Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
Soumyajyoti Ghosh, Subhasri Biswas, Sudipta Maitra
Molecular and Cellular Endocrinology Laboratory, Department of Zoology, Visva-Bharati University, Santiniketan, 731235, India
Keywords:
Luteinizing hormone (LH)
Ovulation
Growth factors
In flammatory cytokines
Prostaglandins
Nitric oxide
A B S T R A C T
Reproduction in bony fish, specifically in the female teleost, encompasses well-de fined stages, including growth and development, final oocyte maturation and finally ovulation – a pre-requisite for forming fertilizable female gametes. The morphological changes encountered during ovulatory response engage a multitude of endocrine,autocrine, and paracrine factors. These include gonadal steroids, growth factors, in flammatory cytokines,prostaglandins, leukotrienes and matrix metalloproteinases. In recent years, the active participation of prostaglandins and their receptors in LH-dependent ovulatory action has received much attention. Considerable evidence also supports the participation of nitric oxide/nitric oxide synthase (NO/NOS) unit in regulating meiotic maturation and ovulatory cascade. However, relatively less information is available on physiological relevance,the inter-relationship and the conjoined efforts of these molecular candidates to trigger ovulation in the fish ovary. The prime objective of the present article is to summarize the potential cross-talk between major molecular candidates downstream of LH action in the follicular layer. Besides, the intraovarian role of prostaglandin(PG) vis-`a-vis NO/NOS system on mammalian and teleost ovulatory response has been emphasised.
1.Introduction
To meet the ever-increasing demand for fish, the aquaculture industries have focused on optimizing the protocols to produce a larger number of eggs with higher efficiency and viability (Lubzens, Young,Bobe, & Cerd`a, 2010). However, the endocrine regulation and underlying molecular mechanisms for the production of high-quality eggs holds knowledge gaps. In fish and most of the vertebrates studied so far,neuropeptides released by hypothalamic neurons regulate ovarian development and function through the action of two major glycoprotein hormones (follicle-stimulating hormone; FSH and luteinizing hormone;LH). The journey of fish eggs encompasses the formation of primordial germ cells, their transformation into oogonia, and subsequently to primary oocytes with the onset of meiosis. At this stage, fish oocytes are arrested at first meiotic prophase, undergo massive growth (vitellogenesis) and accumulate nutritional reserves for future embryonic development (Jalabert, 2005). The binding of maturation inducing steroid (MIS) to its membrane progestin receptor α (mPRα) activates maturation promoting factor (MPF) leading to the withdrawal of prophase (G2) arrest and resumption of meiotic maturation to form fertilizable (haploid) female gametes (Nagahama & Yamashita, 2008).
Mature oocytes are liberated from their surrounding follicle cells to be fertilized through an intricate yet highly coordinated process termed ovulation. This physiological event serves as an indispensable itinerary for the perpetuation of any species. The preovulatory surge in LH signals an oocyte to activate the follicular compartment and synthesize diverse biologically active candidates to release the oocyte through mechanical contraction and retraction of the adjacent wall (Nagahama & Yamashita,2008). The morphological changes encountered during this phase engage a multitude of endocrines (gonadal steroids), locally acting autocrine and paracrine factors (EGFs, IGFs, TGF-β family), as well as immune modulators (in flammatory cytokines, chemokines, nitric oxide,prostaglandins, leukotrienes) facilitating ovulation (Baker & Van Der Kraak, 2019; Goetz et al., 1989; Takahashi et al., 2013; Takahashi et al.,2017). Although the role of maturational gonadotropin (LH) and sex steroids as major regulators of ovulatory response is well established,accumulating evidence from the recent past has demonstrated the involvement of non-steroidal factors to ensure the release of mature oocytes.
Accordingly, the present article aims to summarize the recent advances on the ovulatory response in mammalian and piscine models. The potential role of major molecular candidates underlying LH action in the follicular layer, more specifically the obligatory role of prostaglandins,to aid in teleost ovulatory response has been highlighted (Fig. 1).Additionally, the present overview emphasizes on further in-depth investigations to identify the unexplored molecular and physiological players participating in the formation of “fertilizable eggs” in fish models.
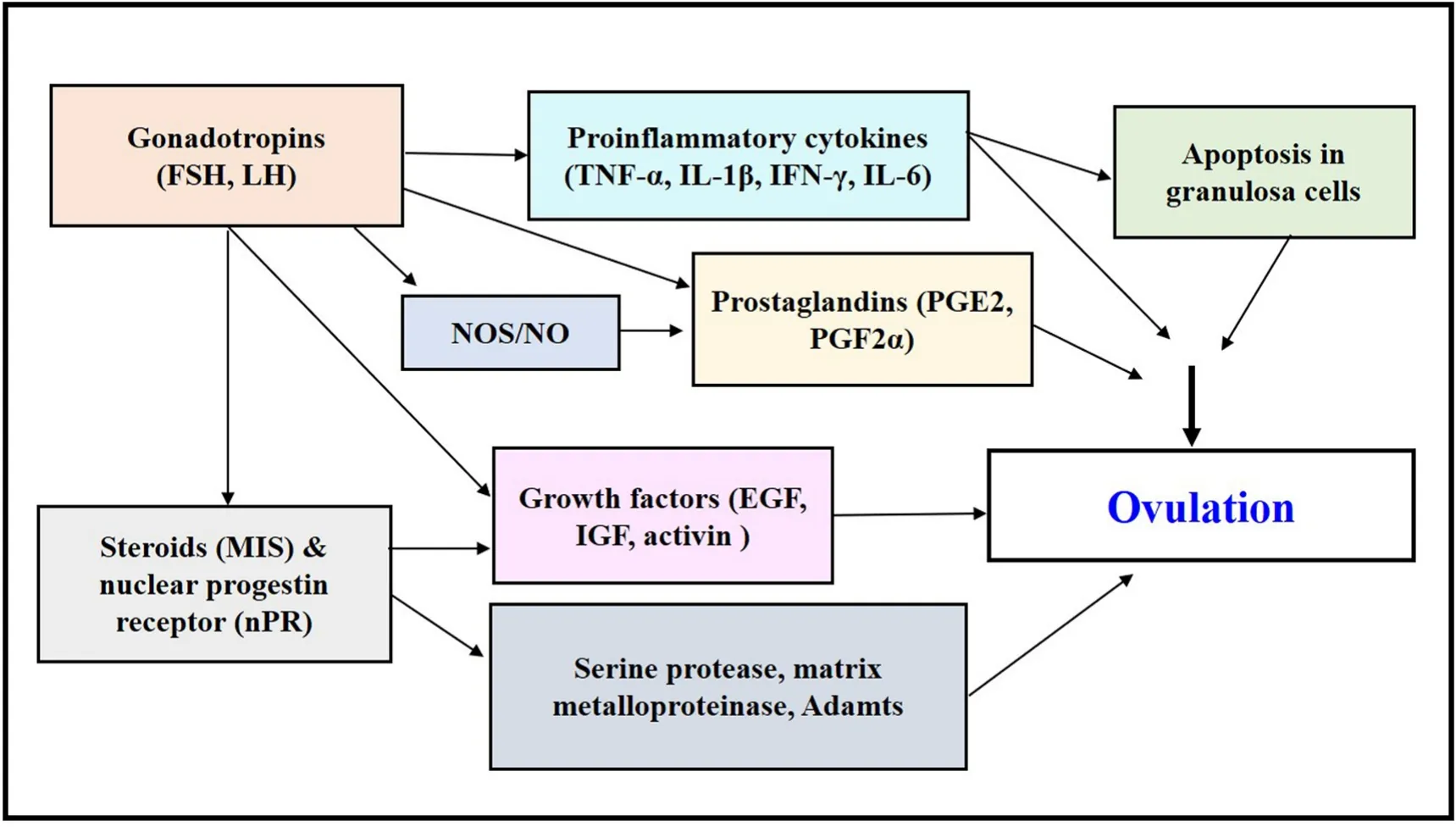
Fig. 1.Molecular candidates governing ovulatory response downstream to gonadotropin action. Participation of multiple ovarian factors including steroids (17,20β-P), growth factors (Egfs, Igfs, Activins), pro-in flammatory cytokines (TNF-α, IL-1β, IL-6), nitric oxide (NO), prostaglandins (PGE2, PGF2α), and matrix metalloproteinases (MMPs, Adamts) in regulation of follicular rupture and ovulation.
2.Maturation-inducing steroid (MIS): regulation of meiotic maturation and ovulation
2.1.MIS, non-genomic steroid action and oocyte maturation
In fish as in other vertebrates, diplotene arrested immature oocytes possess high cyclic-AMP, either from surrounding follicular layers through the gap junctions or by activation of Gαs-mediated adenylyl cyclase (AC) upon 17β-estradiol (E2) binding to G-protein estrogen receptor (GPER) on oocyte membrane (Das et al., 2017). In fish ovary at the end of the vitellogenic growth, LH binding to its cognate receptor LHCGR (luteinizing hormone/choriogonadotropin receptor) at the follicular cell layer activates a series of signalling events to stimulate biosynthesis of maturational steroid, more specifically, 17α,20β-dihydroxy-4-pregnen-3-one (17,20β-P; MIS) the most effective steroid in the resumption offinal oocyte maturation in most teleost species studied till date (Nagahama & Yamashita, 2008). Prior to meiotic maturation, a radical shift in follicular steroidogenesis downstream to LH action promotes 17,20β-P but attenuates E2 synthesis, potentially through decreased P450 aromatase expression concomitant with heightened 20β-hydroxysteroid dehydrogenase (20βHSD) transcription and activity(Senthilkumaran et al., 2004; Nakamura et al., 2005; Bobe et al., 2006).The binding of 17,20β-P to mPRα activates Gαi, thereby attenuating AC activity and cAMP synthesis and driving the oocyte towards final maturation (Zhu et al., 2003). MIS action at the oocyte surface promotes MPF activation, a hetero-dimer of cdc2 andde novosynthesized cyclin B,eventually leads to the breakdown of germinal vesicle (GVBD), first polar body exclusion and finally, the release of the fertilizable (haploid)germ cells, the hallmark of successful ovulation. However, MPF injection into immature oocytes of fish and amphibian triggers maturation with no effect on ovulation, indicating distinct regulation of the two physiological processes. (Nagahama & Yamashita, 2008).
2.2.Nuclear progestin receptor (nPR) and ovulatory response
In addition to meiotic maturation, MIS governs ovulation through its genomic action with nuclear progestin receptor (nPR) (Takahashi et al.,2013; Zhu et al., 2015). Studies in medaka post-vitellogenic follicles demonstrate that gonadotropin induces rapid and momentary expression of nPR in the granulosa cells, and its subsequent binding to 17,20β-P induces ovulation. The authors thus postulated that 17,20β-P is the key hormone required to induce maturation (via membrane receptors) and ovulation (via nuclear receptors) (Takahashi et al., 2013). Further, the functional relevance of nPR relies on the fact that nPR knockout induces anovulation in mice, rats, and zebra fish (Kubota et al., 2016; Lydon et al., 1995; Marvel et al., 2018; Tang et al., 2016; Zhu et al., 2015).Furthermore, oocytes from nPR knockout zebra fish undergo maturation but fails to liberate from the follicular layer just before ovulation (Liu et al., 2017; Tang et al., 2016; Zhu et al., 2015).
Notably, acting as a ligand-dependent transcription factor, LH-induced nPR expression in zebra fish is sensitive to cAMP and its regulatory partner, protein kinases (PKA) inhibition (Tang et al., 2016; Zhu et al., 2015). Mechanistically, in mammals, progestin binding leads to conformational changes in nPR, its release from chaperones, homodimerization and docking to specific progesterone response elements in the target gene promoters (Cheung & Smith, 2000; Tsai & O’Malley,1994). Previously, a series of ovulatory genes including cytosolic phospholipase A2 (cpla2), prostaglandin endoperoxide synthase 2(ptgs2) or cyclooxygenase 2 (cox2), prostaglandin receptor (ptger4b),matrix metalloproteinases (adamts8/9, mmp9) and protease regulators(timp2a) have been recognized as putative targets of nPR in zebra fish(Liang et al., 2015; Liu et al., 2017, 2018, 2020). While MIS-induced metalloproteinaseadamts9gene expression undergoes downregulation in nPR knockout female zebra fish, reducedadamts9expression with impaired ovulation occurs in lhcgr-/-female zebra fish, indicating the potential role of LH in upregulating the expression of matrix metalloproteinases (Liu et al., 2020). Collectively, the molecular mechanisms governing nPR regulation of ovulation have been one of the most extensively investigated research areas in recent years.
3.Role of ovarian growth factors
Studies from several mammalian and non-mammalian models have identified a wide range of locally-acting, non-steroidal, autocrine and paracrine factors, such as the epidermal growth factors (EGFs), insulinlike growth factors (IGFs), and members of the transforming growth factor-β (TGF-β) superfamily (Table 1) in regulating ovulatory response(Ahmad et al., 2020; Chang & Leung, 2018; Shimada et al., 2016). The present section elaborates on the ovarian growth factors and their potential involvement in the process of ovulation.
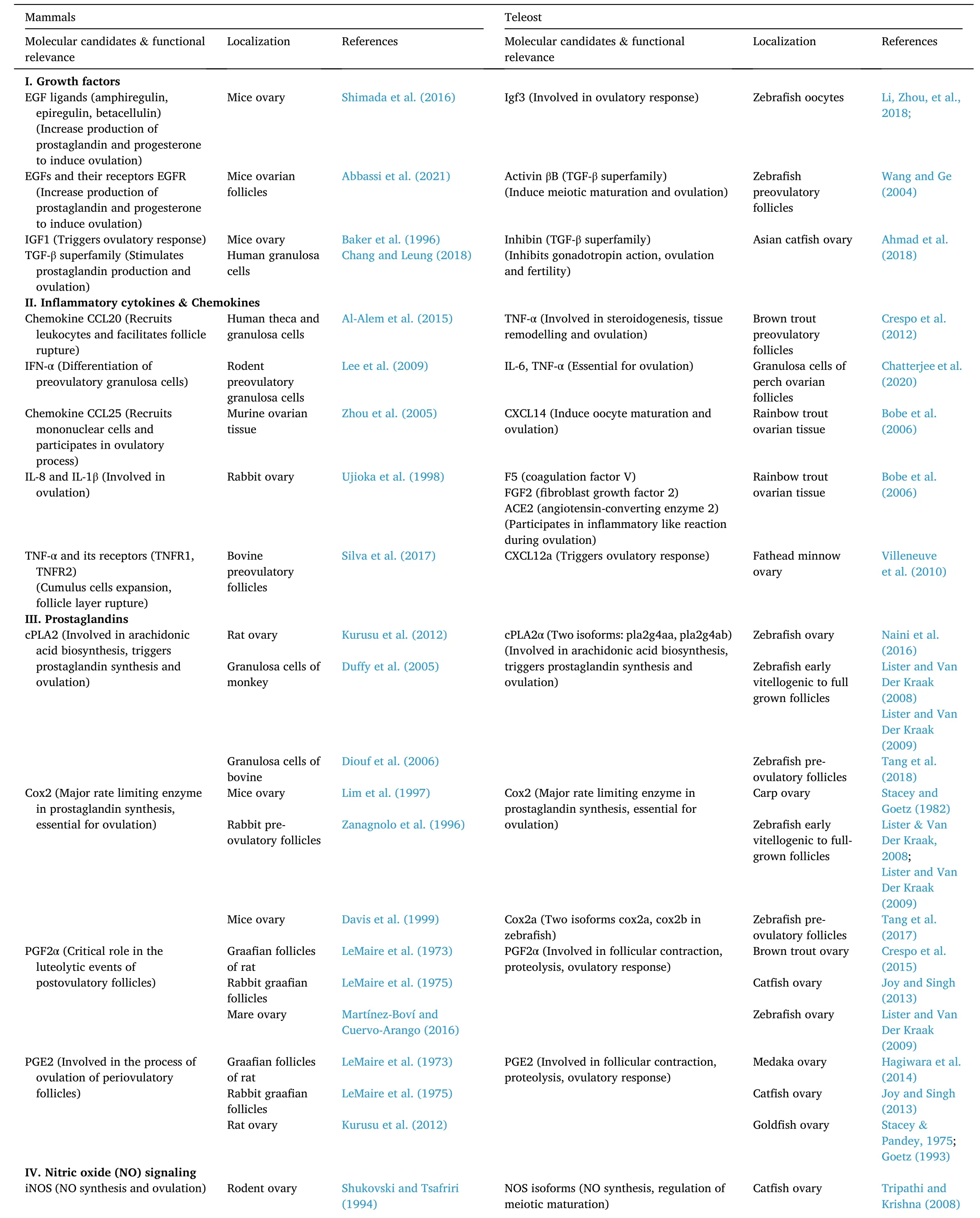
Table 1Intraovarian factors involved in ovulatory response.
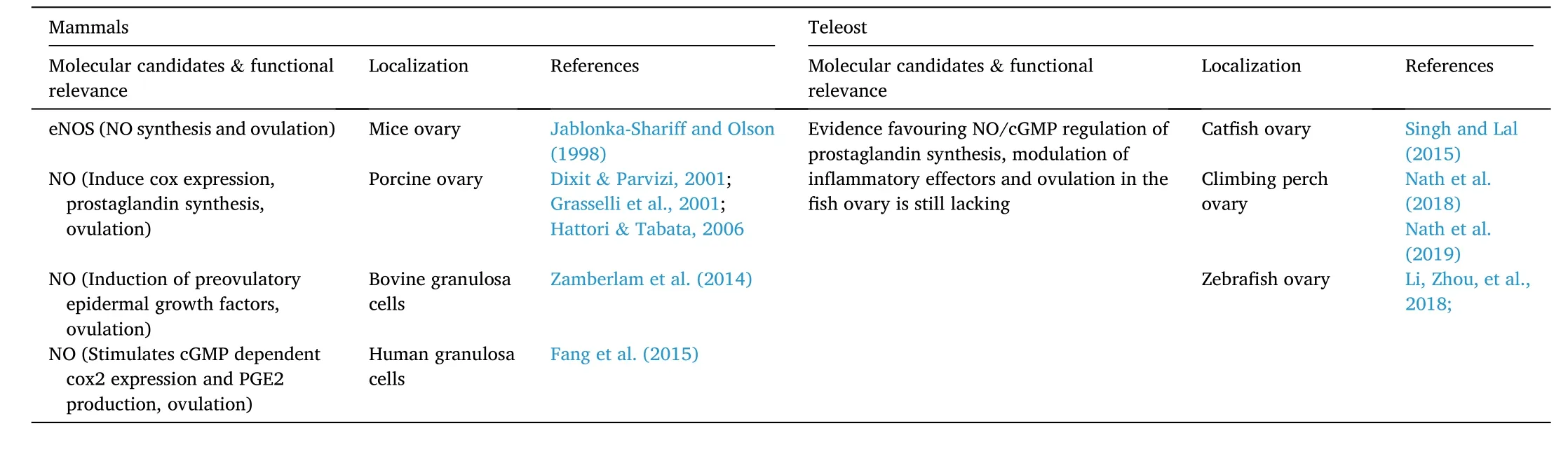
Table 1 (continued)
3.1.Epidermal growth factors (EGFs)
The EGF family ligands including EGF-like factor, amphiregulin(AREG), epiregulin (EREG), and betacellulin (BTC), are integral membrane proteins that undergo a proteolytic process to get released from the murine granulosa cell surface after LH surge (Harris et al., 2003;Shimada et al., 2016). A recent study identified these local intra-ovarian factors as the inducers of the ovulation process in mice, wherein they serve to transfer the stimuli of LH surge from granulosa cells to cumulus cells (Shimada et al., 2016). Signal transduction through EGF receptor(EGFR) stimulates the cumulus cells to activate ERK1/2 and PKC pathways, and their pharmacological inhibition results in suppressed cumulus cell expansion, oocyte maturation, and eventually ovulation and release of fertilizable oocytes (Shimada et al., 2016). A recent finding demonstrates that EGFR-mediated signalling uncouples mouse germ cells from the somatic follicular compartment through a massive and synchronized filopodial retraction at ovulation (Abbassi et al.,2021). In contrast to mammals, participation of EGF signalling in the regulation of fish ovulation has not been reported requiring further investigation in future.
3.2.Insulin-like growth factors (IGFs)
The role of ovarian IGFs in ovarian development and folliculogenesis has been recognized decades back. Any interference with the IGF signaling cascade has been proved detrimental in anovulation and infertility in women (Yoshimura, 1998). The first hint for the physiological relevance of IGF as a potent regulator of ovulation is based on the finding that female mice mutant for IGF1 is infertile and fails to ovulate even after gonadotropin administration (Baker et al., 1996). Although limited, growing research in non-mammalian vertebrates, more specifically teleosts (rainbow trout), demonstrate that the participation of IGFs and IGFBPs (IGF binding proteins) is essential to attain maturational competence in oocytes (Kamangar et al., 2006). Moreover, studies in zebra fish full-grown oocytes show that IGF1 synergizes with MIS to overcome the E2-induced inhibition of oocyte maturation (Das et al.,2016). Although the potential role of IGF family in ovulation remains largely unexplored, recent efforts have identified a novel gonad-specific Igf in teleost (Igf3) to play a crucial role in LH-mediated ovulation in zebra fish oocytes (Li, Niu, & Cheng, 2018). Further studies are warranted to unravel the role of this particular growth factor axis in the process of ovulation.
3.3.Transforming growth factor-β (TGF-β) superfamily
As a member of TGF-β superfamily, activins can in fluence germ cell development, ovarian follicular development, post-menstrual endometrial repair, decidualization, and pregnancy maintenance in humans(Chang & Leung, 2018). Although no direct evidence proves the involvement of the TGF-β superfamily members in the regulatory process underlying ovulation, few studies have shown that in human granulosa cells, activin A could elevate COX2 (cyclooxygenase 2)expression simultaneous to the synthesis of PGE2 (prostaglandin E2), an immune modulator governing ovulation (Chang & Leung, 2018).Interestingly, the zebra fish ovary reveals differential expression of activin subunits and follistatin; the early stage of oocyte maturation shows increased activin βA and follistatin; however, activin βB expression surges dramatically during the final phase of meiotic maturation leading to GVBD and ovulation (Wang & Ge, 2004). These observations may indicate a plausible role of activin βB subtype in the zebra fish ovulatory response. Conversely, another group of TGF-β superfamily,inhibin, can prevent gonadotropin (FSH) synthesis in fish (Ahmad et al.,2020). Interestingly, blocking the action of inhibin with antibodies increased the number of spawned eggs with improved fertility in Asian cat fish (Ahmad et al., 2018). Nonetheless, elaborative research initiative is still required to unravel the functional relevance of local growth factors in piscine ovulation.
4.In flammatory cytokines and ovulatory response
The follicular theca/granulosa layer synergizes with the resident and in filtrating immune cells in the ovary to stimulate chemokines, cytokines, and prostaglandins, the primary in flammatory mediators of ovulatory response (Carlock et al., 2014; Duffy et al., 2018). While chemokines or chemotactic cytokines act as a chemoattractant for leukocytes, the pro-in flammatory cytokines, such as tumour necrosis factors (TNF), interleukins (IL), and interferons (IFN), regulate the leukocyte in filtration through elevated ICAM-1 and E-selectin like cell adhesion molecules in the ovarian endothelial cells (Goetz & Garczynski, 1997; Richards et al., 2002). This section reviews the different chemokines and inflammatory cytokines involved in ovulatory response providing a comparative account between mammalian and teleost ovary(Table 1).
4.1.Evidence from mammalian studies
In human ovarian endothelial cells or macrophages, TNF-α and IL-1β can modulate the expression of matrix metalloproteinases (MMPs)(Saren et al., 1996; Yang et al., 2004). Additionally, treatment with TNF-α induces the expression of different MMPs in pig corpus luteum(Pitzel et al., 2000); administration of TNF-α antiserum in sheep attenuates MMPs expression as well as ovulatory process (Gottsch et al.,2000), suggesting their potential role in follicular layer rupture. Apart from regulating MMPs, pro-in flammatory cytokines can regulate ovulation by modulating caspase-dependent apoptosis in porcine, murine and bovine ovarian follicles (Lei et al., 2016; Silva et al., 2014;Yamamoto et al., 2015). Substantial evidence from rat ovary indicates possible involvement of TNF-α-dependent caspase activation in preovulatory follicles (Mendoza-Rodriguez et al., 2003).
Numerous studies have shown LH induction of pro-in flammatory cytokines during periovulatory stages in the mammalian ovary.Reportedly, hCG induction of chemokine CCL20 in human theca and granulosa cells facilitates recruitment of leukocytes and elevates synthesis of pro-in flammatory cytokines to assist in follicle layer rupture(Al-Alem et al., 2015). Moreover, increased abundance of IFN-α and chemokine CCL25 levels immediately after gonadotropin administration has been documented in rodent preovulatory granulosa cells (Lee et al.,2009) and murine ovarian tissue (Zhou et al., 2005). CCL25 is involved in macrophage differentiation and is chemotactic for macrophages indicating it may be involved in recruiting macrophages to the ovary to produce more cytokines, including TNF-α. Interestingly, hCG injection significantly heightens ovarian IL-8 and IL-1β expression leading to ovulation in female rabbit (Ujioka et al., 1998). Further,in vivotreatment with gonadotropin-releasing hormone (GnRH) increases the transcript abundance oftnfαand its receptors (tnfr1,tnfr2)in porcine preovulatory follicles (Silva et al., 2017). Notably, the dynamic expression of these cytokine genes over the periovulatory period indicate that they may have vital biological functions in ovulation.
4.2.In flammatory network in the teleost ovary
Compared to mammals, research on the role of the immune effectors during teleost ovulation is minimal and is mainly limited to identifying the immune function genes within ovarian tissue. While TNF-α and related genes (receptors of TNF-α gene, TAPI-1; an inhibitor of TNF-converting enzyme) have been identified in the ovaries of the zebrafish, brook trout, rainbow trout, brown trout, and blue fin tuna, interleukin family members, including IL-1β and IL-6, have been characterized in rainbow trout ovarian tissue (Husain et al., 2012; Iliev et al., 2007). Besides, interferons (Type 1 and IFN-γ) have been identified in channel cat fish and rainbow trout ovary (Chaves-Pozo et al.,2010; Long et al., 2004). TNF-α in fluences the onset of ovulation by prompting 17,20β-P production and GVBD in brown trout preovulatory follicles and executes vital role in steroidogenesis, tissue remodelling and ovulation (Crespo et al., 2012). Reportedly, TNF-α and PGF2α initiate a similar response like LH to induce follicle contraction, proteolysis and ovulation in the brown trout ovary. Further, inhibition of TNF-α could prevent LH-induced PG, a potent inducer of ovulatory response, synthesis in this species (Crespo et al., 2015). Interestingly,PGE2 has a stimulatory effect on TNF-α expression in the ovary of Atlantic salmon (Kadowaki et al., 2009). Furthermore, gonadotropin induced IL-6 production in the granulosa cells, in a manner sensitive to TNF-α synthesis, is reportedly essential for ovulatory response in perch oocytes (Chatterjee et al., 2020). Nonetheless, further studies are required to investigate the interaction between LH, PGs, MIS, and pro-in flammatory cytokines in periovulatory follicles; and identify the signalling pathways of these cytokines to enrich our knowledge on the physiology of ovulation in teleost.
5.Prostaglandins and their role in ovulatory response
First identified in human seminal fluid by Ulf von Euler (1935),prostaglandins (PGs) were shown to participate in various reproductive processes, including ovulation, luteal function, implantation and gestation, parturition and resumption of postpartum ovarian cyclicity(Goetz et al., 1989; Takahashi et al., 2017).
The biosynthesis of PGs is catalysed by the concerted action of a series of enzymes. The reaction initiates with phospholipase A2 (PLA2),promoting the breakdown of AA from membrane phospholipids. Among multiple isoforms of PLA2, cytosolic PLA2 (cPLA2) contributes most significantly to AA production in mammalian and non-mammalian vertebrates (Kudo & Murakami, 2002; Murakami et al., 2002). The AA is metabolized to prostaglandin G2 (PGG2) followed by prostaglandin H2 (PGH2), primarily due to the cyclooxygenase activity of PTGS (PG endoperoxide synthase) or COX (cyclooxygenases) enzymes (Vane et al.,1998). PGH2 serves as the precursor for PGE2 (prostaglandin E2) and PGF2α (prostaglandin F2α), formed through the conversion via their respective PG synthases (PGE2 synthase and PGF2α synthase, respectively) (Murakami et al., 2002; Watanabe, 2002). The upcoming sections will discuss the functional relevance of PGs and the enzymes synthesizing them in the light of ovulatory response (Table 1).
5.1.Cytosolic phospholipase A2 (cPLA2)
Based on structural homologies and requirement of Ca2+ions for its catalytic activity, the phospholipase A2 (PLA2) group of enzymes have been divided into five subfamilies (Kudo & Murakami, 2002; Murakami et al., 2002). The detection of major isoforms of cPLA2 in the mammalian ovary and their regulation by gonadotropin prior to ovulation has been an active area of research (Kurusu et al., 2012). Injection with arachidonyl tri fluoromethyl ketone (ATK, AACOCF3), a specific cPLA2 inhibitor, attenuates basal ovulation and total PGE2 synthesis in rat ovary (Kurusu et al., 2012). hCG induces the expression and activity levels of cPLA2 in granulosa cells of monkey and bovine periovulatory follicles (Diouf et al., 2006; Duffy et al., 2005). Importantly, equine chorionic gonadotropin (eCG)/hCG-induced ovarian PGE2 production undergoes attenuation incPLA2-/- (double knockout)but not wild-type mice leading to reduced ovulation and fertilization(Kurusu et al., 2012).
Concerning the participation of the cPLA2 enzyme in gonadotropin induced teleost ovulation, two isoforms of thecpla2α (pla2g4aaandpla2g4ab)gene have been detected in zebra fish in recent years (Naini et al., 2016). In this species, the expression ofcpla2gradually increases from early vitellogenic to full-grown follicles and regulates ovarian PG biosynthesis to stimulate oocyte maturation and ovulation (Lister & Van Der Kraak, 2008; Lister & Van Der Kraak, 2009). Whereas, a recent report demonstrating hCG-mediated cAMP-PKA upregulation ofcpla2expression in zebra fish preovulatory follicles (Tang et al., 2018) indicates cPLA2 as a mediator of LH-dependent piscine ovulatory response.
5.2.PG endoperoxide synthase (PTGS) or cyclooxygenases (COX)
PTGS or COX, the primary enzymes in eicosanoid synthesis, are homo-dimeric in nature. Principally, PTGS undertakes two major reactions, a cyclooxygenase activity, wherein it converts AA into an unstable product PGG2, and a peroxidase reaction transforms PGG2 to PGH2 (immediate precursor of PGs) (Vane et al., 1998). Two major isoforms have been identified in humans with nearly 61% sequence identity, COX1 (70 kDa) and COX2 (72 kDa) (Majed & Khalil, 2012).While theCOX1gene is constitutive,COX2gene expression is induced by several regulators, including MAPK and NF-kB. COX1 provides homeostatic functions including maintenance of gastric mucosa, aiding renal blood flow and blood clotting; whereas COX2 is primarily expressed in response to in flammatory stimuli. Nevertheless, the functional role of COX during pregnancy, parturition and foetal development is indispensable and has been investigated extensively in mammalian species. Although bothCOX1andCOX2genes have been detected in the mammalian ovary (DeWitt et al., 1989; Smith et al., 2000), the COX2 isoform controls the mammalian ovulatory clock (Richards, 1997).
Preovulatory LH surge stimulates prostaglandin synthesizing enzyme(PTGS2 or COX2), catalysing prostaglandins synthesis, PGE2 and PGF2α. PTGS2/COX2 (-/-) de ficient female mice show infertility and anovulation even after exogenous stimulation with gonadotropins;however, PTGS1/COX1 null (-/-) mice exhibits no severe abnormalities except for impaired parturition (Lim et al., 1997). Pharmacological inhibition of COX using aspirin and naproxen blocks ovulation in rabbit preovulatory follicles (Zanagnolo et al., 1996), and PGE2 administration could retrieve ovulatory response in COX2 null mice (Davis et al., 1999).When administered at high doses, non-steroidal anti-in flammatory drugs (NSAIDs) delayed ovulation causing sterility in humans (Akil et al., 1996).
Unlike mammals, limited information is available from research conducted in the teleost ovary. Interestingly, the whole-genome duplication in teleosts culminated in additional copies for thecoxgene(Havird et al., 2008; Ishikawa & Herschman, 2007). Two isoforms ofcox1genes (ptgs1aandptgs1b) and onecox2gene (ptgs2) have been identified in Japanese medaka, green spotted puffer, and fugu (Fujimori et al., 2011; Takahashi et al., 2013), whereas onecox1gene (ptgs1) and two functionalcox2genes (ptgs2a, 2b) have been cloned from the zebra fish and rainbow trout genome (Grosser et al., 2002; Ishikawa et al., 2007; Pini et al., 2005). Like mammals, cox enzymes have been implicated in ovulatory response in teleost. While LH elevatesptgs2amRNA levels in zebra fish ovaries (Tang et al., 2017), a single intraperitoneal injection of indomethacin (nonselective COX inhibitor) could prevent ovulation inCyprinus carpio(Stacey & Goetz, 1982). Moreover,the steep rise incox2expression in spawning zebra fish corresponds well with hCG-induced elevatedcox2before ovulation, indicating potential involvement of cox2 downstream to LH action is conserved among species (Lister & Van Der Kraak, 2009).
5.3.Action of PGs: their receptors and role in ovulation
Using PGH2 as a common substrate, downstream isomerases and oxidoreductases synthesize specific PGs (PGD2, PGE2, PGF2α, PGI2 and TXA2) (Murakami et al., 2002; Watanabe, 2002). Though all cells can synthesize PGs, the terminal product formed depends on the tissue-specific isomerases that convert the endoperoxide intermediates into specific PGs with varied biological functions (Smith, 2006). Available reports from mammalian and sub-mammalian research have identified the essential role of PGE2 and PGF2α in ovulatory response. In mammals, a broad range of receptor subtypes (EP1-4; where EP stands for Prostaglandin E2receptor or PTGER) can bind PGE2 to initiate downstream signalling events (Fujino et al., 2003; Hizaki et al., 1999).PGE2 binding to its receptor EP2 upregulates several genes essential for ovulation, includingAreg,Ereg,Btc,hyaluronan synthase 2 (Has2), and TNF-α-induced protein 6 (Tnfaip6) in the granulosa compartment of human ovarian follicles (Ben-Ami et al., 2006; Liu et al., 2010). Studies on PGE2 receptor type2 (EP2)-de ficient mice showed compromised ovulation, fertilization, implantation, and embryo development (Chakraborty et al., 1996), indicating the importance of PGE2 and its receptors in the ovulatory response. Interestingly, PGs also regulate female sexual behaviour in rats, hamsters, guinea pigs, and amphibians. In rat models,PGE2 stimulates LHRH release from the hypothalamus, and COX inhibition attenuates PGE2 synthesis and LHRH release (Ojeda et al., 1979).
Comparatively, the participation of PGs and its receptor in fish ovulation has long been unknown and relatively less investigated.Emerging evidence provides an insight into the role of PGE2 binding to its cognate receptor, Ptger4b, in the follicular layer rupture and ovulation in piscine models (Fig. 2). Severalin vitroexperiments on yellow perch and Atlantic croaker have established that pharmacological inhibition of COX can block MIS-stimulated ovulation; whereas exogenous PGF2α induces this event (Bradley & Goetz, 1994; Pati?o et al., 2003). In cat fish ovaries, administration of PGF2α and PGE2 has been shown to induce ovulation earlier (Singh & Singh, 1976). Prior to ovulation, PGs are synthesized by brook trout ovaries, which further reference PG involvement in ovulation (Goetz & Cetta, 1983; Goetz & Garczynski,1997). Although the active participation of PGE2 and PGF2α has received much attention (Goetz & Cetta, 1983; Goetz et al., 1989; Goetz& Garczynski, 1997), limited reports in the literature supports the involvement of other isoforms of PGs in teleost ovulation, requiring detailed investigations in future.
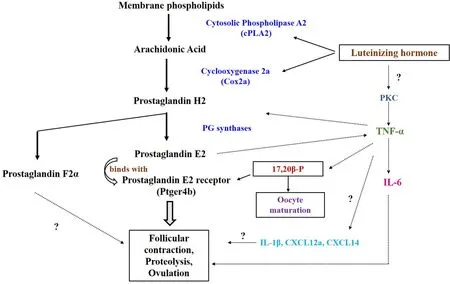
Fig. 2.Inter-relationship between LH-induced pro-in flammatory cytokines and PG synthesis in teleost ovulatory response. LH stimulates prostaglandin synthesis by modulating cytosolic phospholipase A2 and cyclooxygenase 2 expression, which modulates TNF-α expression. In comparison, LH induction of IL-6 production in the follicular cells might result from PKC activation concomitant with elevated TNF-α production. LH-induced 17,20β-P production, prostaglandin synthesis and oocyte maturation eventually lead to follicular contraction, proteolysis and ovulation by the possible action of IL-1β, IL-6 and chemokines (CXCL12a).
To date, six isoforms of prostaglandin receptor genes (ptger 1a/1b/2a/4a/4b/fr)have been detected in the zebra fish ovary. Elevated expression ofptger4buponin vivotreatment with ovaprim (a potent ovulating agent) indicates potential involvement of ptger4b in mediating LH induction of ovulation in this species (Baker & Van Der Kraak,2019). Additionally, recent findings demonstrate LH-mediated upregulation ofptger4breceptor wherein its inhibition can lead to anovulation in the zebra fish ovary (Tang et al., 2016). A most recent study using zebra fish full-grown follicles shows 17,20β-P stimulatesptger4band cPLA2 mRNA and protein expression, a key enzyme in PG biosynthesis(Baker et al., 2021). Congruently, expression ofptger4bmRNA increases upon LH stimulation in an nPR-dependent pathway in medaka ovary(Hagiwara et al., 2014; Tang et al., 2016), and antagonism of the ptger4b receptor in preovulatory folliclesin vitroleads to anovulation in this species (Fujimori et al., 2012).
6.Leukotrienes and its participation in ovulatory response
Like PGs, leukotrienes (LTs) are synthesized from the same precursor AA and are primarily associated with in flammatory and immune functions. From AA, the synthesis of LT is catalysed by the enzyme 5-lipoxygenase (5-LOX) and its co-activator, 5-lipoxygenase-activating protein (FLAP) (McCracken, 2005, pp. 93–111). Several LT isoforms(LTB4, LTC4, LTD4) are generated from the precursor of LT biosynthesis,LTA4, which upon synthesis participate in the in flammatory response by regulating the chemotaxis of leukocytes and neutrophil adherence to endothelial cells (Samuelsson et al., 1987). Whereas, evidences from mammalian models suggest potential involvement of different LT isoforms in mammalian ovulatory response (Priddy & Killick, 1993). Leukocytes, residing in the theca layer of the mammalian ovary, degrades the extracellular matrix by actively producing proteolytic enzymes and ultimately leading to follicle maturation and ovulation (Oakley et al.,2011). In rodents, the level of LTB4 and LTC4 starts to surge during the ovulatory phase, and LTB4 receptor inhibitors can block ovulation(Matousek et al., 2001). Interestingly, female rats and pigs fail to ovulate upon blockade of ovarian 5-LOX pathway using a specific 5-LOX inhibitor, nordihydroguaiaretic acid (NDGA) (Downey et al., 1998; Kurusu et al., 2009). Ovarian follicles of Atlantic croaker and yellow perch treated with inhibitors of 5-LOX enzymein vitroshowed delayed ovulation suggesting its active participation in teleost reproduction(Berndtson et al., 1989; Patino et al., 2003). Furthermore, recent evidence demonstrates that LOX metabolites mediate the 17,20β-P induced ovulatory response in zebra fish (Knight & Van Der Kraak, 2015).Arguably, the potential synergy between PGs and LTs in modulating teleost ovulation is still a less nurtured research topic, and in-depth investigations may provide valued insights into the role of these local mediators in ovulatory processes.
7.Nitric oxide: an emerging player in ovulatory response
A potent, short-lived signalling molecule, NO, regulates diverse physiological processes. Synthesis of NO under the physiological condition from L-arginine and molecular oxygen is catalysed by the enzyme nitric oxide synthase (NOS), an enzyme existing in three isoforms, e.g.,NOS1 (neuronal NOS; nNOS), NOS2 (inducible NOS; iNOS), NOS3(endothelial NOS; eNOS). While NOS1 and NOS3 are constitutive requiring calcium/calmodulin (Ca2+/CaM) for its activation, inducible isoform NOS2 is activated by in flammatory cytokines in a calciumindependent manner (Griffith & Stuehr, 1995; Morris & Billiar, 1994;Snyder, 1995). Although these NOS isoforms are distinct gene products sharing about 50–60% homology in their amino acid sequence (Nathan& Xie, 1994), they are believed to work as a homodimer after activation.
NO binding to its cognate receptor soluble guanylate cyclase (sGC)activates the enzyme resulting in a significant escalation in cGMP concentration and activation of a series of protein kinase (PKG) and phosphodiesterase (PDE). These kinases, in turn, regulate numerous physiological processes in different tissues, such as relaxation of vascular smooth muscle leading to vasodilation, vessel remodelling,coronary blood flow in the heart, natriuresis and renal protection in the kidney. Nonetheless, NO can modulate biological processes through cGMP-independent pathways (Lancaster et al., 1992).
7.1.NO modulation of meiotic maturation
NOS isoforms (NOS1, NOS2, and NOS3) are differentially expressed in the mammalian ovary (Basini & Grasselli, 2015; Tamanini et al.,2003). While attenuation of oocyte-specific phosphodiesterase 3 (PDE3)by cGMP culminating into cAMP build-up maintains meiotic arrest in mammalian oocytes (Conti et al., 2002; Jaffe & Egbert, 2017), the substantial contribution of NO/cGMP signalling in fish oocyte maturation is from recent past (Nath et al., 2019; Nath and Maitra, 2019). The three different NOS isoforms express at different stages of ovarian follicular growth during the annual reproductive cycle inHeteropneustes fossilisandClarias batrachus(Singh & Lal, 2015; Tripathi & Krishna,2008), pointing towards the relative importance of the NOS/NO grid in the piscine ovary. Heightened NO levels could abrogate meiotic maturation by triggering PKA activation and downregulating Mos-MAPK and cdc25 signalling events in perch oocytes (Nath et al., 2018). Further, the functional importance of NO-induced cGMP synthesis vis-`a-vis follicular cAMP level in the modulation of meiotic maturation is also evident(Nath et al., 2019). Recently, the existence of all three NOS isoforms along with four soluble sGC subtypes has been documented in zebra fish ovary wherein a dual regulatory nature for NO/sGC/cGMP pathway has been portrayed to activate or inhibit G2-M1 transition based on oocyte compartmentalization (Li, Zhou, et al., 2018). Reportedly, cGMP dependent protein kinase G (PKG), a serine/threonine-specific protein kinase activated by cGMP, autoregulates the cyclic nucleotide pool through the activation of specific PDEs (PDE5 and PDE9) and modulates oocyte maturation in zebra fish (Li & Bai, 2020; Li et al., 2020).
Notably, another route for enhancing cGMP pool in ovarian follicles is mediated by natriuretic peptide type C (NPPC), a peptide ligand derived from granulosa cells, which interacts with membrane-bound pGC, natriuretic peptide receptor 2 (NPR2) (Robinson et al., 2012;Vaccari et al., 2009). An LH surge or hCG treatment negatively influences this NPPC/NPR2 pathway to initiate resumption of prophase arrest in murine pre-ovulatory follicles (Conti et al., 2012; Kawamura et al., 2011). Recently, in zebra fish, the expression of NPPC and NPR2, at transcript and protein levels, has been detected such that exogenous NPPC-treatment elevates cGMP synthesis in follicle-enclosed oocytes(Pang & Thomas, 2018). To summarize, these findings indicate that the NOS/NO as well as NPPC/NPR2 induced cGMP downstream to LH signalling undertakes intercellular communication within the ovarian microenvironment to modulate phosphodiesterase and cyclic nucleotide-governed signalling cascadesper se.
7.2.Role of NO signalling in ovulatory processes
NO regulation of ovulatory response in mammalian models has been investigated extensively (Hattori & Tabata, 2006). Ovulation fails in rodent ovaries when iNOS activity is inhibited using pharmacological agents and restored through external administration of NO donors(Shukovski & Tsafriri, 1994). Gonadotropin-stimulated rabbit ovary shows halted ovulation upon administration of NOS inhibitor (Hesla et al., 1997; Yamauchi et al., 1997). Furthermore, participation of eNOS is evident from the reduced ovulatory response in hCG-induced eNOS knockout mice (Jablonka-Shariff & Olson, 1998). NO binding to the haem part of COX to regulate PG biosynthesis and ultimately ovulation in porcine ovarian follicles (Hattori & Tabata, 2006). While NOS2-derived NO stimulates the production of PGF2α in these follicles,its pharmacological inhibition prevents the biosynthesis of PGE2 and PGF2α, the key PGs (Dixit & Parvizi, 2001; Grasselli et al., 2001). Insight into bovine ovulation showed the positive in fluence of NO on COX2 synthesis, the primary enzyme responsible for PG biosynthesis (Zamberlam et al., 2014). While externally administered NO and cGMP(through cGMP dependent PKG) stimulates PGE2 synthesis by modulating COX2 expression in human granulosa cells (Fang et al., 2015),studies conducted on rodent ovulation suggest active participation of cGMP in modulating PG biosynthesis (Zor et al., 1977). Available literature from mammalian ovulation has dissected the specific role of cGMP in NOS/NO-mediated PG biosynthesis, while limited information is reported from the teleost ovary. Intriguingly, significant upregulation of ovariancox2corroborates well with the natural spawning of zebra fish in the morning hours (Lister & Van Der Kraak, 2009). Although the participation of PG and their receptors in the ovulation process of bony fish has been shown earlier, the contribution of NOS/NO on the ovulation response deserves a systematic study. Furthermore, this evidence prompts the researchers to investigate the synergy between NOS/NO cascade and PG signaling in modulating teleost ovulation.
8.Conclusion
The follicular layer possesses LH dependent synthetic ability to produce a cohort of molecular determinants ranging from MIS to PGs,in flammatory cytokines and local growth factors, aiding the process of ovulation and release of the mature egg (Fig. 3). Recent studies have paved the way for deciphering the dynamic processes underlying ovulation; however, several grey areas remain unanswered about the complex spatial and temporal expression of various molecular determinants on ovulation. Particular importance is required to understand the role of molecular determinants modulating gonadotropin dependent ovulation in teleost. Substantial evidence from the teleost ovary indicates that gonadotropin-dependent endocrine action (steroids, growth factors) can modulate preovulatory immune response(in flammatory cytokines, NOS/NO, PGs). Nonetheless, further investigation on the participation of endocrine as well as immune effectors is obligatory to explore the regulatory mechanisms of teleost ovulation.Detailed understanding of the ovulatory response will help expand aquaculture industries and enhance our knowledge on anovulation or contraceptive measures.
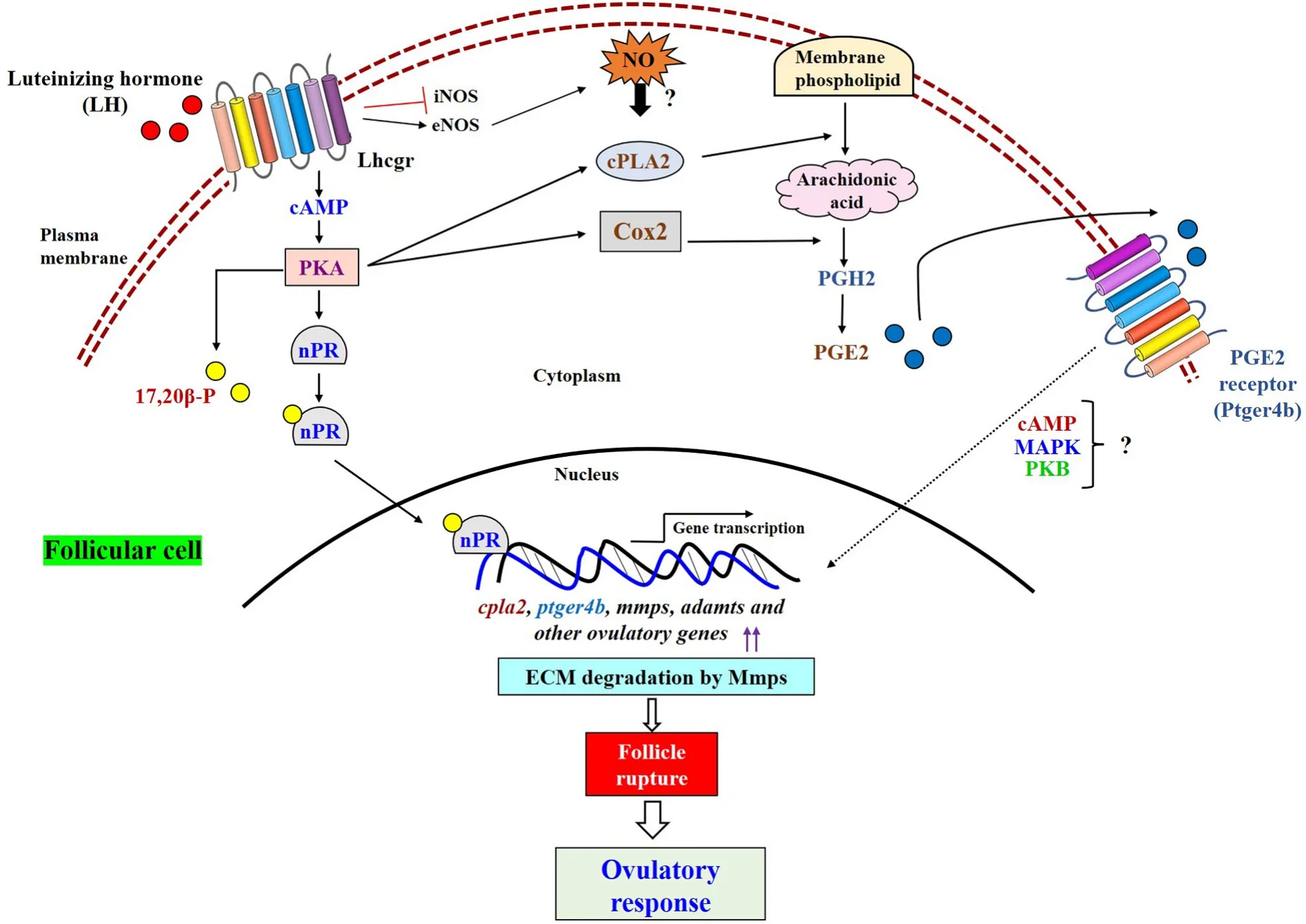
Fig. 3.Potential crosstalk between the molecular candidates involved in regulation of ovulatory response. LH mediated cAMP-PKA activates steroidogenic biosynthetic pathway (synthesis of 17,20β-P), nuclear progestin receptor (nPR) expression, and prostaglandin biosynthesis by modulating the expression of major rate limiting enzymes i.e., cPLA2, Cox2. Binding of 17,20β-P with nPR (acts as transcription factor) promotes several ovulatory gene (cpla2, ptger4b, mmps, adamts etc)transcription. On the other axis, elevated follicular PGs (mainly PGE2) couple with their cell surface receptors (Ptger4b) and triggers ovulation. Further, LH-mediated inhibition of iNOS expression and induction of eNOS expression elevates NO level in follicular cells, which might in fluence teleost ovulatory response, at least in part,and remains to be unveiled.
Declaration of competing interest
None.
Acknowledgements
The authors acknowledge Department of Biotechnology (Grant No.BT/PR28560/AAQ/3/919/2018), Department of Science and Technology under PURSE Program [Grant No. SR/PURSE Phase 2/42 (G)] to SM; University Grants Commission, New Delhi for Senior Research Fellowship (F. No. 16-9 (June 2018)/2019 (NET/CSIR), 15/04/2019) to SG; and DST, New Delhi through the award of INSPIRE Fellowship (NO:DST/INSPIRE/03/2015/005022) to SB. The authors are also thankful to Head, Department of Zoology, Visva-Bharati University, Santiniketan,India (grant number DST-FIST No. SR/FST/LS II-031/2013[C]) for providing infrastructural facilities.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Reproductive farming technology in Japanese eel and chub mackerel
- Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Understanding the impact of stress on teleostean reproduction
- Germ cell markers in fishes - A review
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
