Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
Swathi Tenugu, Balasubramanian Senthilkumaran
Department of Animal Biology, School of Life Sciences, University of Hyderabad, P.O. Central University, Hyderabad, 500046, India
Keywords:
Sex reversal
Sequential hermaphrodite
Sex steroids
Gonadal transdifferentiation
Sexual dimorphism
Teleost
A B S T R A C T
Sex reversal is one of the characteristic properties of sexual plasticity in bony fishes wherein both natural and induced sex change happens at various stages of life cycle in different species. Sex determination in gonochoristic species is genetically regulated, wherein the same sex is retained throughout their life span whereas hermaphrodites change their sex during development or adulthood. In sequential hermaphrodites, serial sex change occurs at different points of life cycle. Concurrently, synchronous hermaphrodites function as both the sexes during spawning. Other variables like temperature, pH and social factors can trigger sex reversal in teleost. Sex reversal through gene mutations and chemicals/hormones, including sex steroids, can be induced mostly at early developmental stages but natural sex reversal can occur at any time. Sex reversal mechanism shows morphological to molecular changes, which are ideal for identification of sex-specific gene markers. In fact, gonadal transdifferentiation occurs at the molecular level through differential expression of transcription factors and steroidogenic enzyme genes vis-a-vis hormones, thereby imparting phenotypic or structural changes. In addition,brain shows sexual dimorphism which is mostly consequential to gonadal sex development and occasionally either causative. The major breakthrough in this line is the identification of sex determining genes such as dmy/dmrt1Yb, gsdfY, sox3 in the Japanese medaka and amhY in Patagonian pejerrey. Incidentally, the induction of mono-sex population by favouring one sex due to sex-specific differences in growth is an important economic boom for aquaculture. This review comprehensively highlights key molecular factors involved in natural and induced sex reversal conditions to illustrate teleostean sexual plasticity and its application perspectives.
1.Introduction
One of the fascinating properties exhibited by bony fishes is the existence of various reproductive strategies in terms of sexuality. Sex determination in teleost is a crucial event comprising a bipotential gonad to develop either into an ovary or testis, but the underlying mechanisms vary differently among taxa (Devlin & Nagahama, 2002;Hayes, 1998). Fishes exhibit gonochorism like most vertebrates,whereby the sexual fate of an individual is majorly determined by genetic, environmental and hormonal interactions (Volff, 2002). They develop into either male or female and retain the same sex throughout the life. Unlike other vertebrates, few fishes are referred to as hermaphrodites, wherein they function as both male and female during the same spawning period (Helfman, Collette, & Facey, 1997, p. 544). In contrast to this, sequential hermaphrodites were found to function as a male and a female at different stages of life (Warner, 1988). Despite of sexual plasticity and various reproductive patterns, the underlying regulatory mechanisms leading to endocrine sex differentiation remain conserved (Kobayashi, Nagahama, & Nakamura, 2013). In this process,sex reversal becomes an intriguing phenomenon most commonly observed in certain groups of teleost. In all mammals, SRY (sex determining region Y in all mammals) is the first sex determining gene discovered that is sufficient for testicular development (Koopman,Gubbay, Vivian, Goodfellow, & Lovell-Badge, 1991; Koopman, Münsterberg, Capel, Vivian, & Lovell-Badge, 1990; Sinclair et al., 1990).Incidentally, the sry-like genes could not be isolated in fish, yet fishes express certain master genes which regulate the formation of gonads that are gender-specific. Among non-mammalian vertebrates,dmyordmrt1bwhich is a duplicate copy of doublesex and mab-3 related factor 1(dmrt1) on the Y chromosome, was the first sex determining gene identified in the Japanese medaka,Oryzias latipesand later inO. curvinotus, which is associated with testicular differentiation (Matsuda et al., 2002, 2003; Nanda et al., 2002). But in other species of medaka,O. luzonensis gsdfYwas the sex determining gene (Myosho et al.,2012). In line with this, sex determining genes identified so far in fishes tend to vary from species to species, for example,amh-Yin the Patagonian pejerrey,Odontesthes bonariensis,amhr2in the puffer fish,Takifugu rubripes,irf9yin the rainbow trout,Oncorhynchus mykissandamhin the Nile tilapia,Oreochromis niloticus(Cáceres et al., 2019; Hattori et al.,2012; Kamiya et al., 2012; Yano et al., 2012). But, the evolutionary conservation of several sex determining genes identified so far is still unclear. These genes specifically regulate some downstream targets,which are highly conserved among different species. Some of the genes involved in gonadal development include doublesex/mab3-related transcription factors (dmrt), Wilms’ tumour suppressor gene (wt1),anti-Müllerian hormone (amh), SRY-box 9 (sox9), aromatase (cyp19) and steroidogenic factor 1 (Ad4BP/sf-1) were identified in the autosomes of several species. In most fishes, sex is determined through factors from somatic as well as sex chromosomes, yet the sexual fate is susceptible to hormones as well as environmental factors leading to sex reversal. In a holistic view, overriding of genetic factors through hormone or environmental in fluence is likely in teleosts.
Sex reversal property was first demonstrated by the Japanese scientist, Yamamoto in medaka through sex reversing the genotypic female to male phenotype and genotypic male to female phenotype (Yamamoto, 1953; 1958). Later, it was postulated that the sex steroids act as natural female and male sex inducers, which led to a framework in research to find the underlying mechanisms of sex change that have been continued till date (Yamamoto, 1969). In addition, aromatase blockers like fadrozole were often used for sex reversal in a few teleosts(Afonso, Wassermann, & Terezinha de Oliveira, 2001; Nakamura,Kobayashi, Miura, Alam, & Bhandari, 2005; Zhou & Gui, 2010). Taken together, the sex determination and differentiation, as well as sexual plasticity in response to sex steroids and environmental factors, is a complex process. The sex reversal phenomenon observed during induction by steroid hormones is perceived only during the critical period of gonadal differentiation. More specifically, sex steroids under natural and induced conditions mediate sex change either to male or female in most of the teleost in a particular time frame. The environmental factors,including temperature, pH and social factors show an impact on sex determination/differentiation in most of the teleosts (Fernandino, Hattori, Moreno Acosta, Strüssmann, & Somoza, 2013). Additionally,certain endocrine disruptors affect sex determination/differentiation and reverse differently. This review will aim to highlight different aspects including sex reversal induced by various steroid hormones,environmental factors and ultimate use of generating monosex population through hormonal treatment followed by genetic cross as well as brain sex dimorphism exhibited by few teleost which were categorized.
2.Sexual development: Morphological changes during normal gonadal development including sex reversal
Sexual development is a conserved process in most of the vertebrates,which de fine particular events that specifically occur during gonadal development by allowing the expression of genetic sex into its appropriate phenotype (Devlin & Nagahama, 2002; Piferrer, 2001; Piferrer &Blázquez, 2005). The molecular mechanisms leading to females and males exhibits wide diversity among vertebrates and the cascade of events during sex differentiation seems relatively conserved (Nagahama,2005). The sex steroids, mainly, androgens and estrogens play a very important role in regulating sex differentiation process (Piferrer &Guiguen, 2008). It commences with the migration of primordial germ cells, followed by their colonization into genital ridge forming gonadal primordium and extends the first process of gametogenesis with the release of gametes upon eventful completion. Due to sexual plasticity,some fishes act as functional males and females during their life-time,referred to as hermaphrodites compared to non-sex-changing species,i.e., gonochoristic which retain only one sex throughout their life. Two terms have been employed for the classification of gonochoristic species by Yamamoto (1969), referred to as (1) differentiated gonochorists where the undifferentiated gonad directly develop into an ovary or a testis during early gonadal development as observed in the European seabass,Dicentrarchus labrax, the common carp,Cyprinus carpioand the coho salmon,Onchorynchus kisuch(Blázquez, Zanuy, Carrillo, & Piferrer,1998; Komen, De Boer, & Richter, 1992; Piferrer & Donaldson, 1989)and (2) undifferentiated gonochorists, in which all the individuals first develop gonads containing oocytes which is termed as ovarian differentiation. Later, the oocytes undergo degeneration through apoptosis in half of the population, followed by masculinization of the gonad proceeding to form an intersexual gonad that eventually develops into normal testis (Maack & Segner, 2003). This phenomenon is termed as juvenile hermaphroditism and was reported inO. masouandGambusia affinis(Koya, Fujita, Niki, Ishihara, & Miyama, 2003; Nakamura, 1984).But in a few species, juveniles possessing a bipotential intersex gonad was found to develop into either ovary or testis as observed in the serranid,Epinephelus striatus, the Japanese eel,Anguilla japonicaand the European eel,A. anguilla(Beullens et al., 1997; Colombo & Grandi,1995; Sadovy & Colin, 1995). Hermaphroditism is a naturally occurring phenomenon observed in most of the species and categorized as simultaneous or sequential (Shapiro, 1990; Yamamoto, 1969). In simultaneous species, the individuals possess both male and female gonadal tissues where both function together. Sequential hermaphrodites are categorized into protandrous, which initially mature as males and protogynic that matured as females (Ross, 1990). In some protogynous species such as the bluehead wrasse,Thalassoma bifasciatum, few individuals differentiate directly into males whereas others first pass through the condition of being functionally females (Munday, Buston, &Warner, 2006; Munday, Wilson White, & Warner, 2006). These species referred to as diandric protogynous which is a rare property observed in protoandric species (Devlin & Nagahama, 2002). In addition, certain species like the rubble gobiid fish,Trimma okinawaehave the ability to undergo serial sex change based on the social colonization behavior.This has been well demonstrated wherein serial sex change of dominant female to male and vice-versa occurred after the introduction of the dominant male in an artificial culture condition (Sunobe & Nakazono,1993). These serial sex changing fish have differential expression pattern of gonad-specific genes. This was the first known teleost where bi-directional sex change was observed which became advantageous wherein a male was forced to become subsidiary, ensuing to take over the social unit by a larger male. Other species such as the saddleback wrasse,T. duperreyalso undergo protogynous sex change where the individuals initially mature as females or males. Later, under social conditions initial phase (IP) female or male fish become terminal phase(TP) males. But in this species, females housed with IP males undergo sex change to become TP males which have high circulating levels of 11-KT and spawn individiually with large female successfully (Morrey,Nagahama, & Grau, 2002). Conversely, females housed with TP males,inhibits sex change but helps in the maintenance of ovarian function and likelihood of successful pair spawning with immediate proximity females. Therefore, under social conditions females change their sex along with gonadal phenotype compared to males which are only restricted in regulating sex change and maintain ovarian function in females(Hourigan, Nakamura, Nagahama, Yamauchi, & Grau, 1991). In such species, during sex change, the ovaries which possess undetectable testicular tissue in females were fully reorganized to a functional testis(Nakamura, Hourigan, Yamauchi, Nagahama, & Grau, 1989). But in the yellowtail clown fish,Amphiprion clarkii, the dominance of the sex change depends on the size. For example, normally female is a dominant and male is the second-largest member in that population based on size.In the absence or death of the female, sex change of male to female occurs, thus, ultimately leading to female dominance (Fricke & Fricke,1977). The sex steroid hormones such as estrogens and androgens induced sex change in several teleost which are categorized as key regulators (Godwin & Thomas, 1993). Thus, due to this sexual plasticity,the underlying mechanisms involved during sex change make it more prominent to identify genes contributing to sex differentiation/determination. In the first instance, sex steroids seem to play an essential role in evoking sex reversal.
3.Sex steroids causing sex reversal
Even though the sex determination in fishes is under genetic regulation, the external factors such as temperature, pH, social behavior induce sex reversal in most of the fishes, where the process of sex determination, as well as differentiation has already been well established (Bushmann & Burns, 1994; Lowe & Larkin, 1975; Pandian &Koteeswaran, 1998; Strüssmann, Calsina Cota, Phonlor, Higuchi, &Takashima, 1996). Additionally, in fishes where the sex differentiation is not yet completed, manipulation of gonadal sex through the administration of sex steroids via diet, immersion, injection or through implantation technique is possible (Devlin & Nagahama, 2002; Frisch,2004; Nagahama, 2002; Pandian & Sheela, 1995). Sex reversal through endocrine mediated techniques is well applied for the production of monosex populations. Steroid hormones are widely used to control and manipulate the sex of the fish (Baroiller, Guiguen, & Fostier, 1999; Borg,1994; Lee et al., 2000; Nakamura et al., 1989). This practice was mostly used in teleosts, among which tilapia and salmonids have received utmost attention. Sex reversal has been limited to only few species such as the channel cat fish,Ictalurus punctatusand the African cat fish,Clarias gariepinusby giving steroid hormone through dietary supplementation or exposure in water (Galvez, Mazik, Phelps, & Mulvaney, 1995; Goudie,Redner, Simco, & Davis, 1983; Liu, Yao, & Wang, 1996; Raghuveer &Senthilkumaran, 2009). In order to advance the maturation in the catfish,C.gariepinus, human chorionic gonadotropin (hCG) induction was performed by implantation of osmotic pump during off breeding season and expression of several steroidogenic enzymes, transcription factors and hormonal levels were measured (Murugananthkumar, Prathibha,Senthilkumaran, Rajakumar, & Kagawa, 2017). However,C.batrachusandC.gariepinusshow distinct difference because of the seasonal pattern variance exhibited and hence, these species seem to be the best model to study any kind of a seasonal in fluence or endocrine manipulation(Rajendiran et al., 2021; Senthilkumaran & Kar, 2021). In some studies of the Asian stinging cat fish,Heteropneustes fossiliswhen eggs were treated with 17α-methyltestosterone (MT) and 17α-ethynyltestosterone(ET) it produced males and upon treatment with 17β-estradiol (E2) and diethylstilbestrol it produced females (Haniffa, Sridhar, & Nagarajan,2004). This sex reversal was amenable as they show sex change at the time of hatching. This feminization is mainly due to the conversion of androgens to estrogens by thecyp19aenzyme. Similarly, this feminizing effect was also found inSalmo gairdneriand occurred only at higher doses of MT treatment (Solar, Donaldson, & Hunter, 1984).
Sex-change is majorly triggered by several steroids such as E2, 11-ketotestosterone (11-KT) and Testosterone (T). E2is one of the most dominant estrogen amongst protandric species that is associated with sex change in most of the teleosts (Kime, 1993). E2is a predominant feminizing sex steroid that can induce permanent sex transition in some teleosts (Chang & Lin, 1998; Lee et al., 2000) and also increase E2production concurrently through progression of natural sex reversal in several species (Godwin & Thomas, 1993; Guiguen, Jalabert, Benett, &Fostier, 1995; Nakamura et al., 1989). In contrast, 11-KT is the predominant sex steroid which is related to sex change to masculinization in most of the protogynous species, whereby permanent sex transition was observed upon treatment of the correlate in many species (Cardwell& Liley, 1991; Grober, Jackson, & Bass, 1991; Higa, Ogasawara, Sakaguchi, Nagahama, & Nakamura, 2003; Kroon & Liley, 2000). Similar to E2, 11-KT levels also showed a progressive increase in synthesis during natural sex change to masculinization in many species (Bhandari,Komuro, Nakamura, Higa, & Nakamura, 2003; Nakamura et al., 1989).11-KT male specific and E2female specific were found to be the dominant products in the incubates of the gonadal tissues that undergo transition (Borg, 1994; Lee et al., 1995; Yeung, Chen, & Chan, 1993).The function of 11-KT in protogynous species is antithetical to that of E2in protandric species facilitating as switches between female and male phenotypes (Hunter & Donaldson, 1983; Pandian & Sheela, 1995;Piferrer, 2001; Piferrer, Baker, & Donaldson, 1993). In transitionalA. melanopus,no apparent E2levels were evident until after oogonial proliferation indicating E2is not required for initiating sex change in this species (Godwin & Thomas, 1993). However, in some other species very low or undetectable levels of 11-KT were observed during transitional sexual phases that led to a point whether 11-KT involved in sex change or else mediated through different combination of steroids (Kime, 1993;Kroon, Munday, & Pankhurst, 2003; Yeung & Chan, 1987). In several studies of hermaphrodite species, similar to 11-KT, T also play a role during sex change by maintaining its levels independent to the sexual stage, indicating T not directly involved during transitions (Cardwell &Liley, 1991; Guiguen, Jalabert, Thouard, & Fostier, 1993; Nakamura et al., 1989). Interestingly, administration T, MT and testosterone propionate induced sex change in few protogynous species (Glamuzina,Glavi?, Skaramuca, & Kǒzul, 1998; Hassin et al., 1997; Kuo, Ting, & Yeh,1988; Lee et al., 1995; Tan-Fermin, Garcia, & Castillo, 1994; Yeh, Kuo,Ting, & Chang, 2003), whereas in estuary grouper small doses of T has stimulated ovarian development. In contrast, T or 5α-dihydrotestosterone inT. bifasciatuminduced ovarian degeneration (Kramer, Koulish, &Bertacchi, 1988), suggesting that sex change is not in fluenced by steroid directly but indirectly affects the precursor’s availability for biosynthesis. However, in most of the species, T shows ubiquitous presence irrespective of the female or male phase. Thus the physiological effect upon T administration majorly depends on enzymes, for example,cyp19aor 11bh which are dominant during the time of treatment. The distinct conversion of the substrates of T into androgens and estrogens was corroborated in few studies when incubated with testicular and ovarian tissues (Lee et al., 1995; Yeung et al., 1993). Incidentally, inC. gariepinusMT treatment did not induce complete sex reversal in entire population which is evident from the occurrence of intersex. On the other hand, in the same species, E2treatment induced almost complete sex reversal (Raghuveer & Senthilkumaran, 2009).
Apart from classical steroids, non-classical steroids such as adrenosterone (Ad) are known to induce sex change in certain species such as black-eye goby,Coryphopterus nicholsii, but this particular effect is more likely due to the presence of 11-KT precursor, as receptors for Ad does not exist (Kroon & Liley, 2000). Aromatase inhibitors (AI) such as fadrozole and exemestane suppress the sex change observed during natural sex reversal in protandric black porgy,Acanthopagrus schlegeli,whereas induced sex change in other species such as protogynous black-eye goby,C. nicholsii,honeycomb grouper,E. merra, threespot wrasse,Halichoeres trimaculatus(Bhandari, Higa, Nakamura, & Nakamura, 2004; Kroon & Liley, 2000) indicating E2dominance. Additionally, E2when co-administered with 11-KT or AI, also suppressed sex-change inH. trimaculatus(Higa et al., 2003). Upon oral administration of MT in carp underwent sex reversal from female to male which was evaluated by using all female gynogenetic carp during sex differentiation (Komen, Lodder, Huskens, Richter, & Huisman, 1989; Nagy,Bercsényi, & Csányi, 1981). In another study, masculinization (mas)mutant which is an autosomal sex-determining gene involved in testes differentiation, induced sex reversal of male development in XX females(Komen et al., 1992). In tilapia, MT administration induced masculinization in XX larvae and treatment with AIs caused sex reversal of functional males (Afonso et al., 2001; Kobayashi, Kajiura-Kobayashi,Guan, & Nagahama, 2008; Kwon, McAndrew, & Penman, 2002; Nakamura, Kobayashi, Chang, & Nagahama, 1998; Ruksana, Pandit, &Nakamura, 2010). However, estrogen treatment did not induce XY sex reversal in tilapia as easily as androgen treatment in XX sex reversal along with AIs whereas 17α-ethynylestradiol treatment from 4 to 6 days post hatch (dph) resulted in complete sex reversal of XY with germ cells proliferation similar to that of XX gonads (Kobayashi et al., 2008;Kobayashi, Kajiura-Kobayashi, & Nagahama, 2003). Similarly, masculinization effect is caused by AI was also observed in the Japanese flounder,Paralichthys olivaceus, the Chinook salmon,O. tshawytschaand the rainbow trout,O. mykiss(Guiguen et al., 1999; Kitano, Takamune,Nagahama, & Abe, 2000; Piferrer et al., 1994).
In female breeding medaka, exposure of AI, the letrozole, affected oocyte development (Sun, Zha, Spear, & Wang, 2007). Ovarian transdifferentiation was evident in female zebra fish upon AI treatment wherein first testis-like tissues containing spermatozoa-like cells developed, which was followed by ovary retraction (Takatsu et al., 2013). Sun et al. (2014) observed the differentiated ovary to undergo transdifferentiation and become functional testis upon long-term AI treatment causing functional sex reversal in tilapia. It was noteworthy that the first morphological change of ovarian structure in this species was observed at 20 dph (Kobayashi et al., 2008). In medaka, estrogen treatment in XY males resulted in ovarian development while XX females upon treatment with androgens induced testis development,causing sex reversal (Yamamoto, 1953). Incidentally, fertilized XY eggs of medaka when immersed in water containing exogenous E2induced sex reversal of few genotypic males to functional females (Iwamatsu,Kobayashi, Hamaguchi, Sagegami, & Shuo, 2005). However, neitherdmynor the germ cells number was affected during the early gonadal development indicating that the Japanese medaka seems not succumbing to external E2treatment during early gonadal sex differentiation(Scholz et al., 2003; Suzuki, Nakamoto, Kato, & Shibata, 2005). The killifish,Kryptolebias marmoratuscapable of undergoing self-fertilization showed skewness to male sex development of embryos after treatment with MT (Kanamori et al., 2006).
4.Factors affecting sex reversal
4.1.Temperature
Temperature-induced sex reversal is one of the properties observed in several teleost by triggering various candidate genes associated with it. The first observation of temperature effect on sex reversal was found in the Atlantic silverside,Menidia menidia, wherein warm fluctuating temperature (17–25 ℃) treatment during larval development resulted in male population (Conover & Kynard, 1981). Among the poeciliids and cichlids families, the blackbelly limia,Poecilia melanogasterand umbrella cichlid,Apistogramma borellishowed a significant effect when larvae were incubated at a temperature range from 23 to 29 ℃, which produced male broodstocks (R?mer & Beisenherz, 1996). In medaka,when embryos were exposed to the high water temperature of 34 ℃,female to male sex reversal was evident, and this simple methodology was used to produce XX males (Kobayashi, 2013; Sato, Endo, Yamahira,Hamaguchi, & Sakaizumi, 2005). The genotypic females (XX) of Hd-rR strain in medaka exhibited sex reversal into male phenotype upon exposure of fertilized eggs at embryonic stages 5–6 to the temperature between 17 and 34 ℃ until hatching, and most of them showed 100% sex reversal at 34 ℃ (Hattori et al., 2007). But this thermal manipulation for inducing sex reversal was ineffective at stage 36 which is at middle embryogenesis stage. But in another study, when Hd-rR and green fluorescent protein transgenic medaka were exposed to 32 ℃ at embryonic stage 25 caused sex reversal suggesting that the process of gonadal sex differentiation induced by high temperature is limited only before the occurrence of morphological sex differentiation stage (Selim,Shinomiya, Otake, Hamaguchi, & Sakaizumi, 2009). In addition to this,high temperature induced expression ofdmrt1in females after stage 36 during embryonic development (Hattori et al., 2007), whereas in normal males, it is expressed in XY gonads after 10 to 20 dph (Kobayashi et al.,2004). Elevated temperature treatment of 32–34 ℃ in tilapia, produced an increased number of males in XX progeny whereas a decreased number of females in XY and YY progenies (Abucay, Mair, Skibinski, &Beardmore, 1999; Baroiller, Chourrout, Fostier, & Jalabert, 1995; Kwon et al., 2002). The thermosensitivity period in this species ranged from 10 to 20 days post fertilization (dpf) or 40 dpf (Baroiller, D’Cotta, & Saillant, 2009). Decreased expression ofcyp19a1andfoxl2with elevated expression ofdmrt1was evident in XX larvae when exposed to a high masculinizing temperature in tilapia (Baroiller, D’Cotta, & Saillant,2009; d’Cotta et al., 2001; Poonlaphdecha et al., 2013). Interestingly,thedmrt1suppresses thecyp19a1atranscription by repressing the activity ofAd4BP/sf1dependent female pathway during sex reversal upon MT treatment in tilapia (Wang et al., 2010). Similarly, decreasedcyp19a1aexpression was observed upon high masculinizing temperature exposure in the European sea bass, the Japanese flounder, the Japanese medaka and zebra fish (Kitano, Hayashi, Shiraishi, & Kamei,2012; Kitano, Takamune, Kobayashi, Nagahama, & Abe, 1999; Navarro-Martín et al., 2011; Uchida, Yamashita, Kitano, & Iguchi, 2004).But the underlying molecular mechanism relating temperature withcyp19a1aexpression is yet to be deciphered. One of the mechanisms linking the temperature in sex ratio shift is the epigenetic modification observed in the study using the European sea bass model in which of increased methylation ofcyp19a1apromoter in females by temperature decreased its expression in masculinized fish during sex reversal (Navarro-Martín et al., 2011). This is due to the prevention of binding ofAd4BP/sf-1andfoxl2to the promoter sites and in turn blockingcyp19a1atranscriptional activation (Wang et al., 2007). The similar epigenetic modification was also observed during temperature mediated sex reversal in zebra fish by methylation and decreased expression ofcyp19a1a, esr1andsox9bas well as enrichment of cAMP-responsive element-binding proteins were observed (Han et al., 2021). In the Japanese flounder, temperature exposure 36 ℃ induced sex reversal from XX female to XX males with increasedgsdfexpression and inhibition of germ cell proliferation leading to testis development (Yang et al., 2019).The African cat fish,C. gariepinus, upon 36 ℃ exposure, showed a masculinization effect when treated for at least 3days between 0 and 23dph and often skewed towards 100% male phenotypes when applied from 6-8dph (Santi et al., 2016). In contrast, full masculinization was observed in common carp when larvae were exposed to 36 ℃, particularly between 7 and 13 dph (Biswas et al., 2021).
The elevated temperature leads to stress and increased the level of cortisol during sex reversal has also received the most attention on reproduction (Consten et al., 2002; Mommsen, Vijayan, & Moon, 1999;Schreck, 2010). The plasma levels of cortisol and androgens were observed in few species during the sex determination and differentiation period. For example, the cortisol levels increased in the pejerrey when larvae reared in warm and male-inducing temperatures compared to that of low temperature (Hattori et al., 2009). Additionally, when treated with cortisol and dexamethasone, agonist, male-biased sex ratios were observed when reared under intermediate temperatures. Interestingly, very high levels of 11-KT and T were evident (Hattori et al., 2009).The three possibilities of inducing masculinization may include (i)cortisol inhibitingcyp19a1aexpression, (ii) cortisol reducing the number of progenitor germ cells (PGC) and, (iii) cortisol affecting 11-KT synthesis. The first possibility was evident in the Japanese flounder and pejerrey where cortisol induced masculinization andcyp19a1adownregulation were seen (Hattori et al., 2009; Yamaguchi, Yoshinaga,Yazawa, Gen, & Kitano, 2010). The depletion of number of PGCs and their proliferation during masculinization was observed in medaka(Tanaka, Saito, Morinaga, & Kurokawa, 2008). Increased incidence of temperature dependent apoptosis in gonadal primordia during testicular differentiation was documented in zebra fish, pejerrey and medaka (Ito,Takahashi, Yamashita, & Strüssmann, 2008; Saito et al., 2007; Saito &Tanaka, 2009; Uchida, Yamashita, Kitano, & Iguchi, 2002). An increase in the levels of androgens was also reported in several teleosts with masculinizing effect (Devlin & Nagahama, 2002). This ultimately indicates the inhibition ofcyp19a1aexpression followed by apoptosis might be a consequence for an increase in the production of androgens to reinsure that cortisol can trigger masculinization in teleost upon high temperature exposure.
4.2.Hypoxia
Hypoxia is a condition of the presence of low levels of dissolved oxygen concentration in water and one of the factors affecting sex differentiation in fish. Hypoxia is more likely perceived as a stressor by fishes as with other environmental factors, specifically during the early stages of development. For example, a male-biased sex ratio was observed in zebra fish when reared under hypoxic conditions compared to normoxic (Shang & Wu, 2004; Shang, Yu, & Wu, 2006). This is due to the modulation of the expression of steroidogenic enzyme genes, which alters T and E2balance (Pollock, Dubé, & Schryer, 2010; Wu, Zhou,Randall, Woo, & Lam, 2003; Yu et al., 2012). It was reported that the African cichlid Egyptian mouth-brooder,Pseudocrenilabrus multicolor victoriaeexhibited higher T levels when exposed to hypoxic conditions compared to normal oxygenated fish however, E2levels were not affected (Friesen, Aubin-Horth, & Chapman, 2012). An increase in cortisol followed by hypoxic conditions was evident during the early stages of development in rainbow trout, suggesting that all are interrelated and majorly active during this particular period (Fuzzen,Alderman, Bristow, & Bernier, 2011). In the Atlantic croaker, hypoxia decreased tryptophan hydroxylase (TPH),cyp19a1bexpression in the hypothalamus.In vivotreatment with AI, 1,4,6-androstatrien-3,17-dione also decreased TPHs, 5-hydroxy tryptophan (5-HTP), serotonin (5-HT)and TPH activity (Rahman & Thomas, 2013). In the same study, E2treatment partially restored TPH activity, 5-HTP, 5-HT, TPHs contents in hypoxia-exposed fish, indicating the role of estrogens in controlling neuronal responses to hypoxic conditions in aquatic vertebrates (Rahman & Thomas, 2013). Combination of environmental stress of hypoxia and xenobiotic exposure of polychlorinated biphenyl mixture (Aroclor 1254) in the Atlantic croaker decreased TPH activity that accompanied with fall of 5-HT in hypothalamus and gonadotropin-releasing hormone(GnRH1) content in preoptic area-hypothalamus (POA-H) region (Khan& Thomas, 2000). This was further associated with a reduction in LH secretion and gonadal development which has been well-reviewed(Khan & Thomas, 2001; Rahman, Khan, & Thomas, 2011). 17, 20β,21-trihydroxy-4-pregnen-3-one, a progestin hormone that induce gamete maturation, were significantly decreased under hypoxic conditions in both sexes of the Atlantic croaker leading to impairment in gamete maturation (Thomas & Rahman, 2009). Hypoxia is also associated with endocrine dysfunction in this species by decreasing endocrine indicators of the estrogen signalling pathway, that regulate vitellogenin production which is a precursor for growing oocytes leading to a reproductive failure (Thomas, Rahman, Kummer, & Lawson, 2006).
4.3.pH
Other environmental factor affecting on sex ratio include pH, mostly studied in poeciliids and chiclids species. Even though the effect of pH is less prominent, but in common, acidic or low pH 5–6 exposurePelvicachromis subocellatus,P. pulcher,P. taeniatus,A. borelli,A. caucatoides,andXiphophorus helleriskewed towards the appearance of males while a high pH value of 7 skewed towards the occurrence of females (Rubin,1985; Sullivan & Schultz, 1986). The sex ratio of 1:1 of males and females was found whenP. pulcherwas exposed to intermediate pH (6.10)(Rubin, 1985). In contrast, the other species,Poecilia sphenopshigher proportion of females was evident when combined with temperature(30 ℃) and pH 6 or 7 exposure. At the same time male ratio increased when exposed to 22 and 26 ℃ with pH 8 depicting opposite gender-bias effect (Benjamín Barón, Fernando Bückle, & Espina, 2002). Exposure of various environmental stress factors and different hormonal treatment and expression of genes in depicting gonadal fate in various teleosts are illustrated in Table 1.
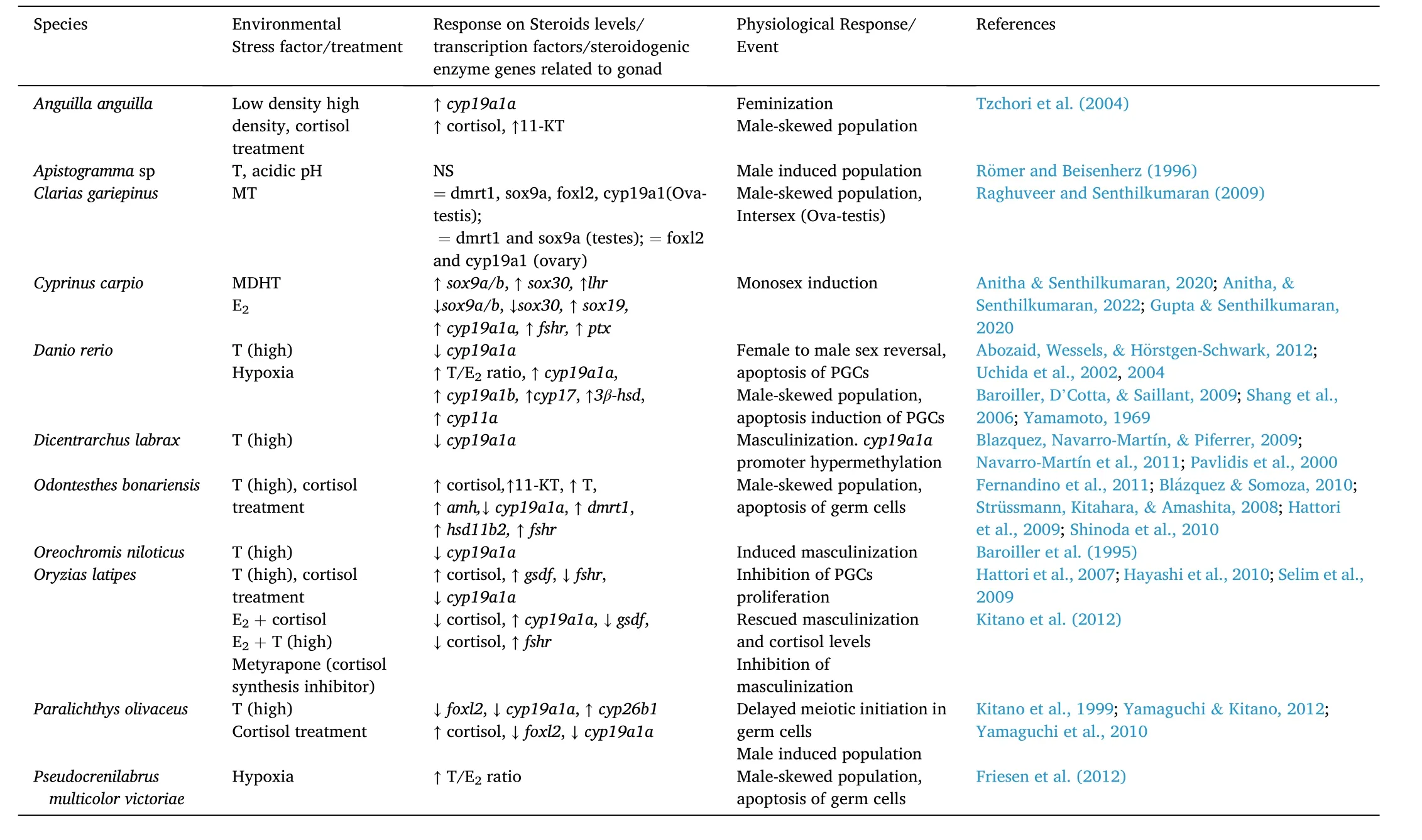
Table 1Exposure of environmental stress factors and different treatment in teleost depicting gonadal fate. A downward arrow pointing indicates decrease in hormone concentration, gene expression, enzymatic activity, an upward arrow indicates increase in these parameters, equal symbol depicts normal expression. “NS”: not studied.
4.4.Social interactions
Population density is an important social factor effecting sex determination in few teleosts. This appeared to be more prominent for sex determination in Anguilliformes (Beullens et al., 1997; Davey & Jellyman, 2005; Degani & Kushnirov, 1992; Holmgren, 1996; Krueger &Oliveira, 1999; Tzchori, Degani, Hurvitz, & Moav, 2004). For example,in the American eel,A. rostrata, low population density was interrelated with a high number of females, whereas male-biased sex ratio was induced by high population density inA. japonica(Davey & Jellyman,2005; Krueger & Oliveira, 1999). Social factors such as size also affects the sex differentiation inCichlasoma citrinellumwhereby larger fishes during the juvenile stage mature into males (Francis & Barlow, 1993).The interaction between growth rate as well as density are essential as both are associated with sex determination (Davey & Jellyman, 2005).Representation of outcome of sequencial hermaphrodites, environmental factors steroid hormones, as well as monosexpopluation performed in various species were depicted in Fig. 1.
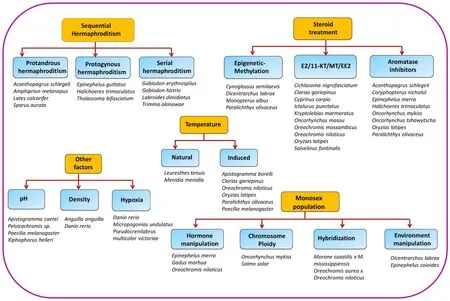
Fig. 1.Schematic representation illustrating outcome of sequencial hermaphrodites, steroid hormones, environmental factors as well as monosexpopluation. Note:References for these with respect to species has been covered in the bibliography of review.
5.Brain sex dimorphism
Fish retain large plasticity on sex reversal that was evident in the brain and also exists this reversibility even during adulthood (Godwin,2010). Brain sex differentiation in mammals and birds usually occurs during embryonic development which are controlled by gonadal steroids as well as genes regulating sex chromosomes, and epigenetic modifications (McCarthy & Arnold, 2011). In teleosts, brain sex dimorphism follows gonadal sex and this may either be a consequential or causative phenomenon. Nevertheless, sexually dimorphic genes sexes at the level of brain serve as a gene marker to understand sex reversal. In non-sex reversal fish like gold fish, gender specific sexual behavior can be induced through hormonal treatment illustrating the dominance of gonadal sex over brain sex (Kobayashi, Saoshiro, Kawaguchi, Hayakawa, & Munakata, 2013).
5.1.Brain cyp19a1b and estrogen receptors
The braincyp19a1bgene is involved as the feedback loop in regulating the steroid production by kisspeptin and hypothalamo–hypophyseal–gonadal (HHG) axis (Hofmann, 2006). The rainbow trout depicted elevatedcyp19a1bexpression in the brain of differentiating males (Vizziano-Cantonnet et al., 2011). Whereas in tilapia, upon exposure to 35 ℃, the expression ofcyp11a1bwas decreased in genetic females and still lower in genetic males than those found in masculinized females (D’cotta, Fostier, Guiguen, Govoroun, &Baroiller, 2001). In another study on tilapia, XY and YY sex reversal into females resulted in increased expression ofcyp19a1bwhich was dependent on ethynylestradiol (EE2) dosage along with increased E2and T levels (Gennotte, Mélard, D’Cotta, Baroiller, & Rougeot, 2014).Increasedcyp19a1bexpression was evident in masculinized XX females of tilapia along with high estrogen receptor-α (erα)anderβexpressions upon temperature (27 ℃) treatment. Increased expression ofcyp19a1b,erαanderβwas observed in tilapia upon MT treatment in 10 and 20 dph(Tsai, Wang, & Fang et al., 2001b). In contrast to this, increased expression ofcyp19a1banderαwas evident upon E2treatment between 0 and 10 dph (Tsai, Wang, & Fang, 2001). Additionally, high temperature induced testicular differentiation after 10 dph in tilapia whereas low temperature before 10 dph resulted in ovarian differentiation and within this thermosensitive period bothcyp19a1banderαwere downregulated relating brain feminization (Tsai, Chang, Wang, & Chao,2003). In medaka brain,esr1mediates male-biased expression, suggesting its role in masculinization whereasesr2βandarβare associated with feminization (Hiraki et al., 2012). The same study depicted the expression of female-specific estrogen and androgen receptors that are activated and inhibited by estrogens and androgens, respectively. In the pejerrey,cyp19a1bexpression was evident in the brain at male producing temperature before the sex differentiation onset (Strobl-Mazzulla et al., 2008). Sex reversal was not evident when adult females were treated with AI in the African cichlidAstatotilapia burtoni, however,partial masculinization was noticed in steroid level changes, body shape,appearance, colour, alterations in brain gene expressions that affected sexual behavior indicating estrogenic in fluence in juveniles (G?ppert et al., 2016).
5.2.GnRH
Hormones released from the HHG axis regulate various reproductive events which lead to fertility by regulating steroidogenesis through feedback at the level of the pituitary and brain. GnRH is known to be a hypothalamic decapeptide neurohormone majorly involved in reproduction by stimulating the release of gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH) by the pituitary gland,that in turn regulates steroidogenesis and gametogenesis (Ando, Hew, &Urano, 2001; Ando & Urano, 2005; Gomes, Costa, & Borella, 2013; Kah et al., 2007; Maruska & Fernald, 2011; Yaron et al., 2003; Zohar,Munoz-Cueto, Elizur, & Kah, 2010). GnRH exists in multiple forms which has resulted through genome duplication (Powell et al., 1994).Species specific and other forms of GnRH localized in various brain regions were found to play a role in reproduction, neuromodulation and during spawning behavior/migration (Senthilkumaran, Okuzawa, Gen,Ookura, & Kagawa, 1999). The Nile tilapia possessing distinguish XY and XX populations showed sexually dimorphic localization of seabream GnRH (Swapna et al., 2008). It was documented that three distinct forms of GnRH in South American cichlid fish were distributed differentially during sex differentiation (Pandol fiet al., 2002). Similarly, it was reported that GnRHir-neurons were appeared in the POA-H during gonadal differentiation (Soga, Ogawa, Millar, Sakuma, & Parhar, 2005).These GnRHir-neurons were found to be increased considerably in the pejerrey during the sex determination period (Somoza, Miranda, Guilgur, & Strobl-Mazzulla, 2006). Whereas in sea bass, during gonadal differentiation, the GnRH levels reached peak in pituitary and brain(Moles, Carrillo, Ma?anós, Mylonas, & Zanuy, 2007). Infact, it was suggested by Baroiller et al. (1999) that the HHG axis is required for complete sex differentiation but not its activation. However, stable GnRH2 and GnRH3 expressions were observed in black sea bass during sex reversal, indicating independence of these two GnRHs in this mechanism (Breton, DiMaggio, Sower, & Berlinsky, 2015). It is important to understand the monoaminergic axis and its crucial role in regulating the HHG axis to analyse sexual dimorphic GnRH localization, in turn GnRH-GTH axis (Goos, Senthilkumaran, & Joy, 1999; Peter, Trudeau, & Sloley, 1991). In teleosts, catecholamines (CA) and 5-HT are the monoamines that are the major components of central nervous system regulating various physiological functions, including reproduction.
The photic signals known to affect the HHG axis primarily through retina and pineal organ in fishes such as the African cichlid,A. burtoniand major carp,Catla catla(Grens, Greenwood, & Fernald, 2005;Moniruzzaman & Maitra, 2012). The two GnRH receptors, type-I and type-II are abundantly expressed in amacrine and ganglion cells respectively. The visual information is processed by amacrine cells and the ganglion cells transmit this processed information to the brain, a neuromodulator in order to in fluence GnRH in this process (Grens et al.,2005; Robison et al., 2001). Exposure of major carp,C. catlato constant darkness and exogenous melatonin treatment resulted in testicular maturation during preparatory phase of reproductive cycle (Bhattacharya, Chattoraj, & Maitra, 2007). It has been reported in the channel cat fish,I. punctatusthat the retinal efferents project towards the suprachiasmatic nucleus (SCN) and further to other brain areas and later these SCN neurons projects to the pituitary (Prasada Rao & Sharma, 1982;Prasada Rao, Job, & Schreibman, 1983). The pineal cells also express GnRH receptors in the European sea bass,D. labrax(Servili et al., 2010).Overall the pineal hormone and melatonin affects dopaminergic system,GnRH, LH, FSH and gonads (Falcón, Migaud, Mu?oz-Cueto, & Carrillo,2010).
5.3.5-HT
The 5-HT neurons have a stimulatory effect in neuroendocrine function by innervating through POA-H upon GnRH-GTH release (Senthilkumaran & Joy, 1996; Senthilkumaran, Okuzawa, Gen, & Kagawa,2001; Zohar et al., 2010). 5-HT appeared to be a contributing factor for sexual dimorphism in brain. This provided clue to authors in order to produce a predominant female population with the use of para-chlorophenyl alanine, a 5-HT synthesis blocker during the crucial window of sex determination/differentiation (Raghuveer, Sudhakumari,et al., 2011; Tsai, Wang, Chang, & Kao, 2001; Tsai & Wang, 1999). Tph is a homotetramer allosteric enzyme that catalyzes the rate-limiting step in 5-HT biosynthesis (Raghuveer, Sudhakumari, et al., 2011; Sudhakumari et al., 2010). In the Nile tilapia,Tph2which is a brain form oftph,showed prominent expression during 5, 10, 15 and 20 dph XY tilapia whereas no expression in 5–20 dph XX tilapia. Additionally, Tph transcripts localized throughin situhybridization was found only in 11 dph male brain specifically in the regions of telencephalon-preoptic area,olfactory bulb and olfactory epithelium but not in the female brain of same age. Tph immunoreactivity positive cells were observed in nucleus preopticus-periventricularis region of male brain of 12 dph but not in female brain of same age (Sudhakumari et al., 2010). Due to this, the dimorphic expression oftphmay indicate a role in brain sex differentiation of the Nile tilapia. In the cat fish,C. gariepinus,tphexpression was increased in males in comparison to females, 50 and 75 dph indicating variance expression in brain during early development (Raghuveer,Sudhakumari, et al., 2011). Increased expression oftph2was correlated with elevated levels of 5-HT and 5-HTP in the MT treated male brain fishes, and on the other hand, pCPA treatment decreasedtph2expression along with 5-HT and 5-HTP levels in the brain of cat fish (Raghuveer,Sudhakumari, et al., 2011).
5.4.Catecholamines
Catecholamines, unlike 5-HT, exerts inhibitory action by dopamine(DA) and facilitatory effect through norepinephrine (NE) on GnRH–GTH synthesis and release (Peter et al., 1991; Senthilkumaran & Joy, 1996).So far epinephrine with detectable levels during resting phase and increased levels during pre-spawning and spawning phases in the Asian stinging cat fish,H. fossiliswas observed, however no role has been indicated in terms of GnRH–GTH release (Senthilkumaran & Joy, 1995).In-depth study on the CAergic system during brain sex development of both sexes in the cat fish,C. batrachusrevealed sex-specific changes whereby the female brain depicted elevated tyrosine hydroxylase (th)expression levels and high copy number compared to males (Mamta et al., 2014). Elevatedthexpression was observed in female brain at 50,75 and 100 dph compared to that of the male brain of same age wherein 50 dph is considered to be as a critical period for gonadal differentiation in cat fish (Mamta et al., 2014; Raghuveer, Senthilkumaran, et al., 2011).However, the changes in the levels of DA and NE also showed sexual dimorphism. Whereas in saddleback wrasse, CAs plays an important role with the commencement as well as completion of sex reversal (Larson,Norris, Gordon Grau, & Summers, 2003). Furthermore, EE2treatment ensued increased CA levels and elevatedthexpression and MT treatment revealed lower CA levels, andthexpression irrespective of sex (Mamta et al., 2014). This indicated 5-HTergic system is prominent in male brain development, whereas CAergic system dominates in female brain development thus exhibiting brain sex dimorphism.
5.5.NPY
Neuropeptide Y (NPY) is considered as the most pivotal orexigenic neuropeptide well expressed in mammals, and it has been reported that NPY stimulates the food intake in teleost fish as in observed in mammals(Halford, Cooper, & Dovey, 2004; Kalra et al., 1999; Volkoff et al.,2005). NPY stimulates the release of GnRH from preoptic-anterior hypothalamic and pituitary fragments also upon T and E2in vivotreatment in gold fish and considered to be one of the mechanisms facilitating the feedback of these two steroids (Peng, Chang, et al., 1993; Peng,Trudeau, & Peter, 1993). Increased expression of NPY was evident in the cat fish,C. gariepinusbrain and testis, and its expression levels were high at 100 and 150 dph during brain development in females compared to males. Predominant expression of NPY was found during the pre-spawning stage of the female brain compared to other reproductive stages (Sudhakumari et al., 2017). NPY also stimulates the release of LH in gold fish through direct action on gonadotroph by stimulating the GnRH release (Trudeau, 1997). Even in the red seabream, NPY stimulatedin vitrorelease of sbGnRH from POA-H slices during immature,spawning and recrudescence stages (Senthilkumaran et al., 2001). The action of GnRH release by NPY is direct as it was not altered by antagonists of NA, gamma-aminobutyric acid and 5-HT. Studies revealed that central injection of NPY caused increased dose-dependent food intake in cohosalmon, channel cat fish and gold fish (De Pedro et al., 2000;López-Pati?o et al., 1999; Narnaware, Peyon, Lin, & Peter, 2000; Silverstein & Plisetskaya, 2000). In another study, pretreatment of T and E2in the gold fish, induced 2–3 fold increase of NPY mRNA expression levels in the forebrain (telencephalon/preoptic area) region. Moreover,T activated NPY synthetic activity in the preoptic area of brain that was revealed byin situhybridization (Peng, Gallin, Peter, Blomqvist, &Larhammar, 1994).
5.6.GDNF
In addition to these factors, glial cell line-derived neurotrophic factor(GDNF), which is a potent survival factor, is majorly involved in controlling several peripheral and central neurons, including dopaminergic neurons (Airaksinen, Holm, & H?tinen, 2006). GDNF signals bind to the GDNF family receptors-α through GPI-anchored receptor, which has a strong binding affinity over GFRα-1 (Trupp, Raynoschek, Belluardo, &Ibá?ez, 1998). Decreased expression of GFRα-1 was observed upon treatment with 1-methyl-1,2,3,6-tetrahydropyridine leading to neurodegeneration with the correlation of L-3,4-dihydroxyphenylalanine,catecholamines (CA), DA and NE levels in cat fish (Mamta & Senthilkumaran, 2018).
Overall, despite of these sex dimorphic markers identified in the brain except 5-HT, which is dominant to shown to induce male population with its 5-HT synthesis blocking drug, pCPA, otherwise all the other neurotransmitters are brain factors and all the brain sex changes are based on gonadal sex (Tsai, Wang, & Fang, 2001). In this context,mismatching sex steroids, i.e., E2to male and MT to female through osmotic pump delivery in the cat fish,C. gariepinus, produced differential effects on certain brain-related genes (Mamta, Sudhakumari, Kagawa,Dutta-Gupta & Senthilkumaran, 2020). This include minimal expression ofcyp19a1b,GnRH1,gfrα-1andthin the brain of MT treated female fish.On the other hand, increased expression ofcyp19a1b,GnRH1,gfrα-1andthin the brain of E2treated male fish (Mamta et al., 2020). However, the transcripts of some of these brain related genes were normally overexpressed in females compared to males (Senthilkumaran et al., 2015)indicating sex steroid based regulation. Thus, sexually dimporphic expression of tph was shown for the first time in tilapia wherein exclusive expression of tph is evident 5 to 20 dph male brain but not in female brain. Furthermore, in cat fish such gender specific differences were subsequently shown in tph, 5-HT and 5-HTP (Raghuveer, Sudhakumari,et al., 2011; Sudhakumari et al., 2010). Differential expression of brain related genes so far studied exhibiting sexual dimorphism play prominent role at brain level with response to gonadal differentiation, may causatively or consequentially responds to gonadal sex (Senthilkumaran et al., 2015). Changes in these brain related-genes along with CAs and serum 11-KT, E2, T levels indicate brain-gonadal interactions and their in fluence on the GnRH-GTH axis through gonadal recrudescence. Some of the sex reversal genetic markers so far identified from brain are useful to understand sex differentiation in addition to the endocrine changes in teleost under various conditions including endocrine disruption (Kar,Sangem, Anusha, & Senthilkumaran, 2021; Rajakumar & Senthilkumaran, 2020; Tenugu, Pranoty, Mamta, & Senthilkumaran, 2021).
6.Monosex population and strategy of a genetic cross
Teleost, most diverse group of vertebrates has increased globally and become attractive animal models for extensive investigation related to sex reversal strategies. In order to overcome certain difficulties exhibited by fishes, for example, lack of growth after maturation, attaining sexual maturity at different age, flesh quality deterioration which may lead to mortality and finally one gender performing better than the other. Considering these, it is worthwhile to produce monosex populations as well as reproductively sterile fish to overcome the hurdles.Sex reversal is one of the strategies/approaches used to produce monosex populations for several purposes in aquaculture. Due to sexual plasticity, fishes can be easily manipulated to induce sex reversal during critical stages by hormonal treatment to understand pubertal and growth related changes and sex dimorphism (Devlin & Nagahama,2002). The efficient and standard treatment widely used by farmers is through dietary administration of MT for 3–4 weeks from 10 dpf to produce monosex male populations in the Nile tilapia (Baroiller &D’Cotta, 2001; Baroiller, D’Cotta, & Saillant, 2009). In the Japanese hirame,P. olivaceus, both low as well as high temperature induced male monosex population whereas intermittent temperature produced 1:1 sex ratio (Yamamoto, 1999). Interestingly other methodologies such as genetic cross made applicable to produce monosex population, which was implied in few species. Initiating with the steroid treatment and combined with hybridization in order to produce sex reversal and sterile fish in cyprinids, cichlids and salmonids, the field has emerged very quickly towards chromosome manipulations, polyploidy and gynogenesis.
In the Nile tilapia,O.niloticusYY male broodstock was produced genetically on a commercial scale. Initially, fry with mixed population was treated with E2, to identify XY neofemales progeny, which were further crossed with XY males to identify YY males. YY males were then crossed to XY neomales, and further offspring were treated with estrogen and then progeny were tested for YY neofemales identification. YY neofemale and YY male were crossed to generate YY male broodstock production (Mair, Abucay, Abella, Beardmore, & Skibinski, 1997).Monosex XY population was generated finally after crossing YY male with normal XX female. A further selection of different strains within the broodstock lines were tested through the GMT? technique (Tuan, Little,& Mair, 1998; Tuan, Mair, Little, & Beardmore, 1999). This methodology was first developed inO.mossambicusfor small-scale supermale production (Pandian & Varadaraj, 1990). Whereas in medaka the monosex population was produced by using Hd-rR.YHNI strain males and crossing with the d-rR strain females and further crossing the males produced in the offspring once again with females of d-rR strain which ultimately resulted in d-rR.YHNI population of males (Scholz et al.,2003). In another study of turbot,Scophthalmus maximustreatment with androgen and crossing of induced classic females (fZW) with neomales(mZW) produced WW female population (Haffray et al., 2009). Female monosex population through the genetic cross was also performed in carp and salmonoids (Cnaani & Levavi-Sivan, 2009; Piferrer, 2001).However, better survival rates of YY males were demonstrated in yellow cat fish, tilapia and rainbow trout compared to low survival rates in other species such as medaka and fighting fish (George, Pandian, & Kavumpurath, 2013). This is a good strategy to develop monosex population across species which should be taken into consideration and make major inputs for research in aquaculture. The pedigree for the production of monosex population in tilapia through hormonal treatment followed by genetic cross and progeny selection is depicted in Fig. 2.
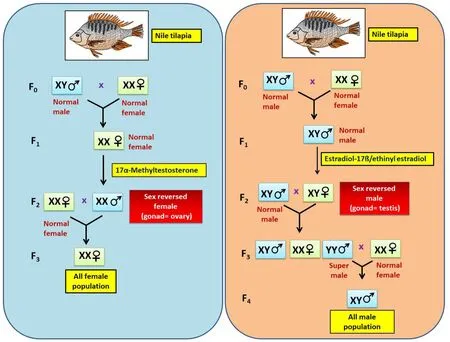
Fig. 2.Illustrating the genetic cross and pedigree selection in tilapia.
7.Conclusion and future perspectives
In both wild and domestic strains of teleost, existence of spontaneous sex reversed and induced sex reversed individuals have been reported.In most of the species, E2and 11-KT considered as the predominant steroids which are linked in the regulation of sex change. The establishment of monosex population in mammals is easy as X is bigger than the Y chromosome whereas in fish no distinguish X and Y chromosomes are present which is often difficult to distinguish through short gun gene approach. Inspite of this, genetic cross technique with XY, ZZ population made it easy to work for inducing sex reversal and this is the best way up to now to obtain monosex population. By combining both natural and induced steroid and environmental factors sex reversal had improved in more advance and used in species such as medaka, tilapia and trout which is often difficult. With the consideration of diversity and plasticity different species have varied mechanism to undergo sex-change by signifying independence evolvement of sex reversal. As reported the spontaneous and induced sex reversal in teleost understandably revealed the amazing sexual plasticity exhibited by fishes. The hormonal treatment warrants to maximize the growth with diverse nutrients also ensuring the meat quality and enhancing the commercial value of the edible fish. The technique or by the combination of ploidy induction provides a scope for the male and female broodstock development,which helps us to understand the underlying mechanisms in the sex determination and differentiation process. Studies on spontaneous and induced sex reversals in fish provides a clue for better understanding the amazing diversity exhibited by fish as well as vertebrate sex determination. However, the steroid hormones, environmental conditions stimulating specific sex bias and genes have an independent parallel differentiating effect which can interrelate to each other but often synergistically leads to sex differences in the brain. Future work in combination with economical relevance on sex reversal strategy ensure development of monosex population for aquaculture bene fits. Furthermore, understanding sex reversal in multiple fish species at the molecular level may provide new gene markers to distinguish endocrine disruption at all levels.
Con flict of interest
The authors declare no con flict of interest concerning to the research report in the manuscript.
Acknowledgements
The research work mentioned in this review was supported by grantin-aid (Ref. No. EMR/2017/000718) from the Science and Engineering Research Board, India to BS. ST is thankful to Senior Research Fellowship support by Council of Scientific and Industrial Research (Ref. No.09/414(1150)/2017-EMR-I), India. Authors acknowledge BUILDER Grant from DBT (Ref. No. BUILDER-DBT-BT/INF/22/SP41176/2020),India to School of Life Sciences, University of Hyderabad.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Reproductive farming technology in Japanese eel and chub mackerel
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Understanding the impact of stress on teleostean reproduction
- Germ cell markers in fishes - A review
