Microstructure and mechanical properties of low-carbon Q & P steel pretreated with intercritical annealing
,
Research Institute,Baoshan Iron & Steel Co.,Ltd.,Shanghai 201999,China
Abstract: The success of obtaining both high strength and high formability in low-carbon quenched and partitioned (Q & P) steels depends on their microstructural constituents.In this regard,the effect of annealing temperature on the volume fraction and distribution of carbon in retained austenite in a low-carbon Q & P steel was studied.The microstructures of Q & P steels subjected to different annealing temperatures were studied in detail by electron microscopy,electron microprobe,and X-ray diffraction techniques.The results suggest that intercritical annealing is beneficial in increasing the volume fraction of retained austenite,which is a consequence of the distribution of alloying elements during intercritical annealing.Moreover,the mechanical properties of intercritically annealed Q & P steel,especially its ductility,are significantly enhanced.
Key words: intercritical annealing; Q & P steel; retained austenite; EPMA analysis; mechanical properties
1 Introduction
The transformation-induced plasticity (TRIP) effect of the transformation of retained austenite to martensite is considered to be effective in developing new grades of steel with excellent mechanical properties.Conventional TRIP steels are typically produced via a process of intercritical annealing followed by isothermal holding in the bainitic region to obtain a microstructure comprising ferrite,bainite,and retained austenite[1-2].Carbon partitioning during the bainitic transformation leads to adequate carbon enrichment of the austenite and increases its thermal stability at room temperature.This is beneficial because the TRIP effect of retained austenite during its deformation can significantly contribute to the elongation and energy absorption ability of steels.However,the heat-treatment parameters,such as the temperatures and time of intercritical annealing and bainitic isothermal holding,influence the volume fraction,morphology,and size of the retained austenite[3-5].
As an alternative to conventional TRIP-assisted steels,a novel heat treatment,quenching and partitioning (Q & P),can produce high-strength steels via the TRIP effect[6-7].This process consists of quenching from the austenitizing (partial or full) temperature to a temperature between the martensite start (Ms) and finish (Mf) temperatures,followed by isothermal holding to promote carbon partitioning from supersaturated martensite to retained austenite.Next,the steels are quenched to room temperature.Generally,the microstructural constituents consist of lath martensite,ferrite,and film-like retained austenite.As is known,a low-carbon content in steels is beneficial for maintaining the good weldability of steels.However,low-carbon Q & P steels generally have a low content of retained austenite due to the insufficient enrichment of carbon in the austenite[8].Thus,there is a need to increase the volume fraction of retained austenite in low-carbon Q & P steels to improve their mechanical properties.
Previous research has shown that a good combination of strength and ductility can be achieved by the use of intercritical annealing in the Q & P process[6-7].However,theoretical predictions have considered the effect of carbon on the stability of austenite but ignored the effect of manganese (Mn).In reality,manganese has an effect similar to that of carbon on the stabilization of austenite and decreasing the martensite start temperature.The distribution of manganese between ferrite and austenite during intercritical annealing increases the manganese content in austenite and improves its stability,which means that retained austenite with a high manganese content consumes less carbon.In this regard,it is feasible to increase the content of retained austenite in low-carbon Q & P steels by intercritical annealing.In this study,intercritical annealing was performed on a low-carbon silicon-manganese (Si-Mn) steel.The objective of this work was to investigate the effect of intercritical annealing on the final microstructure and mechanical properties of a low-carbon Si-Mn Q & P steel.In addition,the effect of the manganese distribution during intercritical annealing on the volume fraction and characterization of retained austenite was investigated.
2 Experimental procedure
The chemical composition of the experimental steel is presented in Table 1.In the present study,the suppression of cementite during the isothermal process was achieved by the addition of silicon[9]and aluminum (Al)[10]and the steel had a sufficiently low-carbon content,based on its good weldability[11].In addition to the above elements,microalloying elements,such as niobium (Nb),play an important role in improving strength without reducing ductility.A 50-kg ingot was cast after being melted in a vacuum induction furnace.The ingot was homogenized at 1 200 ℃ for 2 h,and then hot forged into bars 100-mm wide and 30-mm thick.The bars were then soaked at 1 200 ℃ for 2 h and hot rolled into strips 4-mm thick after eight passes of hot rolling with start and finish temperatures of 1 150 ℃ and 850 ℃,respectively.Subsequently,the strips were air cooled to room temperature and cold rolled into 1.2-mm-thick strips after pickling in 10% hydrochloric acid.
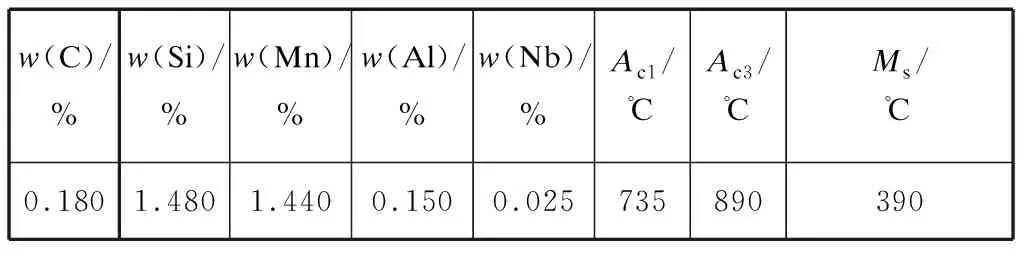
Table 1 Chemical composition and critical temperatures of the experimental steel
To select an appropriate heat-treatment process,the critical temperatures ofAc1,Ac3,andMsin the experimental steel were first obtained by conducting dilatometer tests and are listed in Table 1.Further-more,the phase fractions of austenite and ferrite at different annealing temperatures were calculated using J-Mat Pro commercial software,and are presented in Fig.1.The thermodynamic calculation of the phase fractions by J-Mat Pro commercial software is based on the basic equation for the Gibbs energy of the multi-component solution phase.A schematic of the schedule of the Q-P heat-treatment cycle is presented in Fig.2.Our previous experiments with different annealing time from 3 min to 15 min indicated that the grain size was the smallest and the mechanical properties were the best when the annealing time was 3 min.Thus,we used 3 min as the intercritical annealing time.The specimens were divided into two groups.The first group (named Q-P1) was annealed at 910 ℃ (fully austenitized) for 3 min and then quenched to 220 ℃.Subsequently,these quenched specimens were maintained at 400 ℃ for durations of 5-500 s and quenched to room temperature.The second group (named Q-P2) was annealed at 850 ℃ for 3 min and then quenched to 220 ℃ and partitioned at 400 ℃ from 5 s to 500 s,followed by quenching to room temperature.
The microstructures of the steels were examined using a ZEISS ULTRA-55 scanning electron micro-scope (SEM) and a TECNAI-Q2-20 transmission electron microscope (TEM).The SEM specimens were ground and electrochemically polished in a solution comprising absolute ethyl alcohol,perchlo-ric acid,and distilled water in a ratio of 30∶2∶1.The polished specimens were then etched with 4% nital for 15 s.The TEM foils were prepared by grinding the strips to 50 μm and punching them intoφ3-mm disks followed by twin-jet polishing.Composition analyses of the electrochemically polished and slightly etched samples were performed using a JEOL JXA-8530F electron probe microanalyzer (EPMA).The volume fraction of retained austenite was measured via X-ray diffraction (XRD) using Cu Kα radiation operated at 40 kV and 100 mA.Samples were scanned from 40° to 120° at a scanning rate of 2°/min.
3 Results and discussion
3.1 Microstructural observations
Fig.3 shows SEM micrographs of samples subjected to different annealing treatment.As shown in Fig.3(a),the microstructure of the sample annealed at the fully austenitic temperature (910 ℃) consisted of nearly 100% lath martensite.In contrast,the sample intercritically annealed (at 850 ℃) consisted of martensite (M) and ferrite (F).The ferrite exhibited irregular morphology (as marked in Fig.3(b)) and was uniformly distributed in the matrix,whereas the martensite was lath-type and distributed in clusters.The martensite packet sizes of the intercritically annealed samples were smaller than those in the samples that had been fully austenitized.The reason for this is that the austenite grains generated during annealing at 850 ℃ were smaller than those obtained during full austenitization[12].Retained austenite is difficult to distinguish in the SEM micrographs of both samples.
3.2 Elemental distribution
Fig.4 shows magnified SEM micrographs of samples after Q-P1 and Q-P2 treatment,and the corresponding compositional analysis obtained via line analysis with the EPMA.The red dashed lines in Fig.4 show the typical constituent fluctuations of different phases.Fig.4(a) shows the morphology and elemental distribution in the Q-P1 sample,in which it can be seen that the elemental distributions of silicon,manganese,aluminum,and niobium are homogeneous along the lineAA′.This is attributed to the uniform distributions of the alloying elements in austenite during full austenitization at 910 ℃.During the subsequent quenching process,the austen-ite partly transformed into martensite and there was almost no noticeable diffusion of large atoms such as iron or substitutional atoms during this stage.As reported in previous studies[13-14],the diffusion of carbon from supersaturated martensite to untrans-formed austenite occurs during the subsequent partitioning process.Some carbon-enriched austenite was transformed into fresh martensite during the final quenching process,which led to a high carbon content in the fresh martensite,and the untrans-formed austenite was finally retained at room temperature.As shown in Fig.4(a),the regions with a high carbon content exhibited a somewhat film-like morphology between the martensite laths.
In contrast,Fig.4(b) shows a different distri-bution of the alloying elements,wherein,except for niobium,all the other alloying elements exhibited non-uniform distribution.Ferrite had relatively high concentrations of silicon and aluminum,and low concentrations of carbon and manganese.Previous research has shown that alloying elements diffuse and are redistributed between ferrite and austenite during intercritical annealing[15-16].The austenite-stabilizing elements,such as carbon and manganese,were first concentrated at the austenite grain boundaries and then diffused to the inner region of austenite,which led to a higher carbon content near the austenite grain boundaries than in the inner regions.A relatively high carbon content at the austenite grain boundaries increases the stability of austenite,preventing its transformation to martensite.As shown in Fig.4(b),the austenite near the interface of ferrite and austenite (marked as siteB) had high concentrations of carbon and manganese,and the concentrations of carbon and manganese near the austenite grain boundaries were uniform in the Q-P1 sample (Fig.4(a)).It can be specul-ated that retained austenite was present in the intercri-tically annealed samples near the ferrite/austenite interface.However,in the fully austenitized samples,retained austenite occurred as a film between the martensite laths.
3.3 Volume fraction and carbon concentration of retained austenite
The volume fraction of retained austenite is a major factor governing the TRIP effect in Q & P steels.Fig.5 shows the X-ray diffraction patterns of the Q-P1 and Q-P2 treatment.The retained austenite fraction (Vγ) was calculated using the following equation[17-18]:
(1)
where,IγandIαare the integrated intensities of the austenite and ferrite diffraction lines in the X-ray diffraction patterns,respectively.The position of the maximum diffraction peak of austenite was used to determine the lattice constant of austeniteaγ.This parameter is needed to calculate the concentration of carbon in retained austenite(w(Cγ)) using the following equation[19]:
aγ=0.354 67+0.004 67Cγ
(2)
In this work,the diffraction intensities of the (200)α,(211)α,(200)γ,(220)γ,and (311)γpeaks were utilized to determineVγ[18].The carbon content in the retained austenite was estimated using the average of the values determined for (200)γ,(220)γ,and (311)γ.The calculated volume fractions of the retained austenite and corresponding carbon contents are listed in Table 2.The error associated with this measurement is also included in Table 2.The carbon content of the retained austenite determined using Equation (2) is an average value.The calculated carbon content was not considered to indicate the carbon content at any particular location from the interface to the center,but rather the change trend of the carbon content throughout the retained austenite.
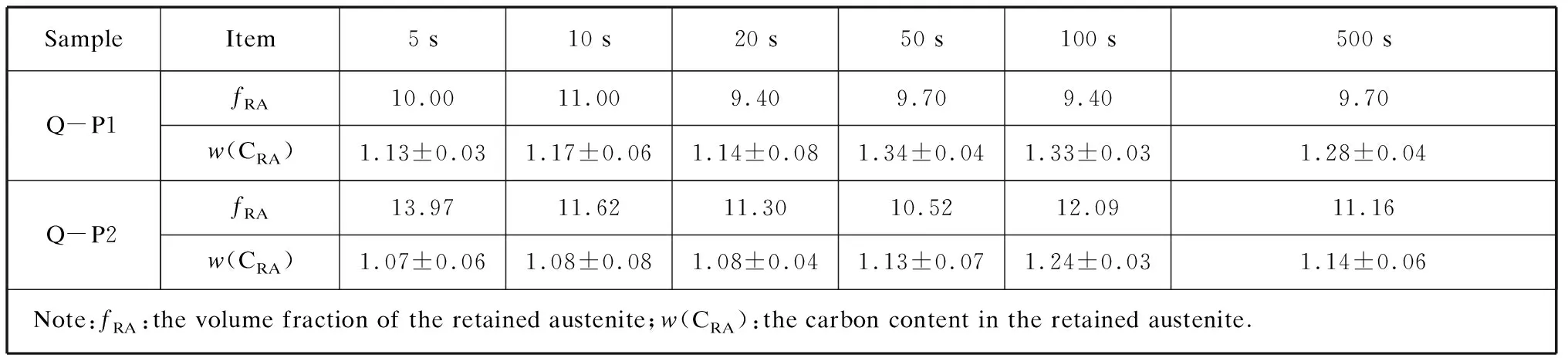
Table 2 Calculated volume fractions and corresponding carbon contents of the retained austenite in steels with different partitioning time after annealing at different temperatures %
It can be seen in Fig.5 that compared to the Q-P1 samples,the retained austenite in the Q-P2 samples exhibited more obvious peaks,i.e.,(200)γ,(220)γ,and (311)γ,and this finding applies to all the partitioning time.The calculated fractions of the retained austenite showed that samples annealed at 850 ℃ (Q-P2) had a higher volume fraction of retained austenite than those annealed at 910 ℃ (Q-P1),irrespective of the partitioning time.However,the carbon content in the retained austenite was higher in Q-P1 than in the Q-P2 samples.As is known,the manganese element has the effect of stabilizing austenite and decreasing the martensite start temperature.In this regard,manganese has the same effect as carbon,which means equivalent stability can be achieved by increasing the manganese content and decreasing the carbon content in retained austenite.In this study,the enrichment of manganese in austenite during intercritical annealing enhanced the chemical stability of the austenite.Therefore,in theory,the retained austenite in the intercritically annealed samples consumed less carbon than those that had been fully austenitized (based on the premise of ignoring other sources of carbon depletion,such as carbide precipitation and carbon solubility in ferrite),which means the intercritical annealing was beneficial for increasing the volume fraction of the retained austenite in the experimental steel.The above statements explain the experimental results in Table 2 very well.
3.4 Characterizations of retained austenite
In addition to the film-like retained austenite mentioned above,other morphologies were present in the retained austenite in the Q-P2 samples.Fig.7 shows TEM micrographs of retained austenite with different morphologies.In Figs.7(a) and (b),some of the retained austenite is surrounded by ferrite with dislocations and has a blocky morphology.Figs.7(c) and (d) show lamellar retained austenite distributed between ferrite grains.Figs.7(e) and (f) show granular retained austenite near the α /γ interface.It is clear that the differences in the morphologies of the retained austenite of these two sample groups are due to the different annealing temperatures.However,an understanding of the formation mechanisms of retained austenite with different morphologies will require further research.
3.5 Mechanical properties
The mechanical properties of the experimental steel are illustrated in Fig.8.Fig.8(a) shows the ultimate tensile strength (UTS) of the samples that had undergone different types of annealing.It is clear that the UTS values of the samples annealed at 910 ℃ are higher than those that had been intercriti-cally annealed.On the whole,as shown in Fig.8(a),the tensile strength of the two groups of samples decreases with extension of the partitioning time from 5 s to 500 s,and the total elongation (TEL) values increase.The degree of martensite tempering is also increased with the extension of the partition-ing time,which decreases the carbon content and recovers the dislocations in martensite.From these results,it can be deduced that the recovery of martensite during the partitioning process is the main reason for the decreasing strength with exten-sions of the partitioning time.The maximum tensile strength of the samples annealed at 910 ℃ reached 1 350 MPa,whereas it reached only approximately 1 050 MPa for the samples annealed at 850 ℃.
Compared with the tensile strength,the corre-sponding TEL,as shown in Fig.8(b),exhibits the opposite change tendency,i.e.,it increases overall with extensions of the partitioning time.As mentioned above,the degree of martensite tempering increases with extensions of the partitioning time,which improves the ductility and toughness of martensite.In Fig.8(b),compared with the TEL of the samples annealed at 910 ℃,the TEL values of the intercriti-cally annealed samples exhibit a significant impro-vement,with the maximum TEL reaching 24.8%.Fig.8(c) shows the product of the strength and elongation (PSE) of the samples treated by the both kinds of annealing.Compared with the samples annealed at 910 ℃,the intercritically annealed samples also exhibit a significant improvement in the PSE.For the samples annealed at 910 ℃,the optimal PSE of 16 359 MPa% appears at the 20-s partition.The corresponding UTS and TEL values are 1 230 MPa and 13.3%,respectively.By con-trast,the intercritically annealed samples exhibit a higher optimal PSE (>24 000 MPa%),with a UTS of 980 MPa and a TEL of 24.8%,respectively.
Although intercritical annealing results in the decreased strength of Q-P steels,it obviously improves the plasticity and PSE of these steels.In the experimental steel,the improvement of TEL and PSE is likely due to two main factors.On one hand,the formation of ferrite during intercritical annealing increases the carbon content in intercritical austenite,which enhances its stability and reduces itsMs.This means that a higher volume fraction of metastable austenite will remain at the quenching temperature (Tq) in the intercritically annealed samples than in the fully austenitic samples.This results in a higher volume fraction of retained austenite at room temperature in the intercritically annealed samples,as shown in Fig.5 and Table 2.On the other hand,a certain amount of ferrite in the experimental steel had a positive effect in improving plasticity.Due to its low strength and good ductility,the ferrite deforms preferentially during deformation.This delays the plastic deformation of martensite and the TRIP effect of the retained austentite,which enhances the plasticity of the experimental steel.
4 Conclusions
We investigated the effect of annealing temperature on the microstructural evolution of retained austenite in a low-carbon Q & P steel using a combination of electron microscopy,XRD,and electron probe techniques.The study results suggest that the fully austenitized Q & P treatment and the intercritical annealing Q & P treatment yield differences in the microstructure and volume fraction of the retained austenite and the mechanical properties of the experimental steel.The microstructures of the intercritically annealed Q & P samples differed from the samples that had undergone fully austenitic Q & P treatment,especially with respect to the morphology and carbon content of the retained austenite.The retained austenite in the fully austenitized Q & P samples was distributed between the martensite laths and appeared as a film,whereas in the intercritically annealed samples,in addition to the film-like retained austenite between the martensite laths,retained austenite was distributed near the α /γ interface,exhibiting blocky,lamellar,and granular-type morphologies.In the fully austenitic samples,the optimal PSE of 16 359 MPa% occurred at the 20-s partition,with corresponding UTS and TEL values of 1 230 MPa and 13.3%,respectively.By contrast,the intercritically annealed samples obtained a higher optimal PSE (> 24 000 MPa%),with a UTS of 980 MPa and a TEL of 24.8%,respectively.The different mechanical properties of these two sample groups were likely due to two main factors:First,the formation of ferrite during intercritical annealing has a carbon-enrichment effect in intercritical austenite,which enhances the stability of metastable austenite.Although increasing the content of retained austenite is beneficial,it results in a significant reduction in strength.Second,the ferrite delays the plastic defor-mation of martensite,which postpones the TRIP effect of retained austenite and improves the TEL of the intercritical samples.
 Baosteel Technical Research2020年4期
Baosteel Technical Research2020年4期
- Baosteel Technical Research的其它文章
- Contributions to Baosteel Technical Research wanted
- Solute redistribution and macrosegregation in continuous casting slab of carbon steel during solidification process
- Deformation behavior of 9Cr-3W-3Co martensitic heat-resistant steel
- Effects of different helium cooling conditions on the structures of GH4169 alloy vacuum arc remelting ingots
- Application and comparison of different anisotropic yield criteria in the formability analysis of aluminum sheet
- Total contents of Vol.14, 2020
