Impact of xenoestrogens on sex differentiation and reproduction in teleosts
Brototi Roy, Reetuprn Bsk, Umesh Ri
aDepartment of Zoology, Maitreyi College, University of Delhi, Delhi, 110021, India
bDepartment of Zoology, Bhaskaracharya College of Applied Sciences, University of Delhi, Delhi, 110075, India
cDepartment of Zoology, University of Delhi, Delhi, 110007, India
Keywords:
Xenoestrogen
Teleost
Sex differentiation
Gonad
Intersex
Reproduction
A B S T R A C T
Among vertebrates, teleosts display a wide array of sex determination and differentiation mechanisms ranging from chromosomal sex determination on one end of the spectrum to environmental sex determination on the other end. However, the interplay of both these mechanisms is also not uncommon. Several gonochoristic fishes exhibit gonadal plasticity often resulting in sex reversal. The major manipulation of sex differentiation in teleost is affected by sex steroids. In this context, the increasing contamination of aquatic ecosystems by estrogen-like compounds, commonly known as xenoestrogens, is of major concern. This often leads to deleterious effects on the reproductive success of fish and thereby adversely impacts aquatic biodiversity. In the present review, we have focused on impact of xenoestrogen at different levels of the reproductive system in fluencing not only gonadal differentiation in teleosts but also their reproductive functions. The review would also explore the mitigation strategies and regulations in place for aquatic xenoestrogen management.
1.Introduction
Xenoestrogens of natural or synthetic origin resemble the female sex steroid 17β-estradiol (E2). They bind to estrogen receptors and initiate the downstream signaling pathway manifesting various physiological effects. Since xenoestrogens interfere with the activities of the natural hormones, they are termed endocrine disrupting chemicals (EDCs).According to Crisp et al. (1998), EDCs are de fined as chemicals that“interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the development, behavior, fertility and maintenance of homeostasis”. Due to various anthropogenic activities xenoestrogens ultimately end up in the aquatic ecosystem endangering all aquatic life forms, especially teleosts. Pharmaceuticals, personal care products, pesticides, food preservatives, UV filters, plasticizers, and coolants represent some of the different classes of potential synthetic xenoestrogens. In addition, there are natural xenoestrogens such as phytoestrogens obtained from legumes, soy, fruits and nuts, and mycoestrogens from fungus, mushroom,algae, and mosses (Fig. 1). They accumulate in the water bodies through run-off water and sewage and have the potential to be toxic at concentrations much lower than endogenous estrogen.
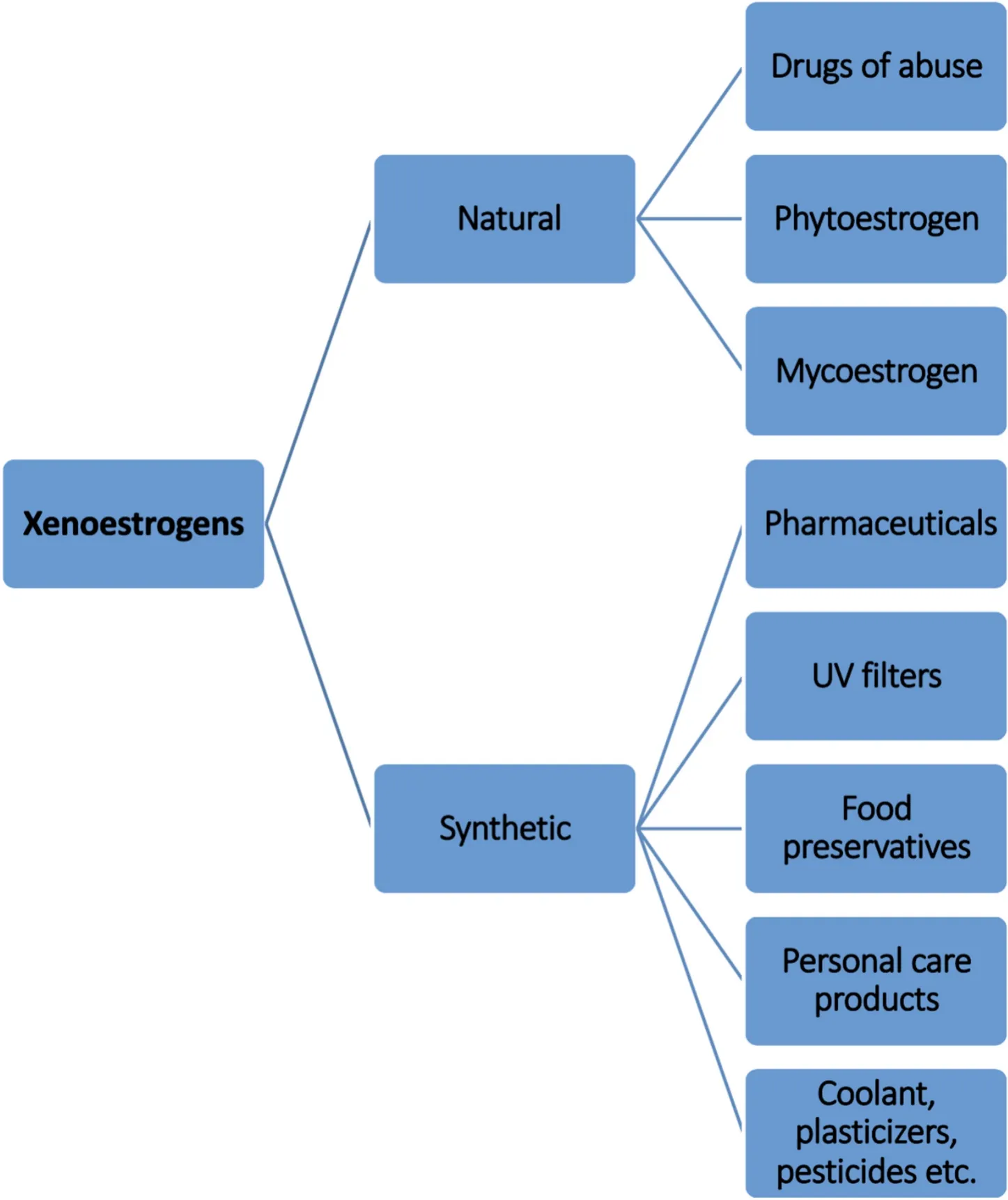
Fig. 1.Different classes of Xenoestrogens. Depending on their origin, xenoestrogens are broadly classified into natural and synthetic xenoestrogens.
2.Sources of aquatic xenoestrogens
The estrogens excreted by humans and other animals constitute the major load of aquatic xenoestrogen. The excreted estrogen is flushed down as wastewater from households, hospitals, and animal shelters and drains into the water bodies. Humans and livestock, especially in areas with concentrated livestock farming are considered as the primary source of estrogen in wastewater discharge (Combalbert &Hernandez-Raquet, 2010). It is estimated that around 4.4 kg/year/million inhabitants estrogen is excreted as urine and faeces. Xenoestrogens such as 17 α-ethinyl estradiol (EE2), mestranol and diethylstilbestrol(DES) present in oral contraceptives and other pharmaceuticals enter as excretory products into the sewage. The xenoestrogens present in sewage are not completely removed or inactivated even by advanced sewage treatment procedures such as nan da magnetic polyacrylic anion exchange resin (NDMP) adsorption, coagulation sedimentation, ozonation, and activated carbon-/electro-adsorption (Sun et al., 2017), and often end up in the water bodies. In fact, less than 10% of synthetic and natural estrogens are estimated to be removed by biodegradation process (Mastrup et al., 2001). Some of the xenoestrogens are further adsorbed to the sludge but majority remain in effluents. It is interesting to note that sewage treatment plants (STP) often increase the estrogenic nature of sewage following chemical modification. STP degrades alkylphenol ethoxylates into nonylphenol (NP) which is more toxic than its precursor (Bhandari et al., 2021; Renner, 1997). Moreover, deconjugation of natural estrogens excreted as conjugates of sulphates and glucuronide requires more time during biodegradation, thereby resulting in discharge of undegraded form of xenoestrogen in ef fluents (Liu et al., 2015). Not surprisingly, municipal sewage water is found to be estrogenic in many places such as Brazil, Canada, China, Germany,Israel, Italy, Japan, Spain, Sweden, Switzerland, Netherlands, the United Kingdom and United States of America (Adler et al., 2001; Caliman &Gavrilescu, 2009) including India (Kumar et al., 2016; Lalwani et al.,2020).
Agricultural activities also contribute largely to xenoestrogens in the aquatic environment. Many agrochemicals such as organochlorine pesticides and hydroxylated polychlorinated biphenyls (PCBs) are estrogenic and of special concern. These organic chemicals are lipophilic and highly stable, thus persistent in the aquatic environment for a very long time (Jayaraj et al., 2016). Most of these pesticides are phenols or may undergo bioactivation to form phenolic structures. Although some of these pesticides such as dichloro-diphenyl-trichloroethane (DDT) and hexachlorocyclohexane (HCHs) have been banned for more than three decades, it is still rampantly used in developing countries due to their low cost and wide spectrum pest killing ability. Faecal and urinary deposits produced during animal husbandry contribute to large amounts of steroidal hormones. The estrone (E1) and E2 concentrations in swine farrowing pits are found to be four times higher than in dairy waste(Raman et al., 2004). However, the manure-borne estrogens from livestock waste depend on the species, sex, age, hormonal status, among other traits of the animal (Hanselman et al., 2003).
Apart from agricultural xenoestrogen contribution, industrial chemicals such as alkylphenols including 4-nonylphenol (4-NP), 4-octylphenol (4-OP), and bisphenol-A (BPA) are added to aquatic ecosystems through ef fluent discharges of manufacturing plant, land fill leachates and sewage treatment plant (Sharma et al., 2009). Bisphenols are synthetic phenols used in the production of polycarbonate plastic products and epoxy resins. Polymers of BPA are used to coat overhead water tanks as well as water pipelines. These are of special concern as they are found in freshwater, sea water, sewage ef fluent and sludge (Liu et al., 2021).Due to increasing demand for BPA based products, the hazard of BPA has increased over the years. Interestingly, the strict regulations of BPA led to an increase in alternative substances such as bisphenol AF (BPAF),bisphenol F (BPF), bisphenol S (BPS) etc. which have similar physico-chemical properties (Vasiljevic & Harner, 2021). In fact, many studies across vertebrates have shown that these analogs including BPAF are more potent than BPA in disrupting estrogen signaling (Pinto et al.,2019; Yamaguchi et al., 2015)
3.Impact of xenoestrogens
3.1.Organizational vs activational effect
The original concept of organizational and activational effect of steroids was proposed by Phoenix et al. (1959) based on the effect of testosterone propionate on mating behavior in guinea pigs. This concept was extended to EDC action by Guillette et al. (1995) based on experiential evidence from other wildlife species including teleosts. The organizational effects are those that occur early in life and could be permanent, whereas activational effects occur in the adult stage and happen to be transient. Most of these studies related to xenoestrogens are activational types though study on organizational impact is equally important to develop a comprehensive understanding of xenoestrogenic effect on development, behavioral and physiological changes in teleost.In view of this, following sections are focussed to describe changes of organizational type such as sex determination and differentiation, and also activational type including gametogenesis, steroidogenesis and egg proteins synthesis in response to xenoestrogens in teleost.
3.2.Sex determination and differentiation
To perceive the impact of xenoestrogen on sex determination and differentiation, it is imperative to understand the mechanisms of gonadal differentiation in teleosts. The sex determination and differentiation in fish show a multitude of mechanisms ranging from chromosomal determination to environmental sex determination at the two ends of the spectrum. Before delving into sex determination and differentiation, it would be of interest to know that fishes are classified into gonochoristic, hermaphroditic, or unisexual based on their sexual characteristics. Gonochoristic fishes have separate male and female sexes and the sex remains stable after sex determination and differentiation. On the other hand, hermaphroditic fishes have gonads capable of producing male and female gametes together, or successively. These fishes exhibit gonadal plasticity and sex reversal. Unisexual fishes are distinct from gonochoristic and hermaphroditic as they exclusively comprise female populations. Among these, gonochoristic fishes are the most prevalent and exhibit environmental sex determination (ESD),genetic sex determination (GSD), and at times a combination of both the mechanism. In teleosts, GSD was first reported in medakaAplocheilus latipes(Aida, 1921) and thereafter in many gonochoristic species.Although male heterogamety (XX/XY) is primarily observed, female heterogamety (ZZ/ZW) is also occasionally seen. However, unlike mammals, sex chromosomes in fishes are homomorphic and cannot be distinguished.
Sex steroids, estradiol and androgens, play a crucial role in sex differentiation in teleosts and at times over ride the genetic sex determination. This was demonstrated long ago by Yamamoto and Matsuda(1963) in Japanese medaka wherein genetic sex of fishes was reversed by feeding estradiol- or androgen-supplemented diet. In view of this,Jafri and Ensor (1979) implicated the chemical contaminants to be responsible for reversal of secondary sexual characters and the appearance of intersex among roachesRutilus rutilus. Similarly, females with male gonopodia were reported in mosquito fish Gambusia affinis holbrooki(Howell et al., 1980). This is substantiated byin situobservations,andin vitroas well asin vivoexperiments using xenoestrogens. Exposures of juvenile fish to xenoestrogens affect the sexual differentiation leading to either feminizing of juveniles and skewing the sex ratio of the population or resulting in intersex. In case of sea lampreysPetromyzon marinus, exposure to 3-tri floromethyl- 4-nitrophenol (TFM) induced a female biased sex differentiation, thereby altered the sex ratio (Purvis,1979). Sex inversion occurred in a large population of XY embryo of Japanese medaka when eggs/juveniles were treated with EE2 or DDT(Edmunds et al., 2000; Scholz & Gutzeit, 2000). Similarly, newly hatched Japanese medaka when exposed to sublethal concentrations of BPA exhibited the absence of males. Moreover, histological examinations showed the presence of testis ova consisting of testicular cells as well as oocytes (Yokota et al., 2000). However, concentration of BPA used in this study was much higher than that observed in water sources. In case of zebra fishDanio rerio, exposure of embryos to 10 ng/l EE2 or 100 μg/L NP during sex differentiation leads to skewed sex ratio(Hill & Janz, 2003; ?rn et al., 2003) and at times 100% female population (Maack & Segner, 2004). In addition, long term exposure of zebra fish embryo to BPA analogue BPF (100 and 1000 μg/L) resulted in female sex ratio bias, and ovo-testis in male zebra fish contained spermatogonia of different stages along with primary oocytes (Yang et al.,2018). A recent study in zebra fish revealed that the feminizing effect of xenoestrogen was highly stage-specific and observed only when fishes were exposed during gonad differentiation stage (Liu et al., 2021).
Xenoestrogen exerts its effect on sex differentiation by altering the expression level of steroidal receptors and steroidogenic enzymes playing critical role in sex differentiation. In South American teleost pejerrey,Odontesthes bonariensis,concurrent with skewed sex ratio, EE2 was shown to downregulate expression of 11β-hydroxysteroid dehydrogenase type 2 (11β2-HSD) responsible for major male androgen 11-ketotestosterone (11-KT) synthesis and also androgen receptors (Arɑ and Arβ). At the same time, the expression of cytochrome P450 aromatasecyp19a1a, a key enzyme involved in estrogen biosynthesis, was signi ficantly enhanced (Pérez et al., 2012). Similar upregulation ofcyp19a1aexpression by 10 μg/L BPF (Yang et al., 2018) was also observed in zebra fish. Moreover, enhanced expression ofcyp19a1bin rohu,Labeo rohitaby 1 and 10 μg/L BPA (Gupta et al., 2018) andcyp 19A2(also referred tocyp19a1b) in zebra fish by nonylphenol (0.01–100 μM) and EE2 (1–100 nM) is also reported (Kazeto et al., 2004). It is worth mentioning that teleosts have two different genes namelycyp19a1aandcyp19a1bthat encode aromatase A and aromatase B, respectively.cyp19a1ais predominantly expressed in the ovaries and plays a crucial role in sex differentiation and oogenesis, whereascyp19a1bexpressed in neuronal tissues is associated with sexual behavior (Bjerselius et al.,2001). The presence of multiple transcription regulatory sites in the promoter ofcyp 19implicates their modulation with several classes of EDCs including xenoestrogen (Cheshenko et al., 2008).
In addition to in fluencing steroidogenesis and steroid receptor expression, xenoestrogens act directly on sex determining genes and thus in fluence sex differentiation in gonochoristic species. The gonadal soma-derived factor (gsdf) gene located on the Y chromosome in male Japanese medaka is an important testis differentiation gene and robustly expressed in testicular somatic cells. It is a member of the transforming growth factor beta subfamily and its overexpression in XX Japanese medaka leads to their testis development (Myosho et al., 2012) while mutation ofgsdfin XY Japanese medaka leads to formation of ovaries(Imai et al., 2015). The suppression ofgsdfexpression has been reported after chronic exposure of fish to BPA, even at low concentration (Horie et al., 2021). Another critical gene responsible for male sex differentiation is seen to be doublesex and mab-3 related transcription factor 1(dmrt1) as marked reduction in its expression has been noticed following treatment of XY larvae of rainbow troutOncorhynchus mykisswith EE2(Vizziano-Cantonnet et al., 2008). Besides, BPF treatment of larval zebra fish downregulates other critical testis differentiation genes such asamhandsox9aexpression (Yang et al., 2018). In case of XX embryos,forkhead box transcription factor L2 (foxl2)gene expressed in ovarian somatic cells (Ijiri et al., 2008) plays an important role in the differentiation of ovaries (Wang et al., 2007). In rainbow trout, strong upregulation offoxl2was observed when XY larvae were fed with 20 mg/kg of EE2 for 2 months (Vizziano-Cantonnet et al., 2008). Although, BPA(45–3406 μg/L) failed to affectfoxl2expression in the XY embryo of Japanese medaka (Horie et al., 2020), BPF (10–1000 μg/L) upregulatedfoxl2expression in zebra fish (Yang et al., 2018).
The pertinent question at this point is whether the effect of these xenoestrogens on gonadal differentiation is permanent or transient.Gimeno et al. (1998) has demonstrated that exposure of male common carpsCyprinus carpioto 4-tert-pentylphenol during the critical period of sex determination resulted in formation of oviduct and this effect was permanent as oviducts were retained even when the treatment was withdrawn. In contrast, a study in zebra fish showed that skewed sex ratio due to exposure to xenoestrogen such as EE2 was reversed during adulthood (Hill & Janz, 2003). Hence it appears that nature of effect,permanent or transient, depends on type of xenoestrogen, treatment regimen, stage of exposure and the species studied. Nonetheless, the exposure of teleosts to xenoestrogens, and at times to a mixture of xenoestrogens, in their natural habitat during sex differentiation and developmental stage directly affect several facets of reproduction or at times might exaggerate the effect of xenoestrogen on the adult stages that ultimately affects the reproductive success of the species. Many xenoestrogens which might not directly affect sex differentiation may have profound effect when present with other molecules or show their effect at later stages of development.
3.3.Gonadotropin-releasing hormone and gonadotropins
For a long time, research on effect of endocrine disruptors on fish reproduction was restricted to analysing and making sense of the visible effect of xenoestrogens on sex differentiation, and alterations in liver and gonad structure and functions. However, in the past decade, a significant number of studies have been performed in fishes to explore the effect of endocrine disruptors, especially xenoestrogens, on hypothalamus and pituitary (Celino-Brady et al., 2021; Le Page et al., 2011).Similar to other vertebrates, kisspeptin-gonadotropin-releasing hormone (Gnrh)- gonadotropins (follicle-stimulating hormone, Fsh and luteinizing hormone, Lh) axis is involved in regulation of gonadal functions, gametogenesis and steroidogenesis in fishes. Further, sex steroids regulate kisspeptin, Gnrh and gonadotropins production following negative and positive feedback mechanisms (Kah & Dufour,2010; Levavi-Sivan et al., 2010; Schulz et al., 2010; Zohar et al., 2010).In light of this, the impact of xenoestrogens on hypothalamo-hypophyseal-gonadal (HPG) axis needs to be envisioned.Exposure of zebra fish embryo to 100 μg/L of BPA and BPS for 25 h post fertilization is shown to increase the expression of kisspeptin gene and its receptor parallel to increase in expression ofgnrh(Qiu et al., 2016).Likewise, a significant increase in expression ofgnrhwas observed in BPAF-exposed adult male zebra fish, though expression remained unaltered in female fish (Shi et al., 2015). Also, exposure of zebra fish to bisphenol analogs and EE2 during developmental stages has shown prominent increase in population of Gnrh-secreting neurons (Qiu et al.,2016; Vosges et al., 2010). Similar observation ongnrhhas been made in sub-adult coho salmonOncorhynchus kisutchexposed to EE2 at a concentration of 12–15 ng/L for 46 days (Harding et al., 2013). On the contrary, exposure of juvenile rainbow trout to NP (0.1–10 μM) and female gold fishCarassius auratusto BPA (1–500 μg/L) significantly decreased the expression ofgnrh(Vetillard & Bailhache, 2006; Wang et al., 2019). The inconsistent effect of xenoestrogens has also been observed on expression of gonadotropins. In most of the female fishes,fshβsubunit expression reduced (Harding et al., 2013; Shirdel et al.,2020; Wang et al., 2019) whilelhβsubunit expression increased (Harding et al., 2013; Johns et al., 2011; Shirdel et al., 2020) under the effect of various xenoestrogens. However, in female zebra fish, exposure to different doses (30, 90 and 270 μg/L) of xenoestrogen fluorotelomer alcohols (FTOH) upregulated the expression of both,fshβandlhβsubunits (Liu et al., 2010). With regard to male fishes, a substantial number of data is not available to derive a conclusion. In NP-exposed Caspian brown troutSalmo trutta caspius, the expression offshβsubunit decreased but no change was observed in expression oflhβsubunit(Shirdel et al., 2020). In male gold fish, exposure to BPA had no effect on either expression offshβorlhβsubunits (Wang et al., 2019). However,male gold fish exposed to xenoestrogen di-(2-ethylhexyl) phthalate(DEHP) and male zebra fish treated with FTOH showed decrease in the circulating level of Lh (Golshan et al., 2015) and expression oflhβsubunit (Liu et al., 2010), respectively. Interestingly, BPAF exposure throughout developmental stages caused upregulation in expression of bothfshβorlhβsubunits in adult male zebra fish (Shi et al., 2015).
In addition, the effect of xenoestrogens has also been explored on expression of aromatase in the brain. This enzyme is known to catalyze the conversion of androgen to estrogen that in turn in fluences HPG axis as well as brain sexualization and sexual behavior (Diotel et al., 2010).Unequivocally, reports demonstrate stimulatory effect of xenoestrogens on expression and activity of aromatase in fishes (Cheshenko et al.,2008; Decourten et al., 2020; Hallgren & Olsen, 2009; Halm et al., 2002;Hinfray et al., 2006; Kortner et al., 2009; Kuhl & Brouwer, 2006; Le Page et al., 2006; Lee et al., 2006; Linderoth et al., 2006; Lyssimachou et al.,2006; Menuet et al., 2005). Altogether, it appears that xenoestrogen is capable of modulating the structure and function of hypothalamus and pituitary though the effect is seen to be xenoestrogen-specific, species-specific and at times, even sex-specific. More detailed and in-depth studies are required to understand how the modulations of this axis by xenoestrogen ultimately affects reproduction and the reproductive fitness of the species.
3.4.Gonad
3.4.1.Structural alterations
Fishes inhabiting water bodies contaminated with xenoestrogens have shown decrease in gonadal size and gonado-somatic index (Alan et al., 2008; Feist et al., 2005; Flammarion et al., 2000; Harries et al.,1997; Hecker et al., 2002; Jobling et al., 1998, 2002a; Lavado et al.,2004). This has been corroborated by exposing fishes to various xenoestrogens such as NP, EE2, E1 and per fluoroalkyls (PFAAs) at concentrations comparable to that observed in contaminated water bodies(Jobling et al., 1996; Kang et al., 2003; Lee et al., 2017; Panter et al.,1998; Van Den Belt et al., 2004; Zha et al., 2007). In fact, it was demonstrated in male rainbow trouts that the xenoestrogen-induced inhibition on testicular growth is a dose-dependent response (Jobling et al., 1996). In addition to gonado-somatic index, xenoestrogens induce deleterious gonadal structural changes in fishes. Presence of necrotic sperm, testicular lesions and decrease in number of sperm within cysts in males while oocyte atresia, ovarian lesions and alteration in number of oocyte stages in females are observed in fishes collected from water bodies contaminated with estrogenic compounds (Alan et al., 2008;Flammarion et al., 2000; Johnson et al., 2008). This has been ascertained by experimental studies by exposing fishes to xenoestrogens(Angus et al., 2005; Decourten et al., 2020; Jobling et al., 1996; Kwak et al., 2001; Miura et al., 2005; Wang et al., 2019; Zha et al., 2007).Exposure to EE2 and NP caused a decrease in the number of spermatogenic cysts in male rainbow trout and ovarian degradation in female rare minnowsGobiocypris rarus(Jobling et al., 1996; Zha et al., 2007).Swordtail fishXiphophorus helleriexposed to BPA (10 ppm) and NP (100 ppb), alone or in combination (0.4 ppm BPA +4 ppb NP), exhibited testicular damage such as appearance of necrotic germ cells in seminiferous tubules (Kwak et al., 2001). In male zebra fish, exposure to 1 mg/L BPAF caused the appearance of acellular areas in the testis (Yang et al., 2016). The study also reported a decrease in the number of sperms and mature oocytes. The effect of xenoestrogens is not restricted to germ cells alone. In male Japanese eelAnguilla japonica, testicular organ culture in 1 pg/mL to 100 g/mL ofpara-nonylphenol (p-NP) led to hypertrophy of Sertoli cells resulting in decrease in germ cell to Sertoli cell ratio (Miura et al., 2005). In male gold fish, exposure to 50 and 500 μg/L BPA induced apoptosis in Leydig cells (Wang et al., 2019). It is evident that xenoestrogens are capable of altering the structure of gonads and thereby impacting reproductive success of fishes.
3.4.2.Functional alterations
Several studies demonstrate the detrimental effect of xenoestrogens on gametogenesis, steroidogenesis, sexual maturation and reproductive success of teleosts. In NP-exposed (184 μg/L) male Japanese medaka,reduced number of primary and secondary spermatocytes suggests its inhibitory effect on differentiation of germ cells (Kang et al., 2003).Similarly, proliferation of spermatogonia type A is seen restricted when Japanese eel testis was cultured withp-NP (Miura et al., 2005). In addition to germ cell proliferation and differentiation, xenoestrogens have been shown to adversely affect spermiation by decreasing the milt volume and altering the spawning timing in fishes (Jobling, Beresford,et al., 2002; Johnson et al., 2008), and sperm quality (Golshan et al.,2015). In adult female zebra fish,in vitroexposure to DEHP (10 and 100 nM) diminished germinal vesicle breakdown indicating the detrimental effect of xenoestrogen on oocyte maturation (Carnevali et al., 2010). A few reports indicate that gonadal alterations caused by xenoestrogens are due to transcriptional changes of genes related to hormone receptors and gametogenesis (Bahamonde, McMaster, et al., 2015; Carnevali et al., 2010; Major et al., 2020). Bahamonde, McMaster, et al. (2015)identified downregulation of genes related to spermatid development,sperm cell adhesion and ovulation in rainbow darterEtheostoma caeruleuminhabiting Grand River basin of Canada that receives municipal wastewater. Similarly, female zebra fish exposed to DEHP (0.02–40 μg/L) exhibited downregulation in ovarian expression of Lh receptor(lhr), membrane progesterone receptor (mpr) and cyclooxygenase-2(ptgs2) while upregulation of bone morphogenetic protein 15 (bmp15)(Carnevali et al., 2010). These observations could be seen in light of the fact that Lh and progesterone are important for oocyte maturation while prostaglandin in ovulation (Armstrong, 1981). Therefore, adverse effect on expression of Lh and progesterone receptors, and enzyme for prostaglandin synthesisptgs2would jeopardize reproductive success. Since Bmp15 inhibits precocious maturation of oocytes by decreasing the level of Lhr and mPr, its upregulation in response to xenoestrogen seems justified (Clelland et al., 2007; Tan, Balofsky, et al., 2009, Tan,Zagrodny, et al., 2009).
With regard to steroidogenesis, it has been observed that fishes populating xenoestrogen-enriched water bodies or exposed to xenoestrogen inin vivostudies show abnormal circulating levels of androgens and E2. Majority of the studies report decrease in level of 11-KT and testosterone (T) while increase in level of E2 in xenoestrogen-exposed male fishes as compared to controls (Bahamonde, Fuzzen, et al., 2015;Feist et al., 2005; Golshan et al., 2015; Hecker et al., 2002; Lavado et al.,2004; Orlando et al., 1999; Schwaiger et al., 2002; Shi et al., 2015;Shirdel et al., 2020; Wang et al., 2019; Yang et al., 2016). The scenario is controversial in female fishes since both increase and decrease of T and E2 is reported after exposure to different xenoestrogenic compounds(Hoffmann et al., 2006; Schwaiger et al., 2002; Shi et al., 2015; Shirdel et al., 2020; Yang et al., 2016). Further, the effect of xenoestrogens has been studied on expression of steroidogenic genes and interestingly,majority of the studies suggest downregulation in expression of steroidogenic genes in gonads. Both male and female adult rare minnows exposed to 25 μg/L of EE2 exhibited significant decrease in gonadal expression of steroidogenic acute regulatory protein (star), cytochrome P450-mediated side-chain cleavage enzyme (cyp11a1), 3β-hydroxysteroid dehydrogenase (3β-hsd), cytochrome P450 17 or 17β-hydroxysteroid dehydrogenase (cyp17a1 or 17β-hsd) andcyp19a1a(Liu et al., 2012,2014). Similar effect was also observed for female rare minnows treated with different doses of BPA (5, 15, 50 μg/L) (Zhang et al., 2014).Further, male gold fish exposed to DEHP (1, 10 and 100 μg/L for 15 and 30 days) have shown downregulation in expression ofstarin testis(Golshan et al., 2015). Even DES caused decrease in ovarian expression ofstarandcyp17in female African cat fishClarias gariepinusand downregulation in testicular expression ofcyp17a1in male zebra fish (Sridevi et al., 2015; Yin et al., 2017) Similarly, a decrease has been recorded in activity of 5α-reductase in male carps exposed to different estrogenic compounds and P450 aromatase in female carps sampled from xenoestrogen-contaminated water body (Lavado et al., 2004; Thibaut &Porte, 2004). Contrary to these reports on adult fishes exposed to xenoestrogens, African cat fish embryos exposed to 50 ng/L of EE2 from 0 to 50 days post hatching showed upregulation in ovarian expression ofcyp17, 3β-hsdand17β-hsd1during their adult stage (Sridevi et al.,2015).
All these alterations caused by xenoestrogens on gametogenesis and steroidogenesis of fishes finally results in diminishing their reproductive success. Studies have shown that exposure to xenoestrogencontaminated water bodies as well as direct exposure to certain xenoestrogens, have caused significant decrease in various reproductive parameters such as fertilization, fecundity, hatching, and survival of larva in several fishes (Aluru et al., 2010; Carnevali et al., 2010;Decourten et al., 2020; Ishibashi et al., 2006; Jobling, Coey, et al., 2002;Laing et al., 2016; Lee et al., 2017; Mu et al., 2018; Qiu et al., 2016;Schwaiger et al., 2002; Shi et al., 2015; Shioda & Wakabayashi, 2000;Sohoni et al., 2001). This deleterious effect of xenoestrogens is often one of the main causes for sudden decline in population of fish species in their natural habitat.
3.5.Vitellogenin and zona radiata proteins as biomarkers
Vitellogenin (Vtg) is a glycolipophosphoprotein synthesized by the liver under the regulation of E2. It reaches ovaries via circulation where it is incorporated into the growing oocytes and processed to form the yolk proteins (Mommsen & Walsh, 1988; Wallace, 1985). However,presence of Vtg in plasma is also demonstrated in male fishes (20 ng/mL)though its level is considerably lower as compared to females(100–1000 mg/mL) (Tyler et al., 1996). Thereby, an increase in plasma Vtg levels in male fishes has been considered as an appropriate indicator or biomarker to assess water bodies contamination with xenoestrogens(Sumpter & Jobling, 1995, 2013). Male fishes inhabiting xenoestrogen-contaminated water bodies exhibit increased levels of plasma Vtg (Bahamonde et al., 2015a, 2015b; Flammarion et al., 2000;Harries et al., 1997; Hashimoto et al., 2000; Jobling et al., 1998, 2002a,2002b; Johnson et al., 2008; Lavado et al., 2004; McGee et al., 2012;Orlando et al., 1999; Purdom et al., 1994; Sardi et al., 2015; Stansley &Washuta, 2007). Further, a positive correlation has been noticed between level of plasma Vtg and concentration of xenoestrogen in water bodies (Adeogun et al., 2016; Allen et al., 1999; Blazer et al., 2014; Feist et al., 2005; Hecker et al., 2002; Hicks et al., 2017; Jobling et al., 2006;McMaster, 2001; Vajda et al., 2008). A similar inference has been drawn in male fishes in which increase in Vtg is seen inversely proportional to the distance of their habitat from the ef fluent discharge site (Bahamonde, Fuzzen, et al., 2015; Blazer et al., 2014; Harries et al., 1997).Numerous experimental studies in fishes have also shown that exposure to E2 or estrogenic compounds such as E1, BPA, BPS, BPAF, NP, EE2,PCBs, dichlorodiphenyldichloroethylene, phthalates, fenarimol, etc.,increased the production of Vtg in females as well as males in a concentration-dependent manner (Andersen et al., 2003; Angus et al.,2005; Dias et al., 2014; Flammarion et al., 2000; Hashimoto et al., 2000;Herman & Kincaid, 1988; Jarque et al., 2015; Jobling et al., 1996; Lee et al., 2017; Panter et al., 1998; Purdom et al., 1994; Verslycke et al.,2002; Zha et al., 2007). In addition, the positive regulation ofvtgexpression by xenoestrogen, especially BPAF, has been shown in male zebra fish though similar effect was not observed in female zebra fish (Shi et al., 2015; Yang et al., 2016). It has been suggested that excess production of Vtg requires over expenditure of energy and as a result other physiological functions get compromised. Also, high plasma level of Vtg can cause damage to liver and kidney, and removal of calcium from scales and bones (Carragher & Sumpter, 1991; Herman & Kincaid,1988).
Like Vtg, zona radiata proteins (Zrp) secreted by hepatocytes under the in fluence of E2 are transported to the ovary to be incorporated into the egg envelope of maturing oocytes in fishes. This protein is also synthesized in males though at much lower levels than females (Arukwe et al., 1997; Arukwe & Goks?yr, 2003). An increase in mRNA and protein expression ofzrpis seen in male fishes from xenoestrogen-enriched water bodies (Adeogun et al., 2016; Arukwe et al., 1997; Jarque et al.,2015). Similar observation has been reported throughin vivoandin vitrostudies where xenoestrogens have caused dose-dependent increase inzrpmRNA and protein levels in male fishes as compared to females(Ackermann et al., 2002; Arukwe et al., 1997; Celius & Walther, 1998).Like Vtg, detection of Zpr at a considerable level in male fishes is also being considered as a biomarker to assess the level of contamination of aquatic environment with xenoestrogens (Arukwe et al., 1997; Arukwe& Goks?yr, 2003).
3.6.Gonadal plasticity (intersex)
Intersex is a reproductive state found in some wild populations of teleosts where male and female gonads exist simultaneously within an individual (Bahamonde et al., 2013). This condition arises either due to appearance of oocytes in testis of male (feminization process) (Nolan et al., 2001) or testicular cells in ovary of female (masculinization process) (Hinck et al., 2007). Several studies have reported xenoestrogens as one of the causative agents for intersex condition in teleosts.
Histological analysis of gonads from fishes inhabiting xenoestrogencontaminated water bodies or exposed to specific estrogenic compounds depict intersex condition (Adeogun et al., 2016; Allen et al., 1999;Andersen et al., 2003; Bahamonde, Fuzzen, et al., 2015, Bahamonde,McMaster, et al., 2015; Hashimoto et al., 2000; Hirakawa et al., 2012;Jobling et al., 2002a, 2002b, 2006; McGee et al., 2012; Nash et al., 2004;Sardi et al., 2015; Schwaiger et al., 2002; Zha et al., 2007). Most of these studies report feminization of male rather than masculinization of female as the major tissue present in the intersex gonad were testis containing oocytes. It would have been convincing if the sex chromosomes in these intersex population would have been determined. To our curiosity, no correlation has been observed between concentration of xenoestrogen and incidence of intersex condition (Kang et al., 2003; Zha et al., 2007). Also, it has been observed that some teleost species do not give rise to intersex individuals when inhabiting a xenoestrogen-contaminated water body even though the other species in the same habitat might have developed intersex condition, indicating species-specific variation in xenoestrogen-induced intersex condition(Bahamonde et al., 2013). It is intriguing to understand whether induced intersexuality has any resultant effect on reproductive success of the fish. A study in roach sampled from various river sites of the United Kingdom showed an increase in plasma level of Vtg and T in intersex fish as compared to non-intersex males, while level of E2 during intersex condition was seen to be higher than normal males but lower than females. In addition, delayed spermatogenesis and decrease in gonadal growth, sperm production, spermiation, milt production, sperm motility and fertilizing ability as well as offspring viability has been demonstrated in intersex roach fish (Jobling et al., 2002a, 2002b). This indicates that the reproductive success of such intersex individuals is very poor and increase in their proportion as compared to non-intersex males might lead to decline in the population of that species.
3.7.Transgenerational effects
The deleterious effects of xenoestrogen on fishes are often multigenerational and severity of the damages increases due to continuous exposure to estrogenic compounds generation after generation. Inin vivomultigenerational studies in Japanese medaka, zebra fish and fathead minnowsPimephales promelas, successive generations exposed to xenoestrogens showed significant reduction in reproductive success (Lee et al., 2017; Nash et al., 2004; Schwindt et al., 2014). In case of Japanese medaka, exposure to PFAAs caused reduction in fecundity, hatching and survival of embryos, and suppressions increased from one generation to the next (Lee et al., 2017). Although exposure to EE2 had species-specific differential effects on F0 generation of zebra fish and fathead minnows, F1 generation of both the species exhibited complete reproductive failure (Nash et al., 2004; Schwindt et al., 2014). In addition, reduced survival of F2 generation embryos of fathead minnows was observed even when they were kept in xenoestrogen-free water(Schwindt et al., 2014), indicating that the harmful effect of xenoestrogen on the reproductive system of fishes is transgenerational. Similar observations have been made in F1 generation of Inland silversidesMenidia beryllina, Japanese medaka and rainbow trout where only F0 generation was exposed to xenoestrogens (Bhandari et al., 2015;Decourten et al., 2020; Schwaiger et al., 2002). The detrimental effects of xenoestrogen in the successive generations ranged from reduction in fertilization, fecundity and hatching to alteration in hormonal levels to decrease in embryo survival to appearance of deformities in larvae. A contradictory observation has been reported in Japanese medaka wherein F1 offsprings obtained from BPA-treated parent generation did not show any change in the sex ratio. However, authors have suggested that F1 generation remained unaffected due to low lipophilicity of BPA used in the study (Kang et al., 2002).
4.Conclusion
Xenoestrogens adversely affect reproductive success of teleost by in fluencing sex differentiation, steroidogenesis, spermatogenesis, oocyte maturation and ovulation (Fig. 2). Moreover, the quantitative risk assessment of xenoestrogen is complicated by the diverse mode of action of different xenoestrogens, cross talk between different pathways of actions, a time lag between exposure and the final outcome, and also transgenerational effects (Fowler et al., 2012; Maffini et al., 2006;Matthiessen & Johnson, 2007; Rubin, 2011). The detrimental effect of xenoestrogens on reproduction in teleost is a matter of concern not only for fishper se, but the entire ecosystem. Hence, effort should be made to minimize the contamination of waterbodies with xenoestrogen. Also,more studies are needed to understand the effect of xenoestrogen cocktail that teleosts are actually exposed to under natural conditions.
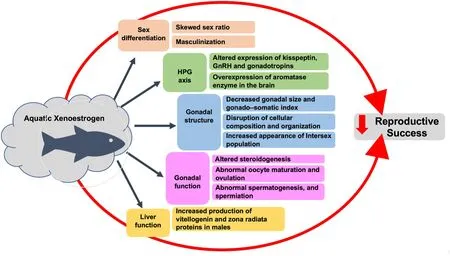
Fig. 2.Schematic representation of the impact of xenoestrogens on teleostean sex differentiation and reproduction. Xenoestrogens affect all aspects of reproduction in fishes, ultimately leading to deleterious effects on the reproductive success of the species.
For effective mitigation of xenoestrogen impact on teleost, a multipronged approach such as detection of xenoestrogen in waterbodies using sensitive and cost-effective methods, treatment of industrial as well as municipal wastewater ef fluents, and strict government regulation, needs to be adopted. To remove EDCs from ef fluents, many treatment methods for removing EDCs are employed such as adsorption,biosorption, electrochemical oxidation, activated carbon, membrane filtration etc. (Kasonga et al., 2021), though these methods are quite expensive. Therefore, a cost-effective sustainable system for waste water management is required. The existing water pollution acts in developing countries including India ignores the menace caused due to xenoestrogens. Further, the concerned acts have been poorly implemented due to inadequate infrastructure including monitoring stations,non-compliance due to the meagre penalty, and inadequate standards.Surprisingly, 17-alpha-ethinylestradiol (EE2), 17-beta-estradiol (E2)and estrone (E1) that were listed in the European Union Watch List of contaminants of emerging concerns (CEC) for monitoring surface water(EU Commission, 2015), was dropped in 2019 (EU Commission, 2020).Hence, to deal with xenoestrogen, a separate act needs to be in place to deal with xenoestrogen and save aquatic biodiversity.
Funding
The authors acknowledge the funding provided under Minor Research Grant, Institution of Eminence (IoE/FRP/LS/2020/27) by University of Delhi.
Declaration of con flict of interest
The authors declare no actual or potential con flict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately in fluence, or be perceived to in fluence,their work.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Reproductive farming technology in Japanese eel and chub mackerel
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
- Understanding the impact of stress on teleostean reproduction
- Germ cell markers in fishes - A review
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
