Understanding the impact of stress on teleostean reproduction
Raju Murugananthkumar, Cheni-Chery Sudhakumari
Department of Animal Biology, School of Life Sciences, University of Hyderabad, P.O. Central University, Hyderabad, 500046, India
Keywords:
Cortisol
Interrenal axis
Steroidogenesis
Gonadotropins
Chromaffin cells
Catecholamines
A B S T R A C T
Fishes exert stress response in a various ways depending on the type of the stressor. The stress responses are activated through a cascade mechanism stimulated by the stressor which involves the hypothalamus–hypophyseal–interrenal (HHI) axis, catecholamines (CA), and gonadotropins. Adaptive stress responses may positively impact the fish survival and reproduction, while continuous or prolonged stress causes adverse effects on the fish reproduction. Corticotropin-releasing factor and adrenocorticotropic hormone are the principal hormones responsible for producing corticosteroids through the HHI axis. Cortisol acts differentially on the stress response as it helps at the early developmental stage; conversely, it impairs the gonadal function. CA have a critical role in maintaining body homeostasis and intermediary metabolism, and they also have a predominant role in reproductive function. Besides hormones, few genetic and epigenetic factors have been identified to understand the molecular responses to stress however, genome-wide associated studies will be initiated to investigate a complete picture of the stress mechanism. Further, recent evidence suggests a growing concern in determining the correlation between the stress hormone level and its associated gene function. Hence, this review highlights the regulation of stress responses in different axes, genetic and epigenetic factors related to stress,and the integration of recent technologies and novel hypotheses to unravel the stress response mechanism in fish reproduction.
1.Introduction
Stress is an important biological phenomenon for the survival of the fish species through its adaptive response to environmental changes. On the other hand, prolonged exposure to stress can cause a maladaptive response that adversely affects physiological processes such as growth,behavior, reproduction, and immunity (Rousseau et al., 2021). Therefore, stress and reproduction are crucial for understanding the successful production, maintenance, and conservation of the species from the global disturbances. Stress has been described in various ways but commonly it is divided into three categories such as primary, secondary,and tertiary responses (See Fig. 1; Wendelaar Bonga, 1997; Consten,2001). The stress response is primarily regulated by producing two major hormones, corticosteroids (mostly cortisol) and catecholamines(CA; epinephrine and norepinephrine). Simultaneously, primary responses are responsible for the secondary stress response, altering various physiological functions of the fish adaptation (Barton, 2002;Consten, 2001; Wendelaar Bonga, 1997). Long-term stress leads to a tertiary response that negatively impacts the fish’s growth, behavior,swimming ability, feeding, and disease resistance. The secretion of cortisol is the primary indicator of stress. Although cortisol is a stress hormone, it helps in reproductive functions such as gonadal maturation,spawning, and progeny development. Cortisol also acts differentially in fish by inhibiting gonadotropin-releasing hormone (GnRH) eventually suppressing the sperm count, delayed ovulation, reduced egg size, and sexual activity (Campbell et al., 1992; Rurangwa et al., 2004). Concurrently, the secretion of adrenaline and noradrenaline from chromaffin cells are responsible for the changes in metabolism, osmoregulatory disturbances, immunity, and cardiovascular and respiratory functions(Wendelaar Bonga, 1997). Besides hormones, several other factors also playing a significant role in the adaptation of fish to the stress response.Recent reports indicated the involvement of certain genetic factors in the stress mechanism in a few fish models. Interestingly, certain environmental factors alter the epigenetic mechanism of fish lead to changes in phenotypes and affecting the disease resistance. Although stress response has been studied for several decades in fishes, molecular mechanisms underpinning the physiological, reproductive, and behavioral changes in the stress response have not yet been completely understood. Further, recent research with the evidence of stressors action and the genetic and epigenetic changes due to the stressors are intriguing to understand the stress response. Hence, this review discusses the stress response in interrenal, gonadal, and chromaffin cells,highlighting the recent technologies and findings to understand the stress mechanism.
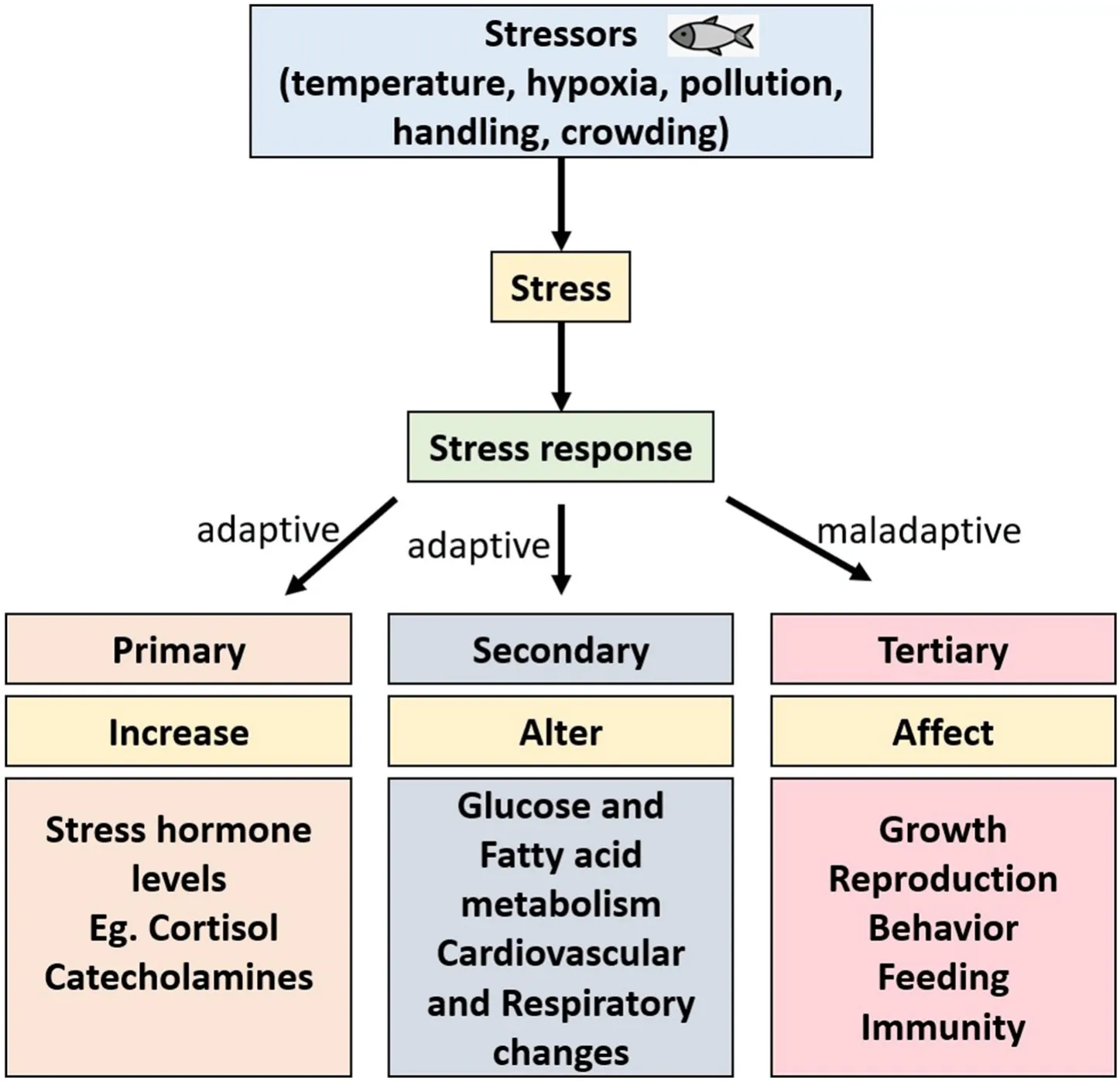
Fig. 1.Stressors in the environment cause stress response categorized into three types such as primary, secondary, and tertiary. Primary and secondary responses are adaptive which release the stress hormones and change the metabolism while tertiary response is maladaptive and prolonged stress response adversely affects the fish’s growth, reproduction, immunity, behavior, and feeding.
2.Regulation of stress response
The regulation of stress response at the hormonal and molecular level has been elaborately studied in several teleosts (Faught et al., 2016;Wendelaar Bonga, 1997). Despite the environmental factors, fishes experience various kinds of stress in aquaculture due to crowding,handling, transport, and during some experimental procedures which are considered as stressors. Stress response leads to an increase in the levels of stress hormones in the blood such as cortisol and CA(epinephrine and norepinephrine), and it increases the plasma glucose level, blood circulation, and muscular activity (Fabbri et al., 1998).Cortisol release into the blood stream is often considered the first step of the molecular stress response. The brain recognizes the presence of stressors in the environment and activates the hypothalamo-hypophyseal-interrenal (HHI) axis to secrete corticosteroids from the post-cardinal vein of the head kidney while chromaffin cells which are equivalent to the mammalian adrenal medulla produce CA (see Fig. 2). HHI axis is like the hypothalamo-hypophyseal-adrenal(HHA) axis in mammals (Wendelaar Bonga, 1997) as it has a compact adrenal gland. The interrenal in fish is comprises of endocrine, hematopoietic, and lymphoid tissue in one organ. The levels of epinephrine and cortisol are diverse in function during a stress response as the rise and fall of epinephrine in the blood is rapid contrastingly the cortisol can stay from minutes to hours in the blood depending on the stressor.Besides, cortisol has an essential role in the secondary response in regulating intermediary metabolism and cardiovascular function to maintain the organism’s homeostasis (Barton, 2002). Upon stimulation,the pituitary releases several polypeptides and regulates normal physiological processes including growth, social behavior, reproduction, and metabolism. The fish pituitary contains several hormone-secreting cells such as the gonadotrophs, thyrotrophs, somatotrophs, and prolactin(PRL) cells. Upon the stressor stimulation, neuroendocrine cells in the hypothalamus release the corticotropin-releasing factor (CRF) which stimulates the anterior pituitary to release adrenocorticotropic hormone(ACTH) that is derived from the hormone precursor proopiomelanocortin (POMC) (De Groef et al., 2006; Wendelaar Bonga, 1997). The secretion of cortisol has been well studied in several fish models. Despite its role during the stress response, cortisol plays an essential role in the early development of fish. In carp, the expression of cortisol was observed during hatching which showed the complete development of the HHI-axis at the early stage (Stouthart et al., 1998).
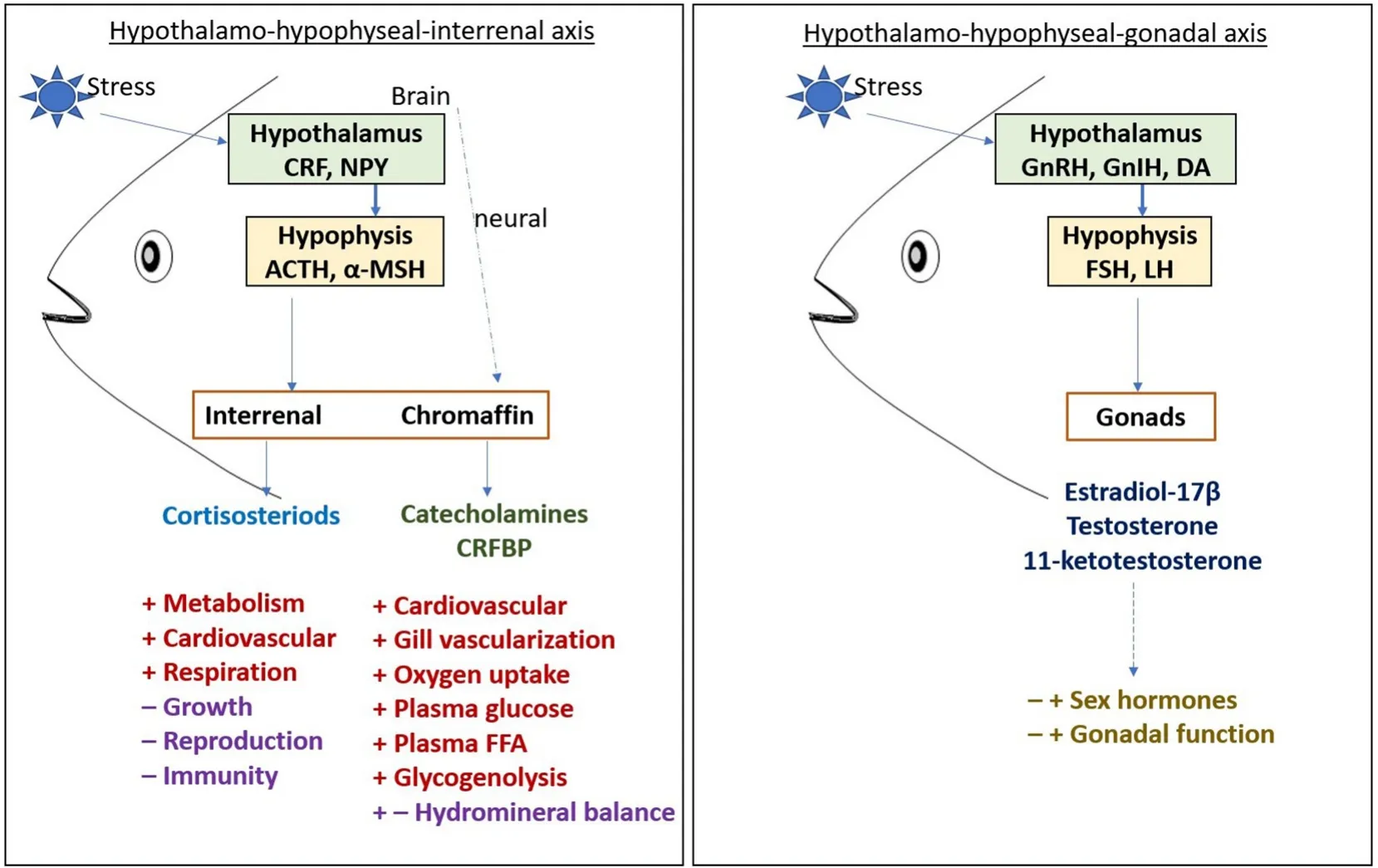
Fig. 2.In the HHI axis, stress causes the release of CRF from the hypothalamus which further activates the ACTH and α-MSH secretion in hypophysis. Corticosteroids and catecholamines produced from the interrenal and chromaffin cells respectively due to the stress response ultimately have positive and negative feedback on the metabolic and physiological functions. In the HHG axis, GnRH and GnIH regulate the FSH and LH secretion in hypophysis which has both the positive and negative feedback on sex hormone production and gonadal function. Abbreviations used in the figure are indicated separately. +indicates positively regulate; – indicates negatively regulate; both +and – indicate positive and negative regulation.
The HHG axis consists of the hypothalamus, hypophysis, and gonads by which the neuroendocrine system regulates the development of gonads, reproduction, and maturation. In fish, the initiation and regulation of puberty are achieved by the complete development of the HHG axis.The expression of sex steroids is the indicator of the onset of puberty.Concerning the HHG axis, the brain receives the signal from the external and internal stimuli to regulate the release of hormones GnRH and dopamine (DA) which helps in the secretion of gonadotropic hormones(luteinizing hormone, LH, and follicle-stimulating hormone, FSH) from the pituitary (See Fig. 2). The gonadotropins (GTH) reach the gonads and helps in the gonadal development and function. LH plays a role in the synthesis and release of sex hormones while FSH helps in germ cell development. The presence of sex steroids is essential for secondary sexual characteristics and behavior. Sex steroids exert either a positive and negative feedback mechanism on the pituitary and regions of brain thereby it helps in regulating gonadal development (Nagahama, 2002;Peter & Yu, 1997). The presence of stress hormone interferes with the HHG axis and disturbs reproduction. Various studies have established that cortisol suppresses the production of sex steroids in that way it inhibits the gamete development. In common carp, elevated cortisol levels decreased the LH pituitary content and androgen levels (Consten et al., 2001). Hypothalamic peptides like GnRH and gonadotropin inhibitory hormones (GnIH) play essential roles in regulating reproduction and gonadal maturation in fish. These peptides have a signi ficant role in stress responses and affect reproductive functions.Intraperitoneal injection of cortisol inAmphiprion melanopusincreased GnIH levels but reduced GnRH, LH, and FSH levels (Choi et al., 2017).The transport of cat fish created a tertiary stress response and showed a significant increase in cortisol, glucose, triglycerides, and total cholesterol levels (Refaey & Li, 2018). Fishes kept at different oxygen levels created a stress response leading to a significant increase in cortisol and glucose levels. A low oxygen levels resulted in reduced feed intake,which caused decreased growth (Klasing et al., 1987). Hypoxia conditions suppress the immune system and make fishes prone to diseases.Crowding and the growth of other aquatic habitats during aquaculture decrease the dissolved oxygen in the water, which causes great stress to the fishes.
3.Hypothalamo-hypophyseal-interrenal (HHI) axis
The hypothalamus and pituitary gland in the production of corticosteroids has been well understood in many vertebrate species. CRF and ACTH are the primary hormones secreted in the brain responsible for controlling corticosteroids in interrenal cells of the head kidney.Therefore, the regulation of corticosteroid production in fish is referred to as the HHI axis. Stress increases the levels of ACTH and cortisol immediately which is the preliminary indication of the stress response.ACTH plays an important role in regulating cortisol secretion in response to stressors in most animals including fishes. Sumpter et al.(1986) reported that plasma ACTH levels were increased in coho salmon and rainbow trout within 2 min of handling and further increased to 8-fold in 30 min. In female sail fin mollies (Poecilia latipinna), ACTH significantly increased cortisol levels. CRF is essential for the release of glucocorticoids there by it regulates the adaptive response in the fish.The mRNA levels of CRF, CRF binding protein (CRFBP), and CRF receptors (CRFRs) are elevated during the primary stress response in rainbow trout (Doyon et al., 2005). Interestingly, CRF, CRFBP, and CRFRs sequences are highly conserved among vertebrates and widely present in various organs and tissues suggesting a prominent role in the adaptation of stress responses (Chen & Fernald, 2008).
Further, the regulation of the HHI axis with the stress response depends on the neuroendocrine factors which include Neuropeptide Y(NPY) (Doyon et al., 2003). In response to social stress, CRF and NPY levels were elevated in the preoptic area of rainbow trout. Both the CRF and NPY levels are positively correlated with the stress response. In gold fish, a cortisol supplemented diet at different levels showed a differential expression pattern of CRF and NPY levels (Doyon et al., 2003).Conversely NPY expression did not alter in the presence of high cortisol levels. A similar result was also noted in the cortisol implantation in rainbow trout reduced CRF mRNA levels while NPY expression did not change. NPY is secreted in the brain and responsible for the feeding behavior and psychomotor activity in gold fish also helps in the release of growth hormone (GH) and GTHs in gold fish pituitary (Matsuda et al.,2013). Besides, NPY stimulated the release of melanocyte-stimulating hormone (α-MSH) in gold fish (Lamacz et al., 1989). α-MSH is secreted in the pituitary from a precursor called POMC, a common precursor for ACTH. Several studies have reported the adaptive stress role of α-MSH in lower vertebrates. Mild stress in salmonid fishes did not change the α-MSH levels, however, severe stress on the fishes resulted in a rapid elevation of α-MSH levels (Rand-Weaver et al., 1993). In certain vertebrates, α-MSH helps in the regulation of pigment migration. The secretion of α-MSH and pigment migration is associated with feeding behavior, energy metabolism, and cortisol release (Takahashi et al.,2014). In tilapia at low pH water, the size of the α-MSH cells in the pituitary has increased (Lamers et al., 1994). In common carp, elevated cortisol and α-MSH levels were seen when the fish were restrained(Stolte et al., 2008). Altered expression of hormones and neuropeptides during stress response suggested its significant role in regulating the HHI axis.
4.Cortisol secretion and reproduction
Biosynthesis of cortisol requires cholesterol as a precursor and secreted in the interrenal upon stimulation by ACTH or α-MSH. Cortisol is the primary stress response hormone expressed during the early developmental period. In common carp, ACTH and cortisol levels were identified during the initial stages of the development. Cortisol, ACTH,and α-MSH levels were present in the unfertilized eggs and were observed in the post-fertilization stages of embryo development(Stouthart et al., 1998). In fish, increased cortisol levels resulted in reduced androgen and estrogen levels conversely it has a role in the spawning and gonadal sex differentiation. The gills, intestine, and liver are the primary sites for cortisol action in fish; therefore, it has both glucocorticoid and mineralocorticoid activities to regulate hydromineral balance and energy metabolism (Wendelaar Bonga,1997). In addition, cortisol helps the organism restore to the original state after the stressor response and acts as an essential mediator for the physiological functions including growth, immunity, and reproduction.
Stress response in fish causes the release of cortisol above the normal level however this condition creates both negative and positive interactions with reproduction. In general, stress causes a rise in cortisol in fish eventually affecting the levels of sex hormones, spawning, behavior,and reproduction. During catching, fishes release cortisol as a stress indicator. In rainbow trout, wild catching and release angling resulted in a significant elevation of cortisol levels, and decreased sex hormone levels can cause impairment in the reproductive process (Clearwater &Pankhurst, 1997). Also, prolonged handling and chronic con finement showed elevated levels of cortisol in the blood, and a significant decrease in the estradiol (E2) levels was observed in the common roach.In rainbow trout, levels of cortisol and vitellogenin were reduced significantly during two weeks of con finement (Carragher et al., 1989).Further, con finement inAcanthopagrus butchericaused elevated cortisol levels with decreased sex hormone levels to prove that stress causes a rapid change in the levels of hormones and inhibition of gonadal steroidogenesis which eventually disrupts reproduction (Haddy & Pankhurst, 1999). Stress induction inOreochromis mossambicustriggered serum cortisol and decreased E2levels which further inhibited the recruitment of preovulatory follicles leads to the interruption of spawning (Foo & Lam, 1993). In another study, repeated acute stress for nine months before spawning showed a significant delay in ovulation and reduced egg size, while in males, lowered sperm counts (Rurangwa et al., 2004). Acute stress response decreased the gamete quality in rainbow trout and the survival rates of progeny are significantly lower than the control. Similarly, inPomacentrus amboinensis, the stress in the female leads to a rise in the cortisol levels and impacted the progeny with reduced larva size (McCormick, 1998). In the Atlantic cod,stress-induced fish with high cortisol levels caused a significant difference in the production of eggs, fertilization rate, and hatching success and continuously released abnormal larvae (Morgan et al., 1999).
Cortisol is not always affecting the fish’s reproductive performance however it helps several fishes to help in spawning and migration. The levels of cortisol in the cat fish reach a peak during the monsoon making the fish spawn. GTHs play a significant role in cortisol secretion. InAnguilla anguilla, cortisol treatment increased the pituitary GTH both in vivo and in vitro (Huang et al., 1999). Administration of ovine LH in cat fish induced higher levels of plasma cortisol which further increased the levels of sex steroids conversely ACTH injection only stimulates cortisol and had no oocyte maturation suggesting GTHs requirement in both the interrenal and ovary (Goswami et al., 1985). Silvering in eel occurs before migration, and increased cortisol levels were found during the process. It helps mobilize energy from the storage and facilitates gonadal growth. Cortisol also plays an essential role in sex differentiation in temperature-dependent sex determination. In preferred larvae reared in high-masculinizing temperature detected with high levels of 11-ketotestosterone (11-KT) indicated that cortisol during the temperature stress acted at the time of sex determination (Fernandino et al.,2013). Further, corticosteroid treatment in rainbow trout showed masculinizing effect and helped in the early development of testis.Higher water temperature during the critical period of sex determination in medaka converted female medaka into phenotypic males and elevation of cortisol (Fernandino et al., 2013). Similarly, in the Japanese flounder and Southern flounder, higher temperatures caused an increase in the levels of cortisol and masculinization (Mankiewicz et al., 2013). In addition, cortisol has an essential role in regulating glucocorticoid metabolism and steroidogenesis.
5.Hypothalamo-hypophyseal-gonadal (HHG) axis
Successful reproduction in fishes depends on several factors,including the environment, and temperature. Fishes are labile to the environmental factors and most fishes show plasticity towards sex determination and differentiation during the early development. Stress response in fishes not only affects growth and immunity but also affects reproduction. Alteration in the environment creates a stress response and leads to the production of stress hormones ultimately affecting the homeostasis during development (Schreck & Tort, 2016). In addition,environmental toxicants cause stress responses and impact the reproductive hormones leading to delayed or precocious gonadal function(Roy et al., 2009). Stress differentially impacts reproduction, for instance, it can either promote or inhibit gametogenesis production depending on the stressor.
The HHG axis regulates gonadal development and gametogenesis. In most of the teleosts, two types of GnRH have been recorded. The gonadotropic hormones are produced in the hypothalamus which stimulates the pituitary gland to secrete two types of gonadotropic hormones, GTH I and II (Kawauchi et al., 1989; Suzuki et al., 1988).Incidentally, GTH I and GTH II are homologous to LH and FSH,respectively. GTH I helps in the gonadal development and onset of gametogenesis while GTH II is involved in the final gamete maturation(Bon et al., 1999; Breton et al., 1998). Sex steroids, E2and testosterone(T) are produced in the gonads upon the stimulation of GTHs. E2promotes oocyte growth, vitellogenin production, and the synthesis of yolk proteins (Clelland & Peng, 2009). Vitellogenin is produced in the liver,transported through the bloodstream, and taken up by developing oocytes. FSH is necessary for the meiotic maturation of teleost oocytes, and its action is mediated through 17α, 20β-dihydroxyprogesterone (17α,20β-DHP) (Pang & Ge, 1999). LH stimulates the testis to produce 11-KT as a potent androgen in regulating spermatogenesis in males. Spermatozoa convert 17α-hydroxyprogesterone, produced by the somatic cells,to 17α, 20β-DHP as a maturation-inducing hormone for the spermiation in many fishes (Lal, 2013).
Differential expressions of GTHs and sex steroids were observed in response to physical and chemical stress. Crowding causes a stress response in fishes, for instance inSalmo truttacrowding affected the reproductive hormones and elevated GTHs, plasma ACTH and cortisol,and decreased T and 11-KT levels (Pickering et al., 1987). Crowding also impacted female tilapia to spawn and oogenesis was affected due to the reduced E2levels which are insufficient for the vitellogenesis (Coward et al., 1998) Exposure to stressors in rainbow trout caused delayed ovulation and reduced egg size however in males, lower sperm counts were found and the offspring from stressed fish had a reduced survival rate. (Campbell et al., 1992). Plasma T and E2levels were decreased inPagrus aratusafter capturing further rainbow trout exerted stress response by decreasing sex hormone levels after 24 h of capture (Pottinger & Carrucj, 2000; Yamaguchi et al., 2006). Cortisol has a major role in regulating reproduction in fishes. In maleMorone saxatilis, low cortisol stress groups showed lower levels of androgens and decreased spermiation response to GTH treatment while high cortisol stress groups began earlier spermiation (Castranova et al., 2005). Cortisol reduced the reproductive ability of the sexually maturing rainbow trout in a dose-dependent manner. Increased cortisol levels in the fishes showed a negative impact on reproduction. Stress and cortisol levels did not block the spawning in fishes however, the levels of stress hormones impacted the reproductive function. In the Atlantic cod, stressed fish showed success in spawning however it yielded abnormal larvae more frequently. Stress caused irregular spawning cycles in cod and low quality in the production of eggs (Morgan et al., 1999). Jalabert and Fostier (1984) described that mature oocyte follicles of rainbow trout secreted 17α, 20β-DHP through GTH stimulation suggesting cortisol’s role in oocyte maturation. Conversely, cortisol reduced the sex hormone secretion in rainbow trout ovarian follicles. Further, injecting cortisol decreased the plasma levels of E2and T with no effect on the plasma GTHs levels. The differential impact of cortisol signifies that cortisol helps in the early development stages and not during the maturation of follicles. Cross talk between cortisol and male steroidogenic pathways triggering masculinization in some fishes. Pejerrey larvae exposed to male-producing temperature increased cortisol and 11-KT, suggesting that cortisol stimulates the hsd11b2 expression to drive testis morphogenesis (Fernandino et al., 2012).
Besides gonadotropins, other peripheral hormones were modulated during the stress response, including PRL and GH. StressedOncorhynchus kisutchshowed elevated levels of circulating PRL for 1–5 days with increased cortisol (Avella et al., 1991). On the other hand, in rainbow trout during con finement, the plasma PRL levels were relatively low to the control (Pottinger et al., 1992). Stress-induced acute handing followed by con finement in rainbow trout caused an increase in cortisol and ACTH levels and a decrease in the GH levels conversely elevated GH levels were observed during the hypoxia condition (Pickering et al.,1991). On the other hand, GH levels were significantly altered during feeding and fasting stress with increased cortisol levels (Farbridge &Leatherland, 1992). Stress also reduced the growth rate of Atlantic salmon parr with an elevation of GH and insulin-like growth factor I levels (McCormick et al., 1998). It is interesting to note that increased cortisol levels have a role in regulating the HHG axis by suppressing reproductive function.
6.Stress and catecholamines
Epinephrine and norepinephrine are the two major CA produced during stress response for the energy metabolism and oxygen transport in the body (Reid et al., 1998). CA are produced by chromaffin cells which are present in the head kidney region in fish. The head kidney region also contains the interrenal cells in fishes (Reid et al., 1998).Unlike cortisol, CA immediately rise during acute stress, reach high, and drop rapidly. However, in chronic stress, the elevation of CA levels may stay for hours or days (McCormick et al., 1998; Perry & Reid, 1993).Oncorhynchus kisutchreleased catecholamines in the blood during hypoxia (Aota et al., 1990). CAs have several effects on the various physiological responses such as respiratory and cardiovascular, blood oxygen transport, glucose, and fatty acids metabolism by entering the bloodstream. Anesthesia during the experiments produces CA at the transient level (Wendelaar Bonga, 1997). DA is essential for regulating various physiological processes however it also has control over the HHG axis for the secretion of gonadotropins. The mechanism is unclear, however previous studies demonstrated that DA levels vary with the reproductive cycle and are crucial for gonadal maturation and reproduction (Dufour et al., 2010). InOreochromis mossambicus, stress-induced fish showed high levels of DA content with a decrease in the LH immunoreactivity in the brain and stage V ovary which suggests the suppressive action of dopaminergic neurons in the reproductive axis (Chabbi & Ganesh,2015). Stress-induced DA secretion in the regions of the brain inhibits the GnRH neurons and affects the LH cells in the pituitary also the DA-nergic action controls the secretion of E2and glucocorticoid receptors (Ganesh, 2021).
Hypoxia causes a significant elevation of plasma CA levels in fishes.In Atlantic cod, a gradual decrease in oxygen levels in water resulted in increased plasma adrenaline levels (Herbert & Steffensen, 2005). During the stress response, epinephrine changes the pH of plasma to acid to increase oxygen affinity to hemoglobin, thereby facilitating oxygen transport. In rainbow trout, the addition of a CA mixture to investigate the Na+/K+exchange in red blood cells resulted in a lowering of blood pH followed by the transient reduction of CO2and O2partial pressure caused binding of oxygen to hemoglobin (Heming et al., 1987). Stress response in fluences blood glucose levels in fish. Repeatedly stressed rainbow trout showed lower post-stressed glucose levels while Eurasian perch after stress treatment showed maximum cortisol and glucose levels (Farbridge & Leatherland, 1992; Jentoft et al., 2005). Similarly,chronic cortisol stimulation on Salvelinus fontinalis lowered blood glucose and hepatic glycogen concentrations (Vijayan et al., 1991). The difference in glucose levels could be due to high energy consumption on repeated stress. However, in rainbow trout, CA regulate the glucose availability in the blood by activating glycogenolysis and inhibiting glycolysis in the liver (Wright et al., 1989). Also, the presence of CA hepatocytes isolated from copper rock fish stimulated glycogenolysis through glycogen phosphorylase (Moon et al., 1999). Several reports in teleost suggest that stress response is related to glucose metabolism which is evident by the oxidation of glucose solves the energy requirement needed to cope with stress. Concurrently, CA facilitate the transport of fatty acids as it is an essential energy substrate after glucose. In common carp, arterially infusion of both epinephrine and norepinephrine resulted in hyperglycemia and free fatty acids levels were increased upon epinephrine infusion while norepinephrine decreased the levels significantly (Van Raaij et al., 1995). Further, in common carp and rainbow trout, hypoxia leads to high blood glucose levels and stimulation of hepatic glycogenolysis however continuous decline of plasma-free fatty acids was observed (Van Raaij et al., 1996). This could be due to reducing lipolytic activity, a specific adaptation during hypoxia. In cannulated carp, norepinephrine inhibited lipolysis through β1-and β3-adrenoceptors while β2-adrenoceptors were needed for lipolysis stimulation (van den Thillart et al., 2002). Quantification of glucose and non-esterified fatty acids in rainbow trout after hypoxia caused a significant decrease in fatty acids turnover rate with an increase in hepatic glucose production (Hu et al., 2018). Unlike mammals,several studies on fishes showed decreased free fatty acids during hypoxia. Some studies warrant the increase of free fatty acids in blood upon CA administration and the difference is due to the differential modulation of CA in various tissues.
7.Recent technologies and molecular approaches to studying stress mechanism
Stress response in fish has been evaluated for several decades by measuring transcripts, protein expression, and cellular response. Several studies have highlighted the expression of genes and protein during cortisol stimulation. The stress response also causes aberrant epigenetic modifications, further changing the gene expression patterns. In recent years, studies on the molecular responses in fish have been increased using current technologies like next-generation sequencing, RNA sequencing, and microarray. Unlike mammals, fishes act differentially with the stressors and cannot be classified due to many species. Interestingly, cDNA microarrays have been developed to determine gene expression patterns for a wide variety of teleost species (Miller &Maclean, 2008). Stress response starts in the brain, and prolonged exposure to stress causes a change in the transcriptome pro files leading to the abnormal expression of genes and change in behavior. In rainbow trout, the microarray technique was used to analyze the differential gene expression in the brain and kidney in response to environmental stressors and concluded that prolonged stress exhibited adaptive response in the brain and slow degradation of extracellular matrix in the kidney (Krasnov et al., 2005). Further transcriptomic response to crowding stress in rainbow trout presented several metabolic genes that were downregulated and upregulated of genes related to stress and apoptosis (Rebl et al., 2017). RNA sequencing of rainbow trout liver after con finement stress showed that genes related to glucose metabolism were enriched compared to the control (Liu et al., 2014).
Gene silencing by morpholino oligonucleotides has been widely used in several fish models to understand the relationship between molecular function and phenotypical variation (Timme-Laragy et al., 2012). Generation of knockout and knock-in models was successfully created and established in medaka, tilapia, and zebra fish (Bedell & Ekker, 2015; Li et al., 2014; Taniguchi et al., 2006; Timme-Laragy et al., 2012; Watakabe et al., 2018). After the emergence of precise genome editing by the CRISPR-Cas9 method, creating a gene knockout model is also feasible in other non-model fishes. Environmental stress-affected red tilapia was genetically modified into complete albinism in Nile tilapia using the CRISPR-Cas9 genome editing (Segev-Hadar et al., 2021). Targeted gene mutation in large-scale loach produced an albino mutant for the ornamental fish having high commercial value (Li et al., 2021). However,taking advantage of these methodologies in other fish models has not been probed due to differences in the growth and reproductive cycle.Nevertheless, transient knockdown of gene expression has been demonstrated in vitro and in vivo using siRNA in a few teleosts species including cat fish and common carp (Anitha & Senthilkumaran, 2020;Murugananthkumar & Senthilkumaran, 2016).
Transcription factors and genes involved in the stress response must be described however, the studies are fragmentary. Genetic screening approaches have been recently developed to address the physiology of stress response in fish. Cortisol treatment in rainbow trout hepatocyte primary culture significantly elevated the genes related to intermediary metabolism, reproduction, and xenobiotic metabolism (Aluru &Vijayan, 2007). Thermal treatments in European sea bass resulted in the release of cortisol and some essential stress genes (nr3c1, nr3c2, and hsd11b2) were affected by the temperature (Goikoetxea et al., 2021).Zebra fish embryos subjected to stress response by stirring and hypoxia resulted in the continuous presence of 11β-hydroxylase, 11-beta-hydroxysteroid dehydrogenase, and glucocorticoid receptor genes from 48 to 120 hpf (Wilson et al., 2013). Further, cortisol-treated zebra fish embryos grown into adults have differential activity of glucocorticoid-responsive regulatory genes klf9 and fkbp5 (Hartig et al.,2020).
Epigenetics plays an essential role in the regulation of gene expression. Epigenetic modifications are considered as DNA methylation,histone modifications, and noncoding RNAs. There is not much evidence to determine the epigenetic regulation of stress response. However,compared to the mammalian models studied with the environmental factors affecting the epigenetic process, stress response may cause significant epigenetic changes in the lower vertebrates like fishes due to its plasticity. Researchers have identified that glucocorticoids cause changes in the gene expression in mice brains. Also, chronic exposure to corticosterone altered the expression of FKBP5 (FKBP Prolyl Isomerase 5) and decreased FKBP5 methylation levels in mice. In the Japanese flounder, the glucocorticoid receptor downregulated the aromatase gene(cyp19a1) by directly interacting with the cAMP-responsive element in the promoter of cyp19a1 (Yamaguchi et al., 2010). In European sea bass,the masculinizing temperature increased the DNA methylation levels in gonadal aromatase to control the conversion of androgens into estrogens. (Navarro-Martín et al., 2011). Several studies showed altered DNA methylation patterns exposed to the chemical stressors (Aluru et al.,2015; Liu et al., 2014; Pierron et al., 2014). Temperature and hypoxia stress in Atlantic salmon affected the methylation sites in the regulatory elements of genes related to stress and metabolism (Beemelmanns et al.,2021). Non-coding RNAs play an essential role in epigenetic regulation which can be categorized into microRNAs (miRNAs), short-interfering RNAs, long noncoding RNAs, and piwi-interacting RNAs depending on the size and function. miRNAs are conserved among the species and participate at the molecular level by regulating the post-transcriptional modifications. Certain miRNAs functions are linked to anti-stressors and anti-in flammation and are used as an indicator for environmental stress response (Raza et al., 2022). Zebra fish exposed to cold stress revealed a significant change in the miRNAs expression which are associated with melanogenesis, GnRH pathway, and circadian rhythm (Hung et al.,2016). The presence of antidepressants and increased temperature inDanio reriocaused significant downregulation of miRNAs related to ovarian pathologies (Ikert & Craig, 2020). InOreochromis niloticus,differentially expressed miRNAs were observed during fishes exposed to acute cold stress (Qiang et al., 2018). Altered plasma miRNA levels were observed inOncorhynchus mykissduring acute stress and the sequence analysis showed that the miRNAs are related to biosynthetic, degradation, and metabolic pathways (Ikert et al., 2021). Based on the existing evidence, more investigations like miRNA pro filing and reverse genetic approach to knockdown specific miRNA are needed to identify and characterize the role of specific miRNAs in regulating the stress response in fish.
8.Conclusions and future studies
Stress creates differential responses in the fishes and has been studied for several decades with respect to concerning metabolism, growth,immunity, and reproduction. In aquaculture, stress plays a critical role in the survival of fish. Due to overcrowding, water quality and dissolved oxygen levels decrease dramatically in stagnant water can cause severe stress to the fish and eventually reduce the growth and quality of the fish. In addition, overcrowding decreases fish immunity leading to bacterial and viral infections. Besides, stress diminishes the gamete quality resulting in a low or abnormal production of offspring. In every stress response, cortisol has a widespread effect on fishes while it exerts both a positive and negative effect on reproductive function. Several studies on teleosts are about the primary stress response and cortisol expression, however, the action of cortisol in reproductive tissues and specific signaling pathways involved during reproductive stress remains unclear. In addition, physiological response to different stressors and impact on hormone production during adverse stress condition are severely lacking in teleosts. Also, several concepts need special attention such as corticosteroid receptor regulation in the target tissues,hypothalamic-pituitary axis response during acute and chronic stress,and genetic and epigenetic changes during specific environmental stress.In recent years, production of knock-out and knock-in models using the CRISPR-Cas9 genome editing are well established in zebra fish, tilapia,and medaka, and behavioral genetic studies were demonstrated using zebra fish. Considering this, generation of fish animal models to study stress mechanism is feasible. Further, genome-wide associated studies,identifying stress biomarkers and its involvement in the stress molecular pathways will shed more light on the unanswered questions. Undoubtedly, the growing evidence suggests that recent technologies will help interpret the stress mechanism well for the successful maintenance of fish species and to preserve the endangered ones.
Declaration of competing interest
The authors declare no con flict of interest.
Acknowledgement
The authors received no financial support from any organizations for writing this review. The authors thank Prof. B. Senthilkumaran (BS) and the Department of Animal Biology, University of Hyderabad (UoH) to work on and compile this review. RM is a former Ph.D. student and CS is a Research Associate in SERB sponsored project (awarded to BS) in the Department of Animal Biology, UoH.
 Aquaculture and Fisheries2022年5期
Aquaculture and Fisheries2022年5期
- Aquaculture and Fisheries的其它文章
- Reproductive farming technology in Japanese eel and chub mackerel
- Molecular determinants regulating the release of the egg during ovulation:Perspectives in piscine models
- Environmental hypoxia: A threat to the gonadal development and reproduction in bony fishes
- Impact of xenoestrogens on sex differentiation and reproduction in teleosts
- Germ cell markers in fishes - A review
- Sexual plasticity in bony fishes: Analyzing morphological to molecular changes of sex reversal
