Germ cell markers in fishes - A review
Sultana Begum, Shabad Modinilla Gnanasree, Narlagiri Anusha,Balasubramanian Senthilkumaran
Department of Animal Biology, School of Life Sciences, University of Hyderabad, P.O. Central University, Hyderabad, 500046, Telangana, India
Keywords:
Germ cell markers
Sexual differentiation
Transplantation
Gonadogenesis
Germ-line development
A B S T R A C T
Identification of germ cell markers in fishes is crucial to track the germ cell differentiation and migration for manipulation of the cells to study sexual differentiation as well as to carry out transgenic transplantation techniques. Several germ cell-specific markers such as vasa, cnbp, dnd, nanos3, cbx2, amh, dmrt1, Ly75/CD205 have been characterized so far in fishes using localization and expression analysis, which have highlighted the spatio-temporal pattern of expression in early gonadal development. Incidentally, seasonal breeders show dramatic changes during gonadal recrudescence, which might also in fluence germ cell differentiation and growth to entrain the reproductive cycle. Hence, an in-depth analysis of the gonadal cycle is required to delineate germ cell progress, differentiation, and maturation explicitly. In this context, fishes undergoing gonadal recrudescence for the seasonal cycle show germ cell proliferation differentially. Most of these germ cell markers belong to the DEAD-box protein family of ATP-dependent RNA helicases sharing consensus motifs and clustering in phylogenetic analysis. These markers were found to be well-conserved throughout evolution. In situ hybridization approaches con firmed the germ cell specific distribution of these molecular markers. In addition, several genes such as fgf and gsdf seem to facilitate germ cell development and differentiation. Hence, more detailed studies on these factors will facilitate a better understanding of germ cell development. This review highlights various germ cell markers in fishes and their immense potential to use these cells for germ cell transplantation. The extensive knowledge of the germ cell markers can also be exploited to carry out other biotechnological experiments aiming at the preservation of genetic information of endangered species or the analytical study of gonadogenesis.
1.Introduction
Several important genes, along with environmental variables, are seemingly essential in sex determination and differentiation, wherein the role of germ cell development achieves greater importance. Highly specialized haploid germ cells are vital for the development of the organisms in contributing genes to the next-generation offspring and conserving the genetic identity of species throughout evolution. The pluripotent germ cell lineage retains the genetic information passing that on to subsequent generation to ensure the survival of species against all odds. Germ cell division conserves genomic identity and lineage. The undifferentiated germ cells undergo further proliferation and differentiation to give rise to haploid male and female gametes, spermatozoa and ova, respectively. The sperm and the ovum fuse via fertilization in a sex-specific manner to generate a diploid zygote that incorporates the genetic information of the parental genomes along with stabilizing some variations via genomic imprinting and recombinase (Nayernia, 2008).The germ cells being haploid, the chromosomal component of an organism is maintained in successive generations (Braat, Speksnijder, &Zivkovic, 1999). Sexual differentiation of germ cells is regulated by the surrounding somatic cells in a sex-specific manner; wherein germ cells are passive in terms of gonadal development. Conversely, studies using germ cell de ficient gonads and germ cells hyperproliferative gonads showed that the variations in germ cell number cause sex reversal,warranting a grave importance to germ cells during sex differentiation in zebra fish and loach (Nishimura & Tanaka, 2013). Gonadal morphogenesis follows different sex-specific developmental pathways,as in spermatogenesis and oogenesis in testis and ovary, respectively(Saito et al., 2007). Pluripotent, undifferentiated spermatogonial stem cells (SSCs) either undergo self-renewal to maintain the stem cell niche or differentiate into type A spermatogonia to commit to spermatogenesis. Later, type A differentiated spermatogonia further differentiate into diploid primary and haploid secondary spermatocytes successively(Lacerda, Costa, & de Fran?a, 2014). The secondary spermatocytes eventually give rise to haploid spermatozoa (Schulz et al., 2010).Whereas in the female ovary, oogonia mature into primary and secondary oocytes that further give rise to the haploid ova (Lubzens, Young,Bobe, & Cerd`a, 2010). Intriguingly, four spermatozoa are generated through each cycle of spermatogenesis while a single cycle of oogenesis gives rise to a single ovum. Pituitary gonadotropins, follicle-stimulating hormone, and luteinizing hormone take control of the gonadogenesis through the hypothalamo-hypophyseal axis in teleost (Chauvigné,Zapater, Crespo, Planas, & Cerd`a, 2014; Goos, Senthilkumaran, & Joy,1999; Senthilkumaran, 2011). The germ cells express several species-specific molecular markers such asvasa,dead end(dnd), nanos,octamer binding transcription factor(oct), soxand anti Müllerian hormone(amh),that were characterized (Nagasawa, Fernandes, Yoshizaki,Miwa & Babiak, 2013a; Tsuda et al., 2003; Sánchez, Camp, Espana,Tassias & Mullor, 2010; Chiang et al., 2001; Kobayashi, Kajiura-Kobayashi, Guan & Nagahama, 2008; Skaar et al., 2011). The expression of the markers can be analyzed further to enhance our knowledge of the role of germ cell markers in driving sexual differentiation. The data can be exploited by implicating bioengineering technologies to make pro fits in aquaculture and fisheries. Moreover, long-termin vitroculture of SSCs has been made possible. Germ cell enrichment and studies involvingin vitroculture will improve our understanding of germ cell development and promote implementation of diverse new generation bioengineering methodologies such as transgenics and cloning strategies (Nayernia,2007).
This review emphasizes the various molecular germ cell markers identified in fish species so far. The follow-up tracing of such markers will also help to identify the phylogenetic relationship among species that have retained the conserved genomic markers. In addition, the germ cell markers can be used to engineer transgenic organisms as well as preserve the genetic information of endangered species, and analysis of basic gonadogenesis along the sexual development pathway (Yoshizaki et al., 2011). This understanding could enhance the bulk production of fishes having high commercial demands to boost the fisheries industry further, and thereby aquaculture.
2.Sexual development and gonadal recrudescence
Reproduction in fishes goes through an intricate cycle of gonadal developmental maturation via pre-spawning, spawning, and postspawning phases. Both male and female gonads are subjected to a series of morphological and histological changes before attaining reproductive maturity (Braat, Zandbergen, van de Water, Goos, & Zivkovic,1999). Certain species exhibit gonadal recrudescence in a single breeding season to allow multiple spawning on induction of temperature, photoperiod, or other environmental signals. Incidentally, these cues regulate sex determination and differentiation as well (Chattopadhyay & Chattoraj, 2017; Rajendiran et al., 2021). Gonadal recrudescence is a period of active gametogenesis, gonadal growth, and substantial increase of sex steroid hormones that culminates with ovulation or spermiation, and spawning in a seasonal or reproductive cycle (DeFalco & Capel, 2009). The periodicity of spawning is predicted by the gonadosomatic index, which reaches the maximum during peak maturity and abruptly diminishes after spawning. This phenomenon is correlated to the gonadotropin-releasing hormone (Boonkusol, Junshum, & Panprommin, 2020; Senthilkumaran, Okuzawa, Gen, Ookura,& Kagawa, 1999). Most seasonal breeders and even the marine teleosts like the Tobinumeri dragonet,Repomucenus beniteguri, undergo gonadal recrudescence, which in fact, replicates sexual development similar to initial gonadal development (Chattopadhyay & Chattoraj, 2017; Zhu,Furukawa, Aida, & Hanyu, 1989). In this context, the cat fish seems to be an excellent animal model to study germ cell progression and gonadogenesis during early sexual development (Senthilkumaran & Kar, 2021;Sudhakumari & Senthilkumaran, 2013). This study also explains the natural phenomenon of gonadal recrudescence, for it shows sexual dimorphism, and the male individuals grow much faster than females of the same age (He et al., 2017; Senthilkumaran & Kar, 2021). Gonadal sex differentiation is controlled by genetic and endocrine regulations after the bipotential gonad commits to a specific fate. In the Japanese medaka,Oryzias latipes,dmrt1bytakes control of the differentiation of primordial germ cells (PGC) and interstitial cells. Cells lackingdmrt1byrather expresscyp19a1and differentiate into female sex precursor cells.nanoswas the earliest marker characterized in PGCs in medaka (Nishimura & Tanaka, 2014). Germ cell migration, proliferation, and differentiation during gonadogenesis were well-studied in the Japanese flounder,Paralichthys olivaceus. The female population was mated with sex-reversed males, and all genetic female (XX) broods were thus artificially produced (Yang et al., 2018). These studies showed mitotically inactive PGCs migrated from the lateral to the dorsal trunk region to form the gonad with differential timing of development eventually. The expression of the germ cell markerpofxl2in the PGCs was evaluated to track the molecular differentiation pathway (Yang et al., 2018).
3.Identification of germ cells
Identification of the germ cell molecular markers seems indispensable to trace the germ cell differentiation, migration and for manipulating the gonadal cells through new age transgenics technologies,including CRISPR-Cas9. But, to move on to further study the germ cell marker characteristics, the concept of identification of a germ cell seems imperative and a preceding step.
Germ cell lineage significantly varies from the somatic cell precursors in its morphology, and can be traced back throughout embryogenesis via light and electron microscopy or histochemistry. PGCs are easily recognized under the microscope by their comparatively large size, distinct nuclear membrane, voluminous, eccentrically located nucleus with homogenous distribution of chromatin in the nucleoplasm,and typical electron-dense ‘nuage’ like structures (Kobayashi,Kajiura-Kobayashi, Guan, & Nagahama, 2008; Deniz Ko? & Yüce, 2012;Dietrich & Krieger, 2009). These dense granular bodies are ribosomes or glycogen granules dispersed in the cytoplasm, mainly surrounding the mitochondria, and can be considered an efficient marker for germ cell identification with the help of Best Carmin or alkaline phosphatase staining (Van Winkoop, Booms, Dulos, & Timmermans, 1992; Deniz Ko?& Yüce, 2012). The nuage-mitochondria association was found to be essential for the differentiation of germ cells inPiaractus mesopotamicus(Abdalla & Cruz-Landim, 2004). PGCs are easily distinguished from the somatic cells in the early embryonic phases of medaka by the presence of granules of melanin pigment in the eyes (Satoh & Egami, 1972).Moreover, eosinophilic granules have been reported as a potential histological marker unique to PGCs in medaka (Gamo, 1961). Germ cell lineage can also be distinguished by detecting the presence ofvasatranscripts or protein in the early embryonic cells, as only the germ cells retain the same (Yoon, Kawakami, & Hopkins, 1997; Braat, Zandbergen,et al., 1999; Knaut, Pelegri, Bohmann, Schwarz, & Nüsslein-Volhard,2000). Expression and subcellular localization studies ofvasamRNA in tilapia introduced the use of anti-vasaantibody (α-TDc) to stain corresponding vasa protein bands to differentiate germ cells from somatic cells. No immunoreactivity was recorded in the somatic cells by immunohistochemistry. This discovery ofvasabeing exclusively expressed in the germ cells paved the way for future research regarding the identification of germ cells directed at the use of germ cell specific markers (Kobayashi, Kajiura-Kobayashi, & Nagahama, 2002). Later,Kobayashi et al. (2008) investigated the expression pro files ofdmrt1andsox9ain comparison with morphological sex differences during gonadal differentiation, with reference to the changes in germ cell number and histogenesis (Kobayashi et al., 2008).
A recent experimental study hinted at an inherent feminizing effect of the germ cells on the gonads, and any manipulation in the number of proliferating germ cells may lead to sex reversal in the teleost species(Nishimura et al., 2018). Three medaka knock out mutants targeting the genes,figlα,meioCanddazl, were generated using TALEN and CRISPR-Cas9 technologies to denote the feminizing effect of germ cells on the gonads (Nishimura et al., 2018). Studies on this line implicated that PGCs are sufficient to support female gonadal development, and there lies a fleeting time frame during which the germ cells induce female sex differentiation of the gonad by imparting its feminizing effect on the somatic cells (Nishimura et al., 2018). The bipotential gonad may develop into a functional ovary after this window, even if the germ cells deplete over time, especially in thedazl-/-mutants (Nishimura et al.,2018).
4.Identification of germ cell markers
Germ cell markers expressed exclusively on the germ-line stem cells,can be characterized byin situhybridization or immuno fluorescence.qPCR analysis showed site-specific expression ofvasaanddndin testis and ovary of the Atlantic salmon,Salmo Salar. The differential distribution patterns of the marker genes were evaluated which depicted that,vasatranscripts were randomly distributed in salmon blastocyst as opposed to the typicalvasalocalization pattern of four clusters as found in the zebra fish,Danio rerio. Thus, the localization of germ cell markers seems to be species-specific (Nagasawa, Presslauer, et al., 2013).dnd,pivotal for PGCs migration and differentiation, was characterized in the Chinese sturgeon,Acipenser sinensis. Gene sequence BLAST and multiple sequence alignment showed six conserveddndortholog regions,furthermore, fluorescentin situhybridization con firmed the abundance ofasdndin germline that tends to gradually diminish as spermatogonia differentiate into late spermatogenic stages. In contrast, expression leveled up in primary oocytes compared to oogonia in the ovary (Yang,Yue, Ye, Li, & Wei, 2015). Gonad specific expression ofnanogandoct4were observed with qPCR analysis in spermatogonia of medaka testis(Wang et al., 2011). Experimental studies carried out to identify the gene markers in the Neotropical cat fish,Leiarius marmoratus, clarified the duration of spermatogenesis, and was used for evaluating the expression of selected stem cell genes in testis (Lacerda et al., 2019). The results showed a significant decrease of germ cell nuclear volume throughout the differentiation of type An undifferentiated spermatogonia to late-type B as well as from diplotene to late spermatids stages.In situhybridization con firmed high levels ofplzfandpou5f3expression in type An undifferentiated spermatogonia (Lacerda et al., 2019). Thus,characterization of the germ cell markers helps in further understanding of the germ cell migration and differentiation, and to study embryology and gonadogenesis.
5.Germ cell markers
5.1.vasa
Incidentally,vasawas the first identified marker inDrosophila, and it contributes to the PGC commitment to differentiate into presumptive gonads (Schüpbach & Wieschaus, 1986; Hay, Ackerman, Barbel, Jan, &Jan, 1988). The gene encodes for the DEAD-box family protein of ATP-dependent-RNA helicase (Hay et al., 1988; Liang, Diehl-Jones, &Lasko, 1994) conserved in the species along with the evolutionary progression. Later, the marker was studied extensively in the Atlantic salmon, Sturgeon, cat fish and many other teleosts (Nagasawa, Fernandes, et al., 2013; Raghuveer & Senthilkumaran, 2010a; Yang, Ye,Yue, Li & Wei, 2016). Localization ofvasaensured the migration of PGCs from mesonephros to genital ridges to further differentiate and develop into germ cell lineage (Knaut, Pelegri, Bohmann, Schwarz & Volhard,2000). Immunohistochemistry and expression analysis by semi-quantitative PCR and qPCR in brown-marbled grouper documented the localized expression ofvasahomologs rich in arginine and glycine repeats in germ cell lineages exclusively (Boonanuntanasarn et al., 2016). qPCR andin situhybridization carried out in the Pacific Abalone,Haliotis discus discus, usingvasaas a control, revealed thatvasawas expressed in the very beginning of germ cell development in both sexes in abundance, and helped to regulate the development of germ cells (Yu et al., 2018). Quantitative increase invasagene expression studies in cat fish con firmed the linear relationship between the abundance of germ cells and gene expression (Raghuveer & Senthilkumaran,2010a). Females recorded higher gene expression owing to the meiotic arrest in the differentiation of ova and thus elevated numbers of primary oocytes in the ovary (Raghuveer et al., 2011). Differential gene expression can also be attributed to distinct phases in reproductive cycles. Marginally highervasatranscripts persisted in spermatogonia during spermatogenesis that eventually reduced with the differentiation into primary and secondary spermatocytes, spermatids and spermatozoa(Raghuveer & Senthilkumaran, 2010a).In situhybridization and qPCR carried out in the summer flounder,P. dentatus, demonstrated thatvasaexpression was substantially higher in spermatogonia and spermatocytes, while no expression was recorded in spermatids and spermatozoa(Du et al., 2022). Similarvasalocalization studies were carried out in striped cat fish to investigate the migration and proliferation of PGCs in early gonadogenesis (Duangkaew, Jangprai, Ichida, Yoshizaki, & Boonanuntanasarn, 2019). In fact, qPCR andin situhybridization analyses revealedphy-vasaexclusively expressed in germ cells of gonads, and migration of PGCs took place at 2–10 days post fertilization (dpf)(Duangkaew et al., 2019). Northern blotting carried out in zebra fish showedvasatranscript in embryos post-fertilization, suggesting it is maternally inherited. Moreover,vasatranscript was found to localize exclusively to the cleavage planes in 2- and 4- cell-stage embryos. This study facilitated the basis for further studies on germ cell marker localization patterns and germ-line maturation and proliferation (Yoon et al., 1997).
5.2.sox9
sox9is one of the most pivotal germ cell markers for the differentiation of male sex from the bipotential gonad across all vertebrates(Chiang et al., 2001; Kobayashi et al., 2008; Nakamura et al., 2012;Raghuveer & Senthilkumaran, 2010b). The gene family codes for several transcription factors containing a DNA binding HMG motif (Sinclair et al., 1990) highly conserved in its constituent amino acid residues throughout the evolution of fishes. Orthologs of the tetrapodsox9gene,that supposedly have arisen through whole-genome duplication, have been discovered in zebra fish and medaka (Amores et al., 1998; Postlethwait, Amores, Cresko, Singer, & Yan, 2004). Variants of thesox9gene persist in different species for experimental studies have documented the presence ofsox9a1andsox9a2in testis, ovary, and ova-testis of rice field eel, whereassox9a2expression is the highest in the mature testis of medaka (Nakamoto, Suzuki, Matsuda, Nagahama, & Shibata,2005). Zebra fish and cat fish showed distinctive expression ofsox9aandsox9bin testis and ovary, respectively (Chiang et al., 2001; Raghuveer &Senthilkumaran, 2010b), andsox9expression was higher in testis of the Nile tilapia,Oreochromis niloticus(Ijiri et al., 2008). This dimorphic expression of gene isoforms supports either testicular or ovarian differentiation from the bipotential gonad through gene duplication(Chiang et al., 2001; Kobayashi et al., 2008). Quantitative PCR analyses were performed in the Nile tilapia to ascertain a de finite period when genes associated with sex differentiation are expressed in the gonad. The study revealed thatsox9had male-specific expression, being abundant only in the later stages of testicular differentiation (Ijiri et al., 2008). The expression ofsox9was documented in the undifferentiated gonad of cat fish days 40 post-hatch (dph) (Raghuveer & Senthilkumaran, 2010b).Seasonal and experimental studies in the common carp,Cyprinus carpio,revealed that the expression ofsoxgenes in a gonadal cycle is gonadotropin and androgen-dependent (Anitha & Senthilkumaran, 2020). The role ofsox9in gonadal differentiation is debatable; it was expressed initially in the supporting Sertoli cells of male testis rather than the spermatids or mature spermatozoa in cat fish (Raghuveer & Senthilkumaran, 2010b). Expression ofsox9bin the germ cells was also evaluated in medaka, which indicatedsox9might be highly involved in the proliferation and survival of both male and female medaka gonadal germ cells without directly in fluencing testicular determination (Nakamura et al., 2012). Furthermore, expression pro filing ofsox9aduring gonadal differentiation and hormone-induced sex reversal in the Nile tilapia revealedsox9adid not show any sexual dimorphism before the determination of sex, and was not expressed in the efferent duct of the testis(Kobayashi et al., 2008). Expression ofsox9in oogonia suggested its ability to express simultaneously in different cell types. The differential expression ofsox9further hinted at co-localization and cross-talks of several germ cell marker genes to impart testicular or ovarian differentiation to the bipotential gonad (Raghuveer & Senthilkumaran,2010b). The regulatory mechanisms ofsox9expression and its interplay with other germ cell markers are yet to be discovered, but enough evidence is available to rendersox9as an efficient germ cell marker(Nakamura et al., 2012; Raghuveer & Senthilkumaran, 2010b).
The interplay ofsox30andsox9in regulating testicular maturation have been demonstrated in the common carp,C. carpio(Anitha & Senthilkumaran, 2020). Furthermore,sox30expression is gonadotropin and androgen-dependent; transient silencing ofsox30with downregulatedsox9affected testicular steroidogenesis in common carp (Anitha &Senthilkumaran, 2020). The role ofsox30in sexual differentiation was con firmed by CRISPR-Cas9 mediatedsox30mutation, that led to dysfunction in spermiogenesis. Moreover, chromatin immunoprecipitation followed by high throughput sequencing revealedsox30regulates the transcriptional efficiency of a couple of genes,ift140andptprb. These genes are involved primarily in spermiogenesis in the Nile tilapia (Wei et al., 2021). Likewise,sox19transient gene silencing bysox19-siRNA treatment resulted in a decreasedcyp19a1activity and sparse expression of a few steroidogenic enzyme genes. This indicated thatsox19plays a regulatory role in ovarian growth and steroidogenesis in common carp(Anitha & Senthilkumaran, 2022).
5.3.gsdf
Gonadal soma-derived factor (gsdf), a member of the TGFβ superfamily, is crucial for male sex initiation, testicular differentiation, and early SSC development in teleosts (Jiang et al., 2019; Yan et al., 2017;Zhu et al., 2018). Tissue distribution studies in the Chinese tongue sole,Cynoglossus semilaevis, have revealed higher expression ofcs-gsdfin the testis than ovary.In situhybridization analysis showed the abundance of thecs-gsdfmRNA in the Sertoli cells, spermatogonia, and spermatids in the mature testis of the Chinese tongue sole. Gene knockdown by transient siRNA interference ofgsdfresulted in dysregulation of a number of sex differentiation genes, namelycs-wnt4aandcs-cyp19a1aindicating an essential role ofgsdffor this correlate in driving male sex differentiation in the Chinese sole (Zhu et al., 2018).gsdfhas been considered a major male sex determinant, and hence use of gene deletion strategies in medaka demonstrated its relevance for maleness (Zhang et al., 2016).Incidentally,gsdfacts as a downstream molecule ofdmrt1to initiate testicular differentiation after the execution of sex determination byDMY, followed bydmrt1.dmrt1bYorDMYin the Y chromosome determines maleness by activatingdmrt1and its downstreamgsdfto initiate testicular differentiation (Kondo et al., 2003; Zhang et al., 2016).Cloning of the genomic DNA andin silicopromoter analysis carried out in the spotted scat,Scatophagus argus, showed that thegsdfpromoter motif contains presumptive transcription factor binding sites fordmrt1andsf1(Jiang et al., 2019).
Moreover,gsdfhas also been demonstrated to play a significant role in androgen induced XX sex reversal in medaka by induction of the androgen-gsdf-dmrt1cascade (Horie et al., 2016). Althoughgsdfpredominantly localized in the male gonad, its expression was evident in the surrounding soma cells of ovary. It regulates the maturation of ovarian follicles, fertility as well as steroidogenesis in female zebra fish(Yan et al., 2017). Overall,gsdfseems to regulate gonadal differentiation by acting as a downstream molecule to various genes (Jiang et al.,2019).
5.4.DMY
DMY, a duplicated copy ofdmrt1, was the first identified gene on the Y chromosome, in the Japanese medaka (Matsuda et al., 2002, 2003)and is considered to be the major sex-determining gene in medaka species (Kobayashi, 2013). Quite a few studies demonstrated thatDMYappears to act as a sex-determining gene in a few medaka species, for example,O. minutillus,O. dancena, andO. luzonensis.(Myosho et al.,2012; Nagai, Takehana, Hamaguchi, & Sakaizumi, 2008; Takehana,Demiyah, Naruse, Hamaguchi, & Sakaizumi, 2007).sox3present upstream togsdfupregulated the expression ofgsdf, which acts onDMYand is indirectly involved in testicular differentiation inO. dancena(Matsuda& Sakaizumi, 2016; Takehana et al., 2014). The mRNA and protein ofDMYwere specifically expressed in somatic cells surrounding the PGCs,indicating that it promotes proliferation and differentiation of PGCs in medaka (Kobayashi et al., 2004). In medaka,dmrt1y,located on the Y chromosome, has 93% nucleotide similarity todmrt1. While,dmrt1is located on the autosome along with its paralogsdmrt2anddmrt3,indicating an ancient duplication event. Thedmrt1yshowed 96% nucleotide similarity to theDMYgene (Matsuda et al., 2002). The expression ofdmrt1ywas seen only in male embryos during embryogenesis, beginning as early as the neurula stage, and persisted throughout larval to adulthood in medaka (Nanda et al., 2002).
5.5.dmrt – double sex and mab 3 related transcription factor
dmrtgene family, consisting of a zinc finger binding motif, has several isoforms encoding transcription factors consisting of a DM domain, that has been well conserved throughout evolution (Zafar,Rather, & Dhandare, 2019).dmrtgenes are involved in sexual development and differentiation (Marchand et al., 2000; Kobayashi et al.,2004). Sevendmrtgenes have been discovered so far in fishesdmrt1,dmrt2a, dmrt2b, dmrt3, dmrt4, dmrt5&dmrt6(Dong et al., 2020; Winkler et al., 2004). In tilapia,DMO,which is similar todmrt, was expressed in the adult ovary, and is responsible for female-specific gene expression and sexual differentiation (Guan, Kobayashi, & Nagahama, 2000).dmrt1is the first conserved gene involved in sex differentiation found in the invertebrates as well as in human (Marchand et al., 2000). In the Nile tilapia,tdmrt1was expressed mainly in Sertoli cells and the epithelial cells of intra-testicular efferent duct in the testis, whereastdmrt1did not seemingly express in the ovary (Kobayashi et al., 2008). Expression ofdmrt1was seen in the Sertoli cells localized nearby the proliferating spermatogonia in the rainbow trout,Oncorhynchus mykiss(Marchand et al., 2000). Similar localization pattern ofdmrt1was observed in the Sertoli cells and the surrounding spermatogonial cells in mature testis as con firmed byin situhybridization analysis in platy fish,Xiphophorus maculatus(Veith et al., 2006). The expression ofdmrt1correlated with the proliferation of spermatogonia in the rainbow trout,O. mykiss, and the Japanese eel,Anguilla japonica, indicating its primary involvement in testicular differentiation vis-`a-vis testicular development (Jeng et al.,2019; Marchand et al., 2000). However, the function ofdmrt2genes was not conserved throughout the evolutionary period. In zebra fish,dmrt2awas involved in symmetric somite formation and pharyngeal cartilage(Lu et al., 2017), whiledmrt2bwas responsible for positioning the organs asymmetrically via the hedgehog signaling pathway (Li et al., 2018;Zhou et al., 2008). The overexpression ofdmrt2acaused defects in left and right asymmetry as well as desynchronization of somite clock genes(Liu, Li, & Gui, 2009). In the Japanese puffer fish,Takifugu rubripes,dmrt3recorded ample expression in the testis and nervous system, and played an essential role in the development of germ cells and nerves in zebra fish (Li et al., 2008; Yamaguchi et al., 2006). Expression ofdmrt4was higher in the ovary than testis, and minimal expression was seen in kidney, spleen, gills, and brain of the Japanese puffer fish (Yamaguchi et al., 2006). In zebra fish,dmrt5was highly expressed in the brain,followed by gonads, indicating its importance in neurogenesis and spermatogenesis (Graf, Wen, Saruise, Rajaei & Winkler, 2015; Guo et al.,2004). On the contrary,dmrt6was believed to be eliminated throughout evolution, and no records of the gene were found in fishes, but recent studies in tilapia revealed that it has a vital role in spermatogenesis(Zhang et al., 2014).
5.6.Wilms tumor suppressor gene (wt1)
wt1family encoding a zinc finger transcription factor, plays an important role in the development of the genitourinary system in mammals as well as regulates the maintenance and survival of PGCs in teleosts (Bollig et al., 2006; Klüver, Herpin, Braasch, Driessle, & Schartl,2009). Twowt1genes –wt1aandwt1bare expressed in teleosts, while three variants of thewt1gene are seen in the rainbow trout (Brunelli,Robision & Thorgaard, 2001; Jiang et al., 2017). In zebra fish,wt1awas expressed in lateral plate mesoderm during early embryonic development, 10–11 h post-fertilization (hpf) in intermediate mesoderm and the expression ofwt1bwas seen 3 h after the expression ofwt1a(Perner,Bates, Nauman & Englert 2016). Immunohistochemistry and immunofluorescent studies showed thatwt1localized in spermatogonia and spermatocytes of adult testis of cat fish (Murugananthkumar & Senthilkumaran, 2016). In medaka,wt1awas expressed in undifferentiated gonads of both sexes but had an elevated expression in testis, whilewt1bwas expressed in developing gonads (Klüver et al., 2009). In the Nile tilapia, expression ofwt1awas seen in the interstitial, theca, and granulosa cells of the ovary, whilewt1bwas expressed predominantly in interstitial cells (Jiang et al., 2017). Morpholino-based knockdown ofwt1aandwt1bin medaka did not show any effect individually, but knockdown of both genes together showed a decreased number of PGCs(Klüver et al., 2009). Deletion of thewt1agene in the Nile tilapia was found to be lethal, whilewt1bdeletion showed normal development.This deletion strategy explains thatwt1bis not essential for testicular development (Jiang et al., 2017).
5.7.Octamer binding transcription factor 4 (oct4)
Octamer binding proteins are a group of highly conserved transcription factors that adhere to the octamer motif and belong to the class V POU domain transcription factor. Expression ofoct4is seen in pluripotent embryonic and germ cells.oct4is expressed aspou51in mammals while it ispou2in teleosts (Sánchez et al., 2010).pou51in mammals evolved by the duplication ofpou2. The expression patterns ofoctwere different in transgenic medaka and zebra fish (Sánchez et al.,2010). In zebra fish,oct4ortholog,spg/pou2, is essential for pre-gastrula morphogenesis, and formation of the mid-hindbrain boundary (Belting et al., 2001; Burgess, Remi, Chen, Hopkins & Brand, 2002). In medaka,oloct4, an ortholog ofoct4, was detected in the early embryo until the blastula stage (Liu et al., 2015). At the mid-blastula stage, it gets limited to the cells around the embryonic streak (Froschauer et al., 2013; Liu et al., 2015). In medaka, expression ofoloct4was comparatively high in the ovary than the testis. Only the germ stem cell population of testis recorded expression ofoct4. In the ovary, all peripheral cells and oocytes expressedoloct4and this had a vital role in gamete maturation (Froschauer et al., 2013). In medaka, reduction ofoct4at high levels affected gastrulation and axis formation; and low-level reduction ofoct4showed defects in the brain, eye, and blood vessels (Sun, Gui, Liu, Hong, & Li,2020 Apr 5). Based on these studies, the role ofoct4seems to extend beyond germ cell function, especially embryogenesis.
5.8.Anti-Müllerian hormone (amh)
amh, a constituent of the TGFβ superfamily, was identified in 1947 by Alfred Jost in rabbits and humans, and is considered to play a regulatory role in gonadal development and gametogenesis in teleost fishes(Pfennig, Standke, & Gutzeit, 2015). It is crucial to testicular differentiation and male sex initiation by inducing caudal to cranial regression of the Müllerian duct, a typical feature of the female sex (Beau et al.,2001; Josso, 2008). Almost all the vertebrates show a distinctive feature of the female urogenital system by having Müllerian ducts. Although most teleosts lack Müllerian ducts, (Skaar et al., 2011), anamhhomolog was discovered to be expressed predominantly in the Sertoli cells of immature testis throughout gonadal differentiation. The orthologs ofamhparticipate in driving testicular development as well. Studies revealed thatamhis an essential marker for male sex differentiation in mammalsin vivo(Beau et al., 2001). Likewise, a mutation in theamhreceptor led to dysregulation of germ cells and sexual development in medaka (Morinaga et al., 2007). In testis,amhregulated the expression of steroidogenic enzymes,cyp11a1&cyp17, which are very much essential for testosterone production in the gonad of zebra fish males(Wang & Orban, 2007).amhsuppressescyp19a1awhich helps in testicular differentiation in zebra fish, also brings about differentiation of Leydig cells to enhance the production of male-specific testosterone(Wang & Orban, 2007). Incidentally,amhwas expressed 6 times higher in testis than in ovaries, and during gastrulation. In contrast, ontogenic expression ofamhwas 25 times higher in male than the female embryos of the mosquito fish,Gambusia holbrooki. This study showed thatamhmodulates the proliferation and migration of PGCs in teleost (Kwan &Patil, 2019). The dimorphic expression ofamhduring sex differentiation was observed in the Japanese flounder in a sex-specific manner (Yoshinaga et al., 2004). Previously it was reported that,amhexpression was observed in undifferentiated gonads of both sexes in tilapia. The expression was upregulated in male gonads remarkably and downregulated in females (Ijiri et al., 2008). Moreover, degeneration ofamhresulted in the transformation of the juvenile ovary into testis during the development. Gene expression analysis ofamhandcyp19a1aduring the gonadal transformation in tilapia showed upregulation ofamhand downregulation ofcyp19a1a(Wang & Orban, 2007). Most of the studies revealed thatamhshowed an inhibitory effect on the germ cell meiotic phase in both sexes, which resulted in negative feedback on the levels ofamhin somatic cells. In addition,amhexpression was reported in the Sertoli cells surrounded by type A spermatogonial cells. The expression diminished in primary spermatogonia after spermatogonial differentiation, indicating an inhibitory effect ofamhin the differentiation of male germ cells of zebra fish gonads (Skaar et al., 2011). These findings indicated thatamhseems to have a significant functional role in sex determination/differentiation. An ortholog ofamh, the y-linkedamhy,was reported in the Nile tilapia, with a missense mutation from C to T,thus replacing the amino acid serine with leucine (Li et al., 2015). Amhy is a non-transcription factor protein, a member of TGFβ, and is quite similar toamh.amhyknock out in XY fishes by using new age transgenic tool CRISPR-Cas9 resulted in upregulation offoxl2andcyp19a1ato induce ovarian differentiation, and eventually, sex reversal from male to female (Hattori et al., 2012; Yoshida et al., 2014). Similarly,amhyoverexpression in XX fishes resulted in the sex reversal from female to male (Li et al., 2015) andamhr2knockout resulted in male to female sex reversal (Liu et al., 2020).
5.9.nanos
nanosgene isoforms encoding for a zinc finger motif protein are considered to be essential for gonadal differentiation in vertebrates.However, the functional implications of expression patterns of the different variants remain not well understood. In zebra fish, three isoforms ofnanoswere found, while four forms were seen in fugu and medaka (De Keuckelaere, Hulpiau, Saeys, Berx, & van Roy, 2018; Aoki,Nakamura, Ishikawa, & Tanaka, 2009). The role ofnanosto facilitate germ cell survival and pluripotency has been conserved throughout evolution. Most of the studies revealed thatnanosare expressed predominantly in the undifferentiated PGCs and a conserved function ofnanosin germ cell development, especially in males, was demonstrated in invertebrates and vertebrates (Wang, Zayas, Guo, & Newmark, 2007;Julaton & Reijo Pera, 2011; Tsuda et al., 2003). Furthermore, functional studies demonstrated thatnanos2andnanos3showed differential expression in PGC. The expression ofnanos2was reported in male germ cells andnanos3in migrating PGCs (Tsuda et al., 2003). Microarray studies reported that few genes related to male germ cell differentiation are affected innanos2-/+andnanos2-/-embryos in mice (Saba, Kato,& Saga, 2014; Suzuki, Tsuda, & Saga, 2007). In zebra fish,nanos1expression was observed in germplasm and PGCs, and localization studies revealed thatnanosare important for germ cell development(K?prunner, Thisse, Thisse, & Raz, 2001). Additionally, the decreased levels ofnanos1in the embryo of zebra fish showed an effect on the migration and survival of the PGCs, indicating the crucial role ofnanos1in PGCs development (K?prunner et al., 2001). In zebra fish, it was demonstrated thatnanos1is crucial for the maintenance and regulation of oocyte production (Draper, McCallum, & Moens, 2007).nanos2is a marker of germline stem cells;nanos2knockout in zebra fish resulted in the absence of gonadal stem cells at 32 dpf, and the mutant showed a transformation of female to sterile male fish at 75dpf. This study disclosed that nanos2 regulates the development of gonadal stem cells in zebra fish (Cao, Yang, & Luo, 2021). In general,nanosseem to play a pivotal role in germ cell development and function, as evident from reports using zebra fish and other teleosts (K?prunner et al., 2001;Presslauer, Nagasawa, Fernandes, & Babiak, 2012).
5.10.Fibroblast growth factor (fgf)
Fgfs are a group of proteins secreted by polypeptide growth factors,and they bind to Fgf receptors and regulate many functions such as cell proliferation, migration, survival, differentiation, sex determination/differentiation (Yun et al., 2010). Similarly,fgfis also involved in germ cell migration and regulates several germ cell functions (Takeuchi,Molyneaux, Runyan, Schaible, & Wylie, 2005). In medaka,fgf2regulates the proliferation and differentiation of the ovarian granulosa and follicle cells by acting as a paracrine factor initiating vitellogenesis (Watanabe,Kobayashi, Ogawa, & Onitake, 1998). In zebra fish,fgf20is involved in fin regeneration (Whitehead, Makino, Lein & Keating, 2005). In the Nile tilapia,fgf16was expressed in all tissues apart from the brain, spleen,and liver; whilefgf20awas expressed in brain, pituitary, and spleen.fgf20b,on the other hand, was found to express in the intestine and ovary. Experimental research conducted in the Nile tilapia revealed that the expression offgf16andfgf20were higher in the normal as well as the sex-reversed gonad (Sun et al., 2012). High expression levels were seen in the XX ovary and 17β estradiol induced XY ovary compared to the XY testis and fadrozole induced XX testis. This study proposed that thefgf16/20subfamily is crucial for oogenesis in the female gonad (Sun et al., 2012). However, no such role of thefgfsubfamily is established in male fish. Nevertheless, additional studies are necessary to implicate a precise role offgfs in teleostean reproduction. In line with this, it has been shown recently thatsox19regulatesfgfisoforms as well as ovarian steroidogenesis related-enzyme genes in the common carp,C. carpio(Anitha & Senthilkumaran, 2022). A schematic diagram of the gene ontogeny of various germ cell markers in teleosts has been represented in Fig. 1.
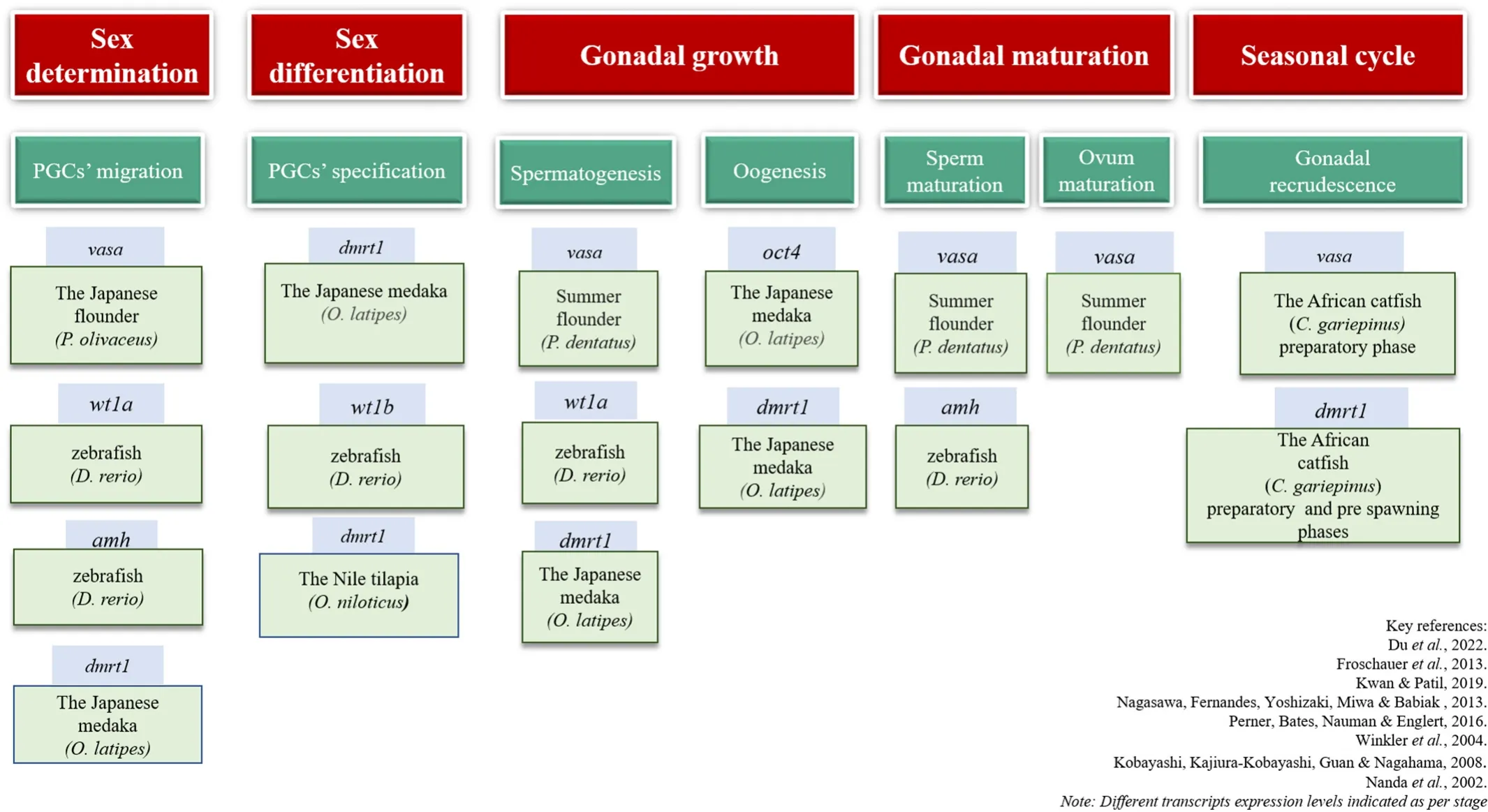
Fig. 1.A schematic diagram of the gene ontogeny of various germ cell markers in teleosts has been represented in the Fig. 1.
6.Germ cell markers interplay
Interaction between germ cell marker variants is indispensable for gonadal maturation and differentiation. The de ficit of any of these gene expressions would hinder the usual molecular differentiation pathway.In line with this, some of the reports indicated the interplay of germ cell markers (Lin et al., 2017). Transcriptome pro filing studies in the Nile tilapia revealed regulation ofsox30promoter by thecis-regulatory element fordmrt1, which is pivotal for male-sex differentiation.Knockdown ofdmrt1resulted in a substantial decrease insox30gene expression (Tang et al., 2019). Visualization of Turbot PGCin vivoby 3′-UTR fluorescent tagging provided an understanding that there lies a sequence homology in 3′-UTRs ofnanos3andvasa, such as GCACs, 62 bp U-rich 187–218 regions nucleotides, that work in association with each other to stabilize the PGCs (Zhou et al., 2019). Furthermore, hybridization experiments revealed a homolog of eukaryotic translation initiation factor-transporter, named OI4E-T, identified in the adult medaka gonads. It interacts with bothnanos3andolvas, and localizes in the similar gonadal regions. This interplay is crucial throughout embryogenesis (Zhao et al., 2013). Incidentally, to our knowledge, the interplay of other germ cell markers such asvasa,oct4,amh,andfgfon germ cell differentiation and function is not reported. Further studies on this line may facilitate a better understanding of the germ cell proliferation and maturation.
7.Potential applications of germ cell markers: highlights
Germ cell markers provide an opportunity to manipulate the embryonic cell lines and perform germ cell transplantation or other transgenic methodologies to preserve the genetic data of endangered species or analytical study of germ cell maturation and differentiation (Li, Fujii,Naito, & Yoshizaki, 2017; Vasconcelos et al., 2019). Germ cell marker gene knockdown strategies performed in the channel cat fish,Ictalurus punctatus, to render sterilization of the species depicted the role of the markers in gonadal differentiation and maturation. PGC marker genes,nanos,anddnd, were suppressed by complementary DNA overexpression and short hairpin RNA interference methods, and the engineered specimens were evaluated for delayed or redundant sexual maturity (Su et al., 2015). These experimental studies showed a significant reduction in sexual maturity in three-year-old brood fishes, in which knockdown constructs are introduced to hinder the expression of the markers.Copper sulfate was used as a repressor compound to restore sexual maturity, and this method seems to be an efficient way of repressible transgenic sterilization (Su et al., 2015). Precautionary measures should be taken while executing such a strategy, as copper nanoparticles can impair testicular development and growth (Murugananthkumar, Rajesh,& Senthilkumaran, 2016; Gupta et al., 2016). Characteristics of germ cell markers were exploited in successful germ cell transplantation in salmonids as well. Experimental studies were carried out to generate germ cell marker specific monoclonal antibodies for rainbow trout and zebra fish (Hayashi et al., 2019).pvasa, exclusively expressed on type A spermatogonia, was tagged with GFP, and the recombinant cell was introduced in mice for bulk production of A-SG specific antibodies(Hayashi et al., 2019). Yoshizaki et al. (2012) came up with the idea of spermatogonial transplantation in the peritoneal cavity of rainbow trout to enhance gametogenesis bydndknockout (Yoshizaki et al., 2012). The technique has gained attention ever since its discovery, for either large-scale production of fishes or characterization studies. After the method of tagging thevasagene by GFP was introduced, it was further localized in the SSCs to carry out intra-peritoneal spermatogonia transplantation in the salmonids (Yoshizaki et al., 2016). A schematic diagram illustratingdndknock out in zebra fish using ZFNs, and its application in genetic conservation of endangered species using Farsi tooth-carp as a model is represented in Fig. 2. In addition toin vitroculture and transplantation, germ cell markers also facilitate extensive study of the phenotype and physiology of spermatogonial cells.gfra1andnanos2were characterized exclusively on type An undifferentiated SSCs that allowed isolation and furtherin vitroculture of the Nile tilapia spermatogonia. Culture of SSCsin vitroin bulk hints at future possibilities of applying biotechnological tools in aquaculture and fisheries(Lacerda et al., 2013). A similar method was used to develop zebra fish embryo cell cultures co-cultured with the cells obtained from a rainbow trout spleen cell-line, RTS34st, that enable the production of germline chimeras.vasagene in the SSCs was characterized, and its expression was evaluated for selection of the recombinant cells (Ma, Fan, Ganassin,Bols & Cerda, 2001). Expression of germ cell markersdazl,dnd,nanos3,vasa,andpiwil1was assessed in zebra fish spermatogonial cell cultures derived from Tg (piwil1:neo); Tg 9(piwil1:DsRed) transgenic fish using an ovarian feeder cell line, that was manipulated to expresslif,fgf2, andgndf. The germ cell markers showed continuous expression in the spermatogonia in the presence of dorsomorphin, a BMP type I receptor inhibitor. The SSCs transplanted were able to inhabit the host gonad to produce functional gametes and get incorporated into the germline(Wong & Collodi, 2013). Much importance has been given tovasaanddndto utilize these germ cell markers for germ cell manipulation,transgenic and biotechnological applications. In this context, it is essential to highlight factors likefgf, sox, nanos,andoctto implicate their significance in terms of germ cell differentiation and gametogenesis.
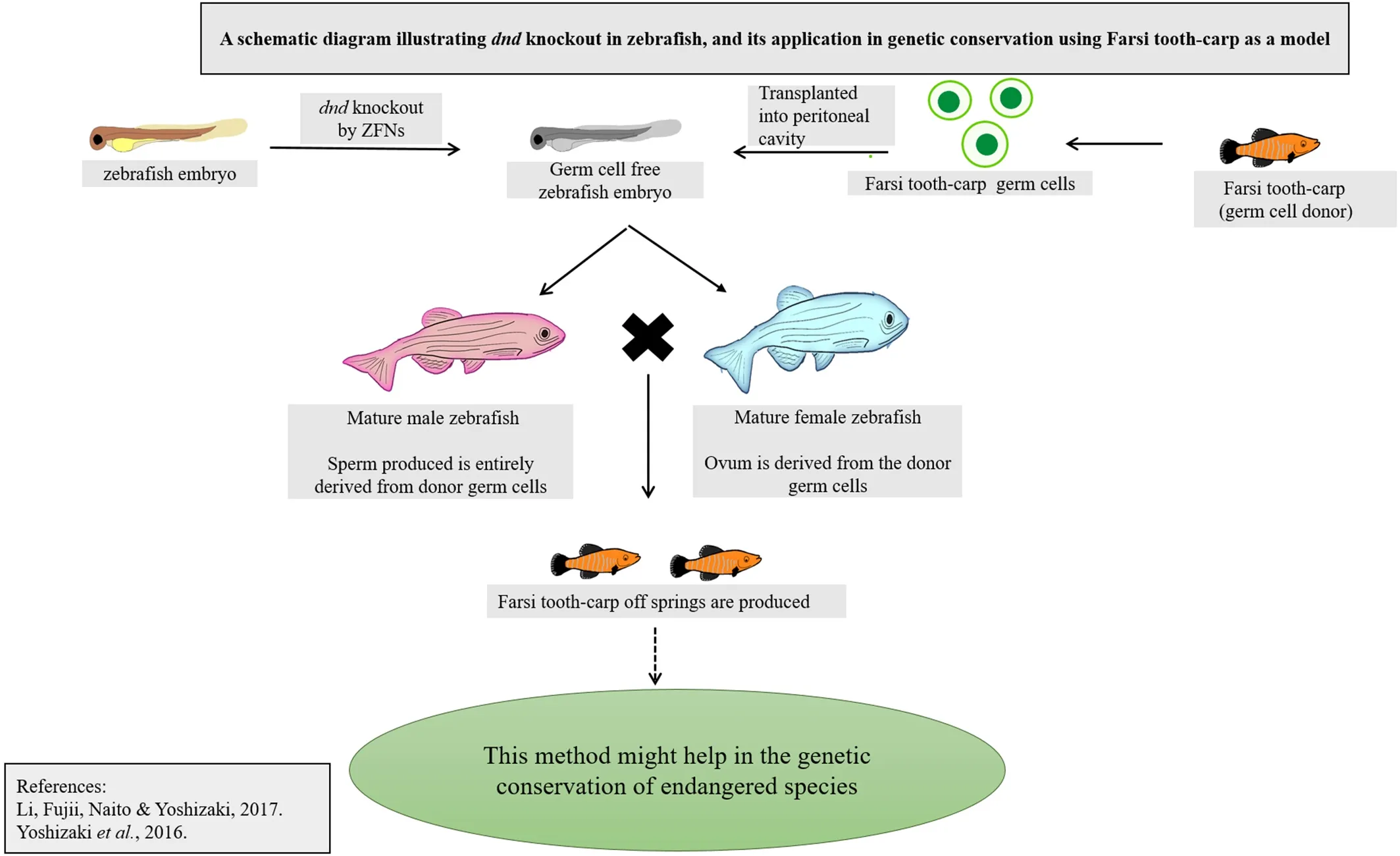
Fig. 2.Schematic diagram illustrating dnd knock out in zebra fish using ZFNs, and its application in genetic conservation of endangered species using Farsi tooth-carp as a model is represented in Fig. 2.
8.Future perspectives
Sex steroids seemingly have a grave importance in germ cell development. More importantly, progestins are known to involve in early gametogenesis, which in turn leads to the meiosis of male germ cells(Kleppe et al., 2017; Miura, Higuchi, Ozaki, Ohta, & Miura, 2006). Later on, androgens and estrogens seem to in fluence germ cell differentiation and development in male and female fish, respectively (Rajakumar &Senthilkumaran, 2020). Incidentally,soxisoforms andap1together,withjunb, is known to regulate sex steroid biogenesis vis-`a-vis gametogenesis (Rajakumar & Senthilkumaran, 2020; Tenugu, Pranoty, Mamta,& Senthilkumaran, 2021), which may indicate an indirect in fluence of these correlating with germ cell development. It is essential to unravel the in fluence of other factors, for example, metabolic hormones and components of the immune system, on germ cell development (Kleppe et al., 2017; Miura et al., 2006). Metabolic peptides seemed to in fluence gonadal development, especially testicular development (Hatef &Unniappan, 2019), which may indicate some role in germ cell differentiation, an aspect worthy to probe. A study using zebra fish demonstrated inhibition of germinal vesicle breakdown by ghrelin (Shepperd,Peng, & Unniappan, 2012). Further research on this line may provide new insights into the role of metabolic hormones, if any, in germ cell development. It is also essential to probe whether the effect of these factors is indirect or direct on germ cell function. Taken together, germ cell differentiation, proliferation, and development till the end of gametogenesis is a complex process warranting more in-depth studies using various teleost models. It is also essential to probe interacting genes, that bring about de finitive sex-dependent regulation. Germ cell enrichment and culturein vitrobecomes indispensable for SSC propagation in rare, endangered teleost species by means of cryopreservation.A number of germ cell isolation and purification methods have been adapted, such as density gradient centrifugation, FACS, MACS etc.(Lacerda et al., 2010; Yoshikawa et al., 2009; Wong et al., 2013; Kise et al., 2012; Ichida et al., 2017; 2019). Discontinuous density gradients,Percoll and Ficoll are most widely used to separate homogenous spermatogonia cell populations before their culturein vitro. Lacerda et al.(2010) used Percoll to segregate the most immature spermatogonia in the Nile tilapia before transplantation (Lacerda et al., 2010). However,the precision of this method is not convincing since it does not differentiate between spermatogonia, Sertoli cells, peritubular myoid cells, or Leydig cells. FACS and MACS seem to fill this void as these techniques are highly specific and recognize the germ cell markers by fluorescence of antibodies (Xie, Nóbrega, & P?eni?ka, 2020). Nagasawa, Shikina,Takeuchi, and Yoshizaki (2010) identified lymphocyte antigen 75(LY75/CD205) as a PGC surface marker in rainbow trout and the Pacific blue fin tuna (Nagasawa et al., 2010). Recent trends such as the use of microbeads or nanobeads have seemed to overpower the traditional methods as these facilitate rapid binding to cells, and being small in size,do not interfere with downstream biotechnological applications (Nayak et al., 2016; Poudineh et al., 2017). Other novel isolation methods such as aptamer-based affinity chromatography can be taken into consideration for isolating germ stem cells (Nery, Wrenger, & Ulrich, 2009). The germ cells thus isolated, are then culturedin vitroby providing a serum containing growth factors, hormones, and other essential components for the proliferation of germ cells or SSC self-renewal (Xie et al., 2020).The germ cell culture can then be used to manipulate the commercially cultured fishes by transgenic or transplantation strategies.
Overall, a more comprehensive research approach is essential to delineate germ cell markers for their specific function. Gene knockout or gene silencing approaches may be employed at different levels of gonadal development for understanding stage-specific effects of germ cell related genes. Identification and characterization of germ cell markers will provide valuable insights into understanding germ cell migration, differentiation, and developmental ontogeny of fishes. It will also help us to comprehend the phenomenon of endocrine disruption and sex reversal, either natural or induced (Kar, Sangem, Anusha, &Senthilkumaran, 2021; Tenugu & Senthilkumaran, 2022).
Localization and expression of several germ cell markers in teleosts are shown in Table 1. Several innovative new-age biotechnological tools such as genomics, gene knockout, transgenic strategies, siRNA, or shRNA transient silencing must be implicated along this line for a better understanding of sexual differentiation and gonadogenesis at the molecular level. Identification of species-specific germ cell markers and their regulation will pave the way to develop new technologies for largescale production of fishes that hold immense economic values and selective breeding of broodstock, as well as production of transgenic fishes. Essentially, fish transgenic strategies and molecular manipulation of the gonads through genetic engineering to bring about gonadal sex reversal, would let us achieve new heights of success, in terms of aquaculture. It is essential to develop genetic sex populations of various fish models and with those germ cell lineages, the distinctive events of male and female sex development can be tracked.
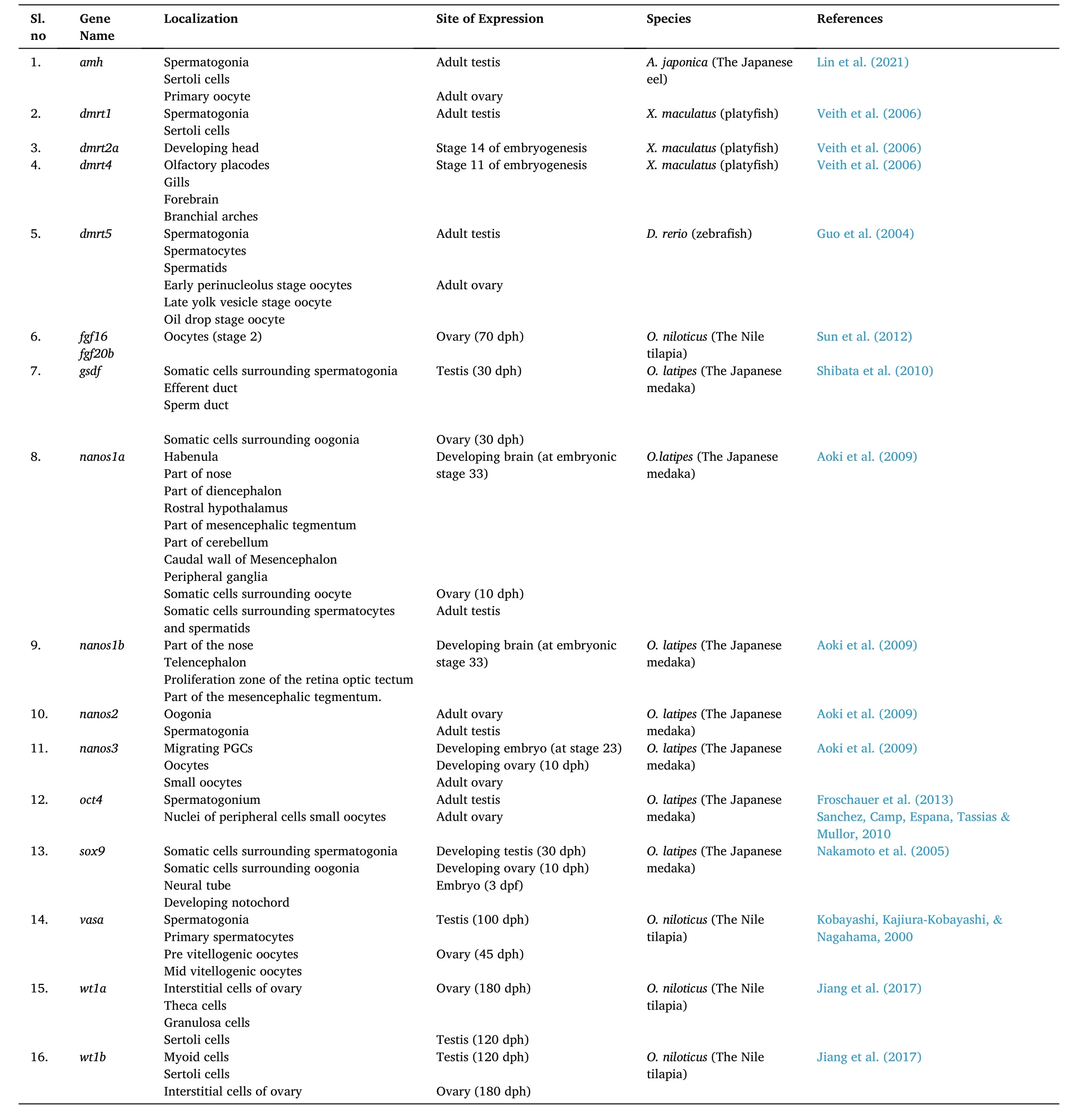
Table 1Showing localization and expression of germ cell markers in teleosts.
Acknowledgements
The research work mentioned in this review was supported by a grant-in-aid (Ref. No. 37(1708)/18/EMR-II) from the Council of Scientific and Industrial Research, India, to BS. NA is thankful to the University of Hyderabad for the Non-NET fellowship. Authors acknowledge BUILDER Grant from DBT (Ref No. BUILDER-DBT-BT/INF/22/SP41176/2020), India, to School of Life Sciences, University of Hyderabad. Authors also acknowledge Ms. Sonika Kar for proofreading the manuscript.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Reproductive farming technology in Japanese eel and chub mackerel
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Understanding the impact of stress on teleostean reproduction
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
