg?rd et al., 2015; Tolo et al., 2021).The gill preparation was made from about 0.5 cm ×0.5 cm pieces of the ascending part of the gill branches.Gill samples were dissected from formalin-fixed specimens and decalcified in 10% ethylenediaminetetraacetic acid (EDTA).After decalcification, the samples were dehydrated in an ethanol series to 100% ethanol, infiltrated with toluene, and then immersed in paraffin.Paraffin sections were cut at 6 m thickness using a Leica Jung 820 Histocut Rotary Microtome and mounted on slides.The sections were stained with Hematoxylin and Eosin.All experimental animal protocols in this study were reviewed and approved by the Animal Care and Use Committee of Brawijaya University, Malang, Indonesia (Approval Letter No.130-KEP-UB-2021,dated December 17, 2021).
2.2. Probiotic production
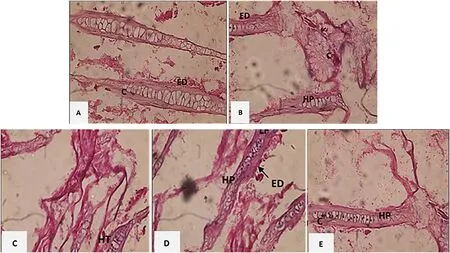
Fig.6.Histology of gill tissue in koi.(A) Healthy Koi.(B) Myxobolus infected koi.(ED: Edema.HP: Hyperplasia.C: Congestion).(C) Myxobolus infected koi with probiotic 0.55 mL/30 L administration.(D) Myxobolus infected koi with kutuklin 1 μL/g feed administration.and (E) Myxobolus infected koi with dimilin 0.02 mg/5 L; (ED: Edema.HP: Hyperplasia.LF: Lamella Fusion.C: Congestion.HT: Hypertrophy).
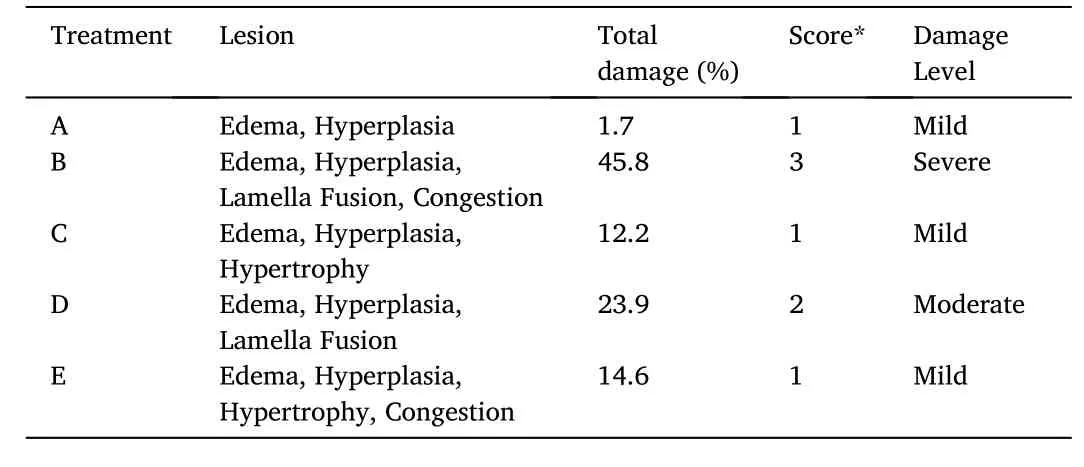
Table 3 Types and levels of tissue damage to koi gill.
In this study, the probiotic formulation consisted of local herbal ingredients combined with three genera of bacteria –Lactobacillussp.,Nitrosomonassp.andBacillusspp.each with concentration of 106CFU/mL.Probiotic production refers to Soltani et al.(2019) and (Ministry of Marine Affairs and Fisheries, 2019) by combining microorganism and local herbs with other ingredients such as molasses, aquadest in a container.Then, fermentation process was conducted for about 2 weeks and probiotic was ready to be used.
2.3. In-vivo treatment of koi
The koi samples were acclimatized and fasted for 24 h.Probiotic and dimilin treatments were conducted using the immersion method by adding substances to the koi live media.Kutuklin treatment was given by using the oral method.The administration of kutuklin was conducted ad libitum by giving feed into the water on day-0.The immersion method for probiotic and diflubenzuron was carried out once for 6 h on day-0.Determination of probiotic formulations, kutuklin, and diflubenzuron administration according to previous studies (Yanuhar et al.,2019b, 2020a, 2020b) following the local use.The administration of probiotics based on preliminary research using an initial dose of 1.1 mL/30 L of water, 0.55 mL/30 L of water, and 1.65 mL/30 L of water.The minimum treatment dose was 0.55 mL/30 L refers to the previously reported method (Jahangiri & Esteban, 2018).The Committee for Veterinary Medicinal Products recommends the inclusion of deltamethrin compounds in fish with a maximum residue limit is 10 μg/kg and diflubenzuron is 1000 μg/kg based on Annex III Council Regulation(EEC) No.2377/90 (EMEA, 1998; EMEA, 2000).Treatment A was a negative control, healthy fish without treatment; Treatment B was a positive control, Myxobolus sp.infected koi without treatment; Treatment C was Myxobolus sp.infected koi with probiotics treatment at a dose of 0.55 mL/30 L; Treatment D was Myxobolus sp.infected koi with kutuklin treatment at a dose of 1 μL/g of feed; Treatment E was Myxobolus sp.infected koi with dimilin treatment at a dose of 0.02 mg/5 L.The treatments were given periodically for 10 days while observing their development.Dissolved oxygen of water was maintained using aeration manually.A multiparameter water quality meter was used daily to test and maintain the optimal value according to Yanuhar et al.(2021),temperature (25–30?C), pH (6.5–7), carbon dioxide (4–10 mg/mL) and dissolved oxygen (>5 mg/mL).Siphoning was conducted everyday.Fish were fed twice a day, in the morning and evening.
2.4. PCR (Polymerase Chain Reaction)
2.4.1.Extraction
DNA (deoxyribonucleic acid) was extracted from tissue preserved in absolute ethanol solution using a Genomic DNA Mini Kit (Geneaid Biotech Ltd., Taiwan).Each sample of gill tissue, identified as eitherMyxobolus-infected or healthy, was inserted into a 1.5 mL microtube.GT Buffer (Geneaid Biotech Ltd., Taiwan) (900 μL) was added, the tissue pulverized using a grinder pestle, and the solution centrifuged (Hettich Zentrifugen Mikro 22R, Germany) at 12,000 rpm for 3 min.Next, 600 μL of coating solution was added to a new 1.5 mL microtube.Silica (40 μL)was added to the solution, vortexed to make it homogeneous, and centrifuged at 12,000 rpm for 15 s.After centrifugation, researchers discarded the solution, washed the silica pellet with 500 μL of GT Buffer,vortexed the silica pellet until it formed a suspension, and centrifuged the suspension at 12,000 rpm for 15 s.After discarding the solution, 1 mL of 70% ethanol (Merck, Germany) was added to wash the silica, and the pellet was again vortexed until the silica pellet formed a suspension.The suspension was then centrifuged at 12,000 rpm for 15 s, the ethanol solution was discarded, and a micropipette was used to remove any remaining 70% ethanol.Next, ddH2O (1 mL) was added to the silica pellets.The silica pellets were vortexed until a suspension formed.The suspension was incubated at 55?C for 10 min, vortexed until it was homogeneous, then centrifuged at a speed of 12,000 rpm for 2 min.Finally, 500 μL of the supernatant was transferred into a new microtube(Gupta & Kaur, 2020; Nurekawati, 2016).
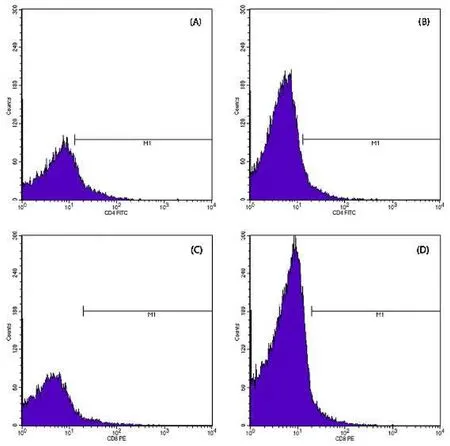
Fig.7.Relative Count Profile.a) CD4 +cells in healthy fish (17.58%); b) CD4 +cells in Myxobolus infected fish (7.33%); (c) CD8 +cells in healthy fish (4.4%); d)CD8 + cells in Myxobolus infected fish (4.25%).
2.4.2.Amplification
The amplification process was conducted using a specific primer 18S SSU rDNA (Gupta & Kaur, 2020).The 18S SSU rDNA gene was amplified with the primer pairs shown in Table 1.The total volume of the PCR reaction mixture was 25 μL, consisting of Master Mix (KAPABiosytems,KK510).Forward Primer (ERB1) 2 μL, Reverse Primer (ERB10) 2 μL,DNA Template 2 μL, Nuclease free water 19 μL.Amplification was carried out by setting the predenaturation temperature at 94?C (2 min),followed by denaturation at 94?C (1 min), annealing at 58?C (1 min),extension at 72?C for (1 min 30 s) for 35 cycles and added final elongation at 72?C (5 min) (Soelistyoadi et al., 2019).
2.4.3.Electrophoresis
The DNA amplification was conducted using 1.5% agarose gel immersed in 1X TAE buffer (SYBR? Safe, Thermo Fisher, Germany).The holes in the gel were filled sequentially with a marker, 8 μL of amplification result, and a control blank.Electrophoresis (Mini-PROTEAN, Bio-Rad, USA) was carried out for 45 min at 100 V.The agarose mixed with SyBr Safe (Invitrogen) and was immersed in 1 ×TAE buffer for 15 min.The gel was placed on the gel documentation and observed under UV light.
2.5. DNA sequencing
TheMyxobolusgene sequence was analyzed using the Mega ver.6.06(software) program and adjusted to the database or library stored in GenBank – the National Center for Biotechnology Information (NCBI)(http://www.ncbi.nlm.nih.gov/blast) – with the BLAST program (Gupta& Kaur, 2020; Saha & Bandyopadhyay, 2018) to obtain the percentage of similarity to the database.Homologous sequences of the DNA gene nucleotide sequences and the BLAST program tracing were aligned(multiple alignments) using ClustalW.Specimens were identified by constructing a family tree and the percentage of similarity index.Genetic markers, genetic diversity and genetic distance from the nucleotide sequences of theMyxobolusDNA partial genes were analyzed using MEGA version 6.06.
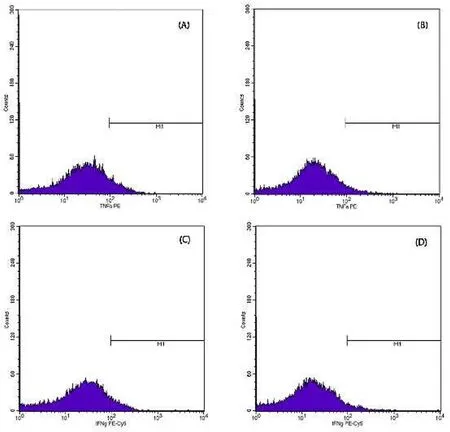
Fig.8.Relative Amount Profile.a) TNF-α response in healthy fish (11.82%); b) TNF-α in Myxobolus infected (4.72%); (c) IFN-γ response in healthy fish (8.84%); d)IFN-γ in Myxobolus infected fish (3.69%).
2.6. Fluorescence
In this study, to identify whether DNA is present in the mitochondrial myxozoan, we stained the stages of multicellular development of the koi carp (C.carpio) gill tissue with the fluorophore 4′, 6-diamidino-2-phenylindole (DAPI) as was done by (Yahalomi et al., 2020) onM.squamalisandH.salminicola.Myxobolusinfected gill tissue slices were stained with DAPI (Fluoroshield using DAPI, Sigma-Aldrich Finland Oy,Espoo, Finland).DAPI is the counterstain for DNA and RNA, and it penetrates cell membranes to stain the DNA and RNA ofPlasmodiumparasites in intact erythrocytes.The DAPI stain solution was applied to the sample and distributed over the surface of the slide (Holmstrom et al., 2020).Fluorescence staining involved heating the paraffin slices at 40?C overnight in an oven then, soaked it in Xylol 1 (10 min) and absolute ethanol-1 (5 min) twice each.Next, the paraffin slices were soaked sequentially in 90% ethanol and 70% ethanol, each for 5 min.The preparation was then soaked in PBST (Bio-World, USA) three times for 5 min each, followed by citrate buffer 10 mM pH 6, 120?C (15 min).The preparations and containers were removed from the oven and left for approximately 10 min.Next, the preparation was soaked in in PBST(3 ×5 min) and dipped in a solution of DAPI: PBST (1:100) for 5–30 min,washed in PBST (3 ×5 min), dried and covered with a glass cover, and dipped in 10% glycerol.Observations of the preparation were made with a fluorescence microscope (Bright Field Microscope, Nikon/Eclipse;Japan).
2.7. Morphology observation using SEM
TheMyxobolusinfected tissue was preserved in a 2.5% glutaraldehyde solution at 4?C for 2 h.The infected tissue was dehydrated with ethanol, dried and washed in a mixture of absolute acetone and amyl acetate (3:1, 2:2, and 1:3 ratio, and 100% amyl acetate).The tissue was dried with CO2in an HCP-2 Critical Point Dryer (Hitachi).Finally, the tissue was coated with metallic gold using an IB-2 ion coater and the tissue observed by SEM (Hitachi TM 3000 Microscope, Japan) at 15 and 20 kV (Saha & Bandyopadhyay, 2017).
Fig.9.Relative Count Profile.a) CD4 +and CD8 +profiles in C probiotic treatment (4.88%; 13.98%); b) CD4 +and CD8 +profiles in treatment D kutuklin (1.93%;5.1%); (c) CD4 +and CD8 +profiles in treatment E dimilin (10.54%; 16.86%); d) TNF-α and IFN-γ profiles in C probiotic treatment (15.57%; 8.23%); e) TNF-α and IFN-γ profiles in the D treatment kutuklin (29.26%; 5.66%); f) TNF-α and IFN-γ profiles in treatment E dimilin (4.66%; 0.65%).
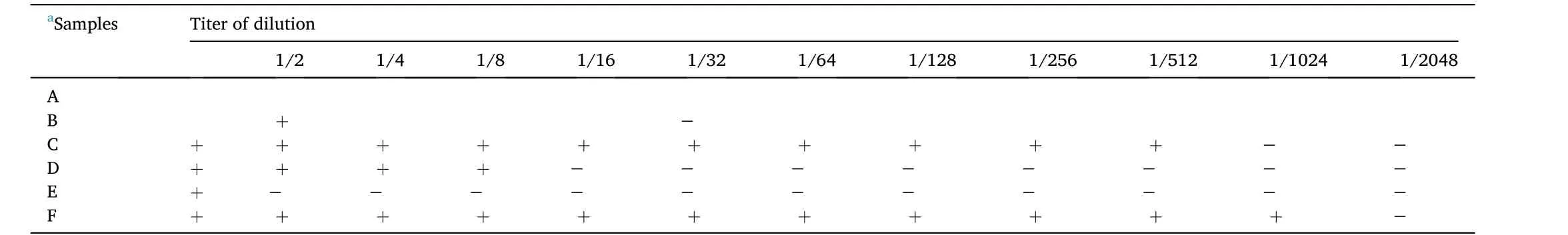
Table 4 The results of the agglutination response tests on erythrocytes of each treatment.
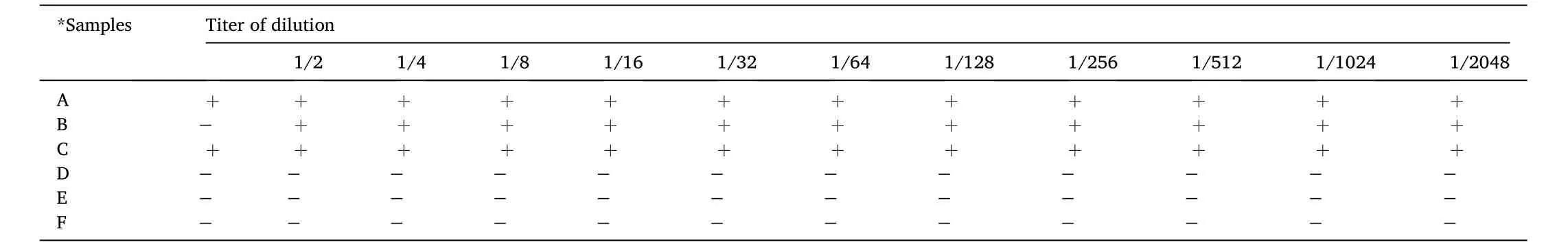
Table 5 The results of the agglutination response tests in the blood plasma of each treatment.
2.8. Histological observation of koi gill tissue
There are 3 fish representing each treatment that were dissected and prepared using the method of Riisg?rd (Riisg?rd et al., 2015).Preparations were observed under a light microscope with a magnification of 100–400 ×.Observations were made based on the field of view showing abnormalities (lesions) in the tissue.Assessment of tissue damage for each organ used a semi-quantitative scoring method (Bariˇsi′c et al., 2015;Cavicchioli et al., 2015).The assessment was based on the percentage of damage in the field of view.Assessment scores were 0 for no damage(0%), 1 for mild damage (<15%), 2 for moderate damage (15%–30%)and 3 for severe damage (>30%).Observations were made of 3 tissue sections in the observed organ samples based on the 5 visible fields of view.
2.9. Flowcytometry
Flow cytometry analysis applied to samples of freshly dissected gill organs.The gill tissue was crushed with the base of a syringe in a container with 2–3 mL of PBS solution then placed in a 15 mL centrifuge tube and centrifuged at 2500 rpm and 10?C for 5 min.The supernatant was removed, and 1 mL of PBS added into the cell pellets.The cell pellets were divided into several 1.5 mL microtubes containing PBS solution,then centrifuged at 2500 rpm and 10?C for 5 min.Extracellular specific molecular staining was carried out by add 50 μL of Foxp3 PE/Cy5 antimouse specific antibody solution, anti-mouse PE/Cy5 CD4+, anti-mouse PE/Cy5 CD8+, TNF-α and anti-mouse PE/Cy5 IFNγ to cells (pellets).All antibodies were supplied by BioLegend, Inc.(San Diego, CA).The cell pellets were incubated for 20 min at 4?C (darkroom).Next, 400 μL of PBS was added, and the cell pellets were transferred to a cuvette for flow cytometry analysis.The cell morphology, viability, and physiological status of cells were monitored and characterized throughout the culture using a flow cytometer (BD Biosciences, USA).Data were processed using BD Cell Quest ProTM software (Lestari & Rifa’i, 2018).
2.10. Agglutination test
The procedure of hemagglutination and inhibition were performed using previously described methods (Jiang et al., 2019; Kaufmann et al.,2017) with a U-shaped microplate (U-bottomed 96-well microtiter plate,Anicrin, Italy).The test serum was diluted twice on the eighth tube using sterile PBS.Next, 50 μL of serial dilution serum and koi blood volume was added for each treatment to each hole of the 96-well plates.The test serum was added to each plate as a control.The plates were incubated overnight at 28?C in a humid environment.Circular diffuse deposition with obscure edges at the bottom of the ’U’ was determined as a positive reaction, and clear, compact, circular deposition was determined to be a negative reaction.The agglutination titer was defined as the lowest dilution.
2.11. Statistical analysis
All data were analyzed by Statistical Package for Social Sciences(SPSS) version 23.0 software (SPSS Inc., Chicago, IL, USA).The result of Immune response (CD4+, CD8+, TNF-α, and IFN-γ) were obtained to determine the difference effect of treatment using one-way ANOVA (sig.95%).If there is a significant difference, then the Least Significant Difference (LSD) test is carried out between the treatment and the observed variables.
3.Results
3.1. Observations of clinical symptoms
Myxobolus-infected fish obtained from quarantine tanks as a collective sample had a different appearance than healthy fish.Clinical symptoms in the aquaculture container for each treatment were observed for 10 days.Myxobolusinfected fish had a protruding operculum, as it was not completely closed.The operculum in infected fish moved faster than in healthy fish.Severely infected fish often went to the surface of the water to breathe.Furthermore, there were nodules or white parts, like boils, on the gill organs of koi fish.Myxobolusinfected fish had paler scales and produced more mucus than healthy fish.General symptoms are white nodules on the gills (operculum) and pale gills containing thousands of spores.Furthermore, healthy fish exhibited no wounds on the body, were bright in color, moved actively, and did not swim on the surface of the water.The comparison between infected fish before and after treatment seems not too significant.Infected fish showed different morphological appearances compared to healthy fish(Fig.1).
White nodules on the gills (operculum) and pale gills were similar to the previous parasitology examinations revealed that the infected gill lamellar tissue contained the whitish plasmodia (Guo et al., 2018; Yuan et al., 2015).This parasite is usually confined to fish gills, skin, and areas adjacent to the fins.There is thickened dermis covered with mucus or patches of creamy-white plasmodia located on the tissue.In some cases,many 1 mm round white cysts (plasmodia) infect the tips of the gill filaments and the dorsal and lateral fins.Infected fish experience failure of osmoregulation and malabsorption of nutrients.Previous studies(Ahmed et al., 2019; Swaminathan et al., 2016; Yanuhar et al., 2019b)found that the external symptoms ofMyxobolusinfected fish depended on the type of fish.As a result, the fish suffers from clinical manifestations of diseases such as anorexia, enophthalmia, bone protrusion on the head, thinning of the body, cachexia, and death (Saha & Bandyopadhyay, 2017; Sekiya et al., 2016).Gupta and Kaur (2017) investigatedMyxobolus holzeraespecies as the cause of severe gill disease in India.They found that infectedLabeo rohita(carp) showed moderate symptoms with creamy-white colored gills.Furthermore, the body surface and gills produced mucus.The parasite caused degeneration of the gill filaments and other cellular elements.
3.2. PCR results for Myxobolus koi
3.2.1.Sequence results analysis of Myxobolus koi
Myxosporean attacks specific hosts, organs, and tissues organism.The host and site of infection are the main features for detecting a specific disease mechanism (Abraham et al., 2015).Generally, myxobolus infects fish at the spore stage, then develops into nodules on the specific organ as a host.This parasite mostly found attached to the koi gill filaments (Lamela).PCR test only used as initial identification to identify the presence of myxobolus before treatment on infected fish.PCR tests using 18S SSU rDNA on gill samples of healthy andMyxobolusinfected koi were visualized by electrophoresis (see Fig.2).Whereas viewed based on PCR results, the band’s appearance showed the genomic copy content of theMyxobolusparasite’s DNA for the target organ sample.Infected fish appeared at the specific band of the positive control (2000 bp).Molecularly, the gills showed positive infection withMyxobolussp.After the amplification process, sequencing was carried out to determine the DNA structure of the parasite.The DNA sequencing results of the Myxobolus koi parasite (Table 2), which infected koi fish(Cyprinus carpio) in Blitar have a similarity (percent identity) to the Gen Bank data for several organisms.Furthermore, theMyxobolus koiisolate from GenBank was used as a reference for analyzing the genomic data.The phylogenetic analysis is shown in Fig.3.
Phylogenetically, the 18S rDNA nucleotide sequence ofMyxobolus koi(Blitar), which causes gill infection, was grouped with otherMyxobolusspp.[Myxobolus koi(FJ10800); (FJ841887); (KT240127) andMyxobolustanakai (LC228235); (LC228236)].The percentage of similarity to the closest reference order was compared with the taxonomic classification for deep-branched lineages using the phylogeny-based method.The percentage of similarity or level of homology between the 18S SSU rDNA sequences obtained from theM.koi(Blitar) sample and other representative myxosporean was 95.99%-9.37% with an E value of 0.0.The species that are phylogenetically most closely related toM.koi(Blitar) isM.koi(KT.240127.1), with a similarity of 98%.
3.3. Observations through fluorescence staining and SEM
Fluorescence and SEM observation are only used as initial identification to identify the presence ofMyxobolusbefore treatment and only done on infected fish.Fluorescence staining is used to detect antigens or localization of antigens in tissue or clinical specimen cells.Plasmodia shaped cysts isolated from koi gill lamellae are small, elongated, and pale white.A positive expression ofMyxobolusparasites shows a blue glow in the cell area, while a negative expression of theMyxobolusparasites does not fluoresce.Visualization of theMyxobolusparasite captured in the koi gill tissue is shown in Fig.4(a) and moderately clear in Fig.4(b) at a magnification of 100 ×.The respiratory functional component of the gills, the gill lamellae, is affected by a parasitic infection that causes histological changes in the infected tissue (Saha &Bandyopadhyay, 2017).Permeable fluorescent staining can be used to visualize intracellular plasmodium parasites.In addition, lower magnification reduces the need for high-power microscope equipment(Holmstrom et al., 2020; Poostchi et al., 2018).
The SEM results showed that theMyxobolusparasite in infected fish could be confirmed in the koi gill tissue and was used in the diagnostic process.Furthermore, the diagnosis showedMyxobolusspores partially covering the gill lamellae, as shown in Fig.5(a) and Fig.5(b).Fig.5(c)and (d) show theM.koispores measuring 10.4–11.5 μm (n = 3) using 5000 × magnification.Using morphometric and morphologic methods revealed that the shape ofM.koiin this study resembled the shape ofM.koiin the gill lamella ofCyprinus carpio(Kato et al., 2017).Yokoyama et al.(2016) conducted a fluorescence staining study of myxosporean parasites using a double vital stain test with Hoechst 33,342 and PI.The myxosporean often caused a mixed staining pattern on the nucleus,showing blue and red fluorescence, causing difficulty in assessing spore viability.A single myxosporean forms an elongated ellipse in front view,a narrow fusiform in lateral view, and is pyriform.However, the spores found were 15.6–19.7 (17.3 ± 1.0) μm length, 5.7–9.3 (6.7 ± 0.7) μm width, and 4.4–5.7 μm thickness (5.0 ± 0.26) (n = 50) with fine spore valves, and symmetrical with thin straight lines.The three-dimensional structure of myxozoan parasites and the tissue damage caused disintegration and erosion of cartilage tissue.The damage can be observed using SEM (Abraham et al., 2015; Liu et al., 2016; Saha & Bandyopadhyay, 2017).
3.4. In-vivo test results
3.4.1.Gill tissue histology
Based on observations using a light microscope, there was a difference in the gill tissue of healthy fish,Myxobolusinfected fish, and treated fish.Healthy fish had fewer lesions than infected fish and treated fish.The appearance of lesions in healthy fish may be due to stress and difficulty adapting to the new environment.Observations were made on 3 parts of the tissue in the observed gill organ samples based on the 5 visible fields of view.Changes in the gill tissue were more intense in the positive control.Gill tissue inMyxobolusinfected fish with and without treatment are shown in Fig.6.Gill tissue from healthy fish (control -)shows few lesions from edema and congestion.The lesions on the gill tissue of untreatedMyxobolusinfected fish (control +) were edema,hyperplasia, lamellae fusion, and congestion.Histology changes in hypertrophy and hyperplasia on the gill filaments are similar to histology features found in treatment D (kutuklin) and treatment E (dimilin).
There are also some changes in the tissue structure similar to (Dar,Kaur, & Chisti, 2017), which found pathological changes inCirrhinus mrigala’s gills infected byMyxobolusspp.from Bangladesh.The pathological changes were hypertrophy and hyperplasia.There were inflammatory cells and an accumulation of blood cells at the base of the secondary gill lamellae.Some of these changes can interfere with gill function, thereby reducing respiratory capacity and ion exchange.Edema can causes cell swelling or excessive fluid buildup in the tissues.The accumulation of fluid causes cells to become irritated and swell.The degree of damage to the host usually depends on the intensity of the infection.The developing plasmodia press the surrounding tissue and can cause circulatory disorders (L¨ovy et al., 2018; Yanuhar et al.,2019b).In some areas, lamellae fusion occurs as a result of the proliferation of the epithelial filament (degeneration and mucus) (Frasca et al., 2018; Gupta & Kaur, 2020).
The damage assessment based on observations of the gill tissue for each treatment was shown in Table 3.This observation illustrates the effect of each treatment onMyxobolusinfected koi gills.The total damage found in treatments A to E were 1.7%, 45.8%, 12.2%, 23.9%,and 14.6%, respectively.Treatment B (control +) had a high score (severe damage) compared with other treatments due to severe damage to the infected fish.Compared to the control’s treatment, treatments C(probiotic administration), D (kutuklin administration), and E (dimilin administration) had mild to moderate category levels of damage.TheMyxobolusinfected koi treated with probiotics 0.55 mL/30 L showed lower damage than the fish treated with either kutuklin or dimilin.Therefore, probiotics can reduce pathogenic organisms in the digestive tract of fish due to their antagonistic activity at the colonization sites in the host’s intestine.Furthermore, probiotic usage may prevent and control disease, enhance fish immunity, and is environmentally friendly.
3.4.2.Immunomolecular response
The immune response observed from the activity of CD4 +, CD8 +,TNF-α and IFN-γ cells showed a different response from each treatment.Fig.7 showed the results of CD4+and CD8+responses as a control in healthy and infected fish, respectively.The gills of healthy fish andMyxobolusinfected fish treated with probiotics, kutuklin, or dimilin were assessed.The immune response explains the path of infection and the defense mechanism ofMyxobolusinfected fish.The value of the CD4+count profile in healthy fish (control -) was 17.58%, which was higher than the other treatments.However, the CD8+value in healthy fish was 4.4% and in infected fish was 4.25% lower than other treatments.Healthy koi andMyxobolusinfected fish (controls) showed a significant immune response of TNFα and IFNγ (Fig.8).
The TNFα profile measurements for healthy fish (control -) and infected fish (control +) were 11.82% and 4.72%, respectively.The IFNγ profile measurements for healthy fish (control -) and infected fish(control +) were 8.23% and 3.69%, respectively.Treatment with probiotics, kutuklin, or dimilin caused CD4+molecular immune response values of 4.88%, 1.93%, and 10.54%, respectively (see Fig.9).The CD8+profile measurement for treatments (C) probiotics, (D) kutuklin, and (E)dimilin were 13.98%, 5.1%, 16.86%, respectively even higher than healthy fish.Dimilin (E) treatment had the highest values for the CD4+and CD8+profiles.Probiotics (C), kutuklin (D), and dimilin (E) treatments ofMyxobolusinfected fish produced a molecular immune response in the form of TNF-α and IFN-γ, as shown in Fig.9.The TNF-α responses to probiotics (C), kutuklin (D), and dimilin (E) treatments were 15.57%, 29.26%, and 4.66%, respectively.The IFN-γ responses to probiotics (C), kutuklin (D), and dimilin (E) treatments were 8.23%,5.66%, and 0.65%, respectively.The dimilin (E) treatment had the lowest values for the TNF-α and IFN-γ profiles.
Analysis using one-way ANOVA show that all treatments results difference significant response on CD4+, CD8+, TNF-α, and IFN-γ with significancy of 95%.Analysis using the LSD test show that treatment of probiotic caused significantly different (sig.95%) on CD4+responses in healthy fish, while treatment of kutuklin and dimilin produced significantly different (sig.95%) on CD4+responses in both healthy and sick fish.Treatment of probiotic and dimilin resulted in significantly different (sig.95%) on CD8+responses in both sick and healthy fish,whereas treatment of kutuklin did not produce significantly different(sig.95%) on CD8+responses.Treatment of probiotic and kutuklin produced significantly different (sig.95%) on TNF-α responses in sick fish except for dimilin treatment.All treatments produced significantly different effects (sig.95%) on IFN-γ expression in both sick and healthy fish.The high CD4+profile value is similar to the condition of healthy fish.CD4+cells are lymphocytes that produce cytokines involved in regulating inflammation and the immune response to various types of pathogens (Takizawa et al., 2016).CD4 helper T cells recognize exogenous pathogens through interactions with Major Histocompatibility Complex (MHC) II molecules expressed on Antigen Presenting Cells(APC).Furthermore, protease breaks the antigen into smaller molecules.MHC I binds the molecules for presentation to the cell surface.The molecules turn into CD8+T cells (cytotoxic T cells) (Jung et al., 2020;Nakanishi et al., 2015; Soleto et al., 2019; Yanuhar et al., 2012).The fish cells expressing CD4-related genes are activated in the presence of pathogens and release cytokines against pathogens.CD4 response is higher because the number of lymphocytes is higher to attack pathogens.Piazzon et al.(2018) and Xing et al.(2020) also showed that the activation of CD4+T cells against parasitic infection increases helper T cells.CD4+cells may stimulate CD8+cells, which are crucial for cellular immunity against intracellular microorganisms.Therefore, fish possessing CD8+cells or a higher activation level have a higher chance of avoiding or clearing the infection.
In contrast to the previous results, the dimilin (E) treatment had the lowest values for the TNF-α and IFN-γ profiles.In general, there are three mechanisms of immune response to eliminate viral infections, through antibodies, cytotoxic mechanisms, and interferons.Increased expression of both TNF and IFN can occur due to biological stimuli such as viruses.However, the rapid reproduction of the virus makes the amount of interferon produced not comparable to fight the virus.The value of IFNγ was higher in myxobolus infected fish without treatment.Meanwhile,the concentration decreased after all treatment.The low production of IFN-γ indicated decreasing pathogen development.Fish possessing less response to social stimuli have reduced INF-γ expression (Kirsten et al.,2018).The TNF-α level increase during the inflammatory process and cellular response to phagocytosis, chemotaxis, and increased expression of proinflammatory cytokines.Several immune gene expression studies(Boltana et al., 2018; Huang et al., 2020; Mohammadian et al., 2018)show that increased levels of TNFα expression are associated with different probiotic treatments.At low levels, TNFα can inhibit the growth of the blood stage of the parasite by activating the cellular immune system and killing the parasite directly, but its activity is only weak.At the right level, TNFα will provide protection and healing.IFN-dependent leukocyte differentiation mechanisms can be activated by the inflammatory response from the innate response to infection.However, the inflammation causes tissue damage, as shown in previous histological responses, and sometimes develops into chronic inflammatory disease, affecting immune cell dynamics (Ashfaq, Soliman, Saleh, &El-Matbouli, 2019; Langevin et al., 2019).
3.4.3.Agglutination response test
Immunological techniques based on binding between specific antibodies and antigens have been widely used in the detection and serological characterization of several fish pathogens.Agglutination is a mechanism in the host system to prevent pathogens from entering the host cell.The antigen-antibody reaction is considered to be the basic immunodiagnostic method very specific and sensitive (Rani et al.,2021).This method is used to estimate protective pathogens or antibodies Agglutination occurs in wells where the antibodies are too dilute to neutralize the antigen.The antigen clotting is caused by administering fluids or serum to the microplate.The hemagglutination occurs at the highest dilution.However, agglutination occurs as the antibody value of fish.Parasite-specific antibodies inhibit agglutination, shown as red dots at the bottom of the well.
The highest concentration at which agglutination occurs is the antibody titer in the fish sample serum.Table 4 shows the agglutination titer dilution on the red blood cells of fish, the fish treated with probiotics had an endpoint titer of 1/16 dilution.The fish treated with kutuklin had a titer of 1/2 dilution, and the fish treated with dimilin had a titer of 1/2048 dilution.Table 5 shows the results of the agglutination titer dilution in fish blood plasma.The microplate showed deposition between theMyxobolusprotein antigen and samples from fish treated with probiotics, kutuklin, and dimilin until the end of dilution.Treatment with myxobolus protein antigen in normal plasma showed a titer of 1/2 dilution.Although antibody titers are not related to disease severity,titers tend to decrease in individuals who respond successfully to immunotherapy.No hemagglutination occurs in a negative control situation without antigens (parasites) and antibodies.Hemagglutination erythrocytes only occur in the presence of antigens.However, parasitespecific antibodies block the hemagglutinin of theMyxobolusparasite,deterring hemagglutination.In the control sample, the red blood cells did not bind together and sank to the bottom of the well that shown as a red dot in the center of the well.On the other hand, several antigens containing hemagglutinin cause agglutination of red blood cells,resulting in hemagglutination and the formation of a lattice structure that produces a red color throughout the well.The myxobolus protein antigen added to the well plate inhibits agglutination and is visible as a red dot at the bottom of the well (Kim et al., 2020).
4.Conclusion
Histopathology of myxobolus infected koi gill tissue from probiotic,kutuklin and dimilin treatments exhibited edema, hyperplasia, hypertrophy, lamellae fusion, and congestion.Based on the scoring analysis,probiotic treatment of myxobolus infected fish resulted in fish with less damage (12.2%) than those given other treatments.The degree of damage to the host and the fish immunological response generally depends on parasite infection intensity.The immune response observed from the activity of CD4+, CD8+, TNF-α and IFN-γ cells showed a different response from each treatment.The dimilin treatment increased the production of CD4+and CD8+, the kutuklin treatment increased TNFα production, and probiotic treatment increased IFN-γ production.Meanwhile, hemagglutination erythrocytes only occur in the presence of antigens.The myxobolus protein antigen added to the well plate inhibits agglutination and is visible as a red dot at the bottom of the well.Probiotic, kutuklin, and dimilin treatments had a positive influence on the koi immunomolecular response.However, excessive use of synthetic drugs is not recommended.Therefore, the balanced use of probiotics is highly recommended as an alternative, environmentally friendly treatment for disease prevention and control and for strengthening the immune system of myxobolus infected fish.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Declaration of competing interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors would like to express their gratitudes to the Institute for Research and Community Service, Universitas Brawijaya, Indonesia through the “Doktor Mengabdi 2018" [grant DIPA number: DIPA-042.01.2.400919, 2018].
 Aquaculture and Fisheries2023年5期
Aquaculture and Fisheries2023年5期
- Aquaculture and Fisheries的其它文章
- The effectiveness of light emitting diode (LED) lamps in the offshore purse seine fishery in Vietnam
- Effects of dietary phosphorus level on growth, body composition, liver histology and lipid metabolism of spotted seabass (Lateolabrax maculatus)reared in freshwater
- Effects of transport stress on immune response, physiological state, and WSSV concentration in the red swamp crayfish Procambarus clarkii
- Expression of gastrin and cholecystokinin B receptor in Lateolabrax maculatus
- The first draft genome assembly and data analysis of the Malaysian mahseer(Tor tambroides)
- The competitiveness of China’s seaweed products in the international market from 2002 to 2017
