A meta-analysis of 1-year outcomes of transcatheter versus surgical aortic valve replacement in low-risk patients with severe aortic stenosis
Aaqib H Malik, Syed Zaid, Hasan Ahmad, Joshua Goldberg, Tanya Dutta, Cenap Undemir,Martin Cohen, Wilbert S Aronow, Steven L Lansman
1Department of Medicine, Westchester Medical Center and New York Medical College, Valhalla, NY, USA
2Department of Cardiology, Westchester Medical Center and New York Medical College, Valhalla, NY, USA
3Section of Cardiothoracic Surgery, Department of Surgery, Westchester Medical Center, Valhalla, New York, USA
Abstract Background Transcatheter aortic valve replacement (TAVR) for the treatment symptomatic severe aortic stenosis (AS) is indicated in patients with intermediate or higher surgical risk. Latest trials showed TAVR, and surgical aortic valve replacement (SAVR) perform similarly at 1-year for the composite outcomes of mortality, stroke and rehospitalization. We performed a comprehensive meta-analysis to compare individual outcomes at 1-year for TAVR compared to SAVR in low-risk patients. Methods PubMed, Embase, and Cochrane central were searched for all the randomized controlled trials (RCTs) that reported 1-year comparative outcomes of TAVR and surgical aortic valve replacement (SAVR). Our conclusions are based upon the random-effects model using DerSimonian-Laird estimator. Results Data from 4 trials and 2887 randomized patients showed that TAVR had lower rates of all-cause mortality, cardiovascular mortality, and atrial fibrillation compared to SAVR at 1-year follow-up (P < 0.05 for all). Also, TAVR was also associated with a significantly higher risk of permanent pacemaker implantation and moderate-severe paravalvular leak (P < 0.05). Conclusions The latest randomised trial data demonstrates that in short-term, TAVR is safe and effective in reducing all-cause mortality or stroke. Longer follow-up of RCTs is needed to determine the durability of clinical benefits in TAVR over SAVR in low-risk patients.
Keywords: Aortic stenosis; Low surgical risk; Meta-analysis; Transcatheter aortic valve replacement
1 Introduction
Severe aortic stenosis (AS), when left untreated, is associated with an increased risk of morbidity and mortality including heart failure,[1]stroke, perioperative complications,[2]and worsening of coronary artery disease.[3-5]Transcatheter aortic valve replacement (TAVR) is currently indicated in intermediate or higher surgical risk patients with symptomatic severe AS.[6-12]In high surgical risk patients, TAVR has been found non-inferior to surgical aortic valve replacement (SAVR) at 5 years for both balloon-expandable[7]and self-expanding transcatheter prostheses.[13]In stroke or all-cause mortality, TAVR was found to have an absolute risk reduction of 9.4% compared to SAVR at 3 years (P= 0.006).[14]Among intermediate-risk patients, TAVR was non-inferior to SAVR for both Sapien XT (Edwards Lifesciences LLC, Irvine, CA)[15]and Core-Valve (Medtronic Inc, Minneapolis, MN),[16]while Sapien 3(Edwards Lifesciences LLC, Irvine, CA) performed via transfemoral approach was superior to SAVR in a propensity-matched analysis.[17]Currently, in patients with lowsurgical risk, guidelines still support SAVR as the standard treatment of AS.[14]Low-surgical-risk patients represent four out of five severe AS patients that will eventually undergo SAVR.[18]Given the significant technological and procedural advancements, as well as favourable outcomes in intermediate-risk patients, there is speculation that TAVR may become a reasonable alternative to SAVR for low-surgical risk patients.
As a result, recent randomised controlled trials (RCTs)have reported favourable composite outcomes, including mortality and stroke, with TAVR similar to those seen with SAVR.[19]Accordingly, the objective of this study is to synthesise and collate the evidence from the available RCTs to assess the relative efficacy and safety of TAVR, for the individual outcomes of mortality and stroke, compared to SAVR in symptomatic severe AS patients with low surgical risk.
2 Methods
2.1 Data sources and searches
We searched PubMed, Embase, and Cochrane Central Register of Controlled Trials (from inception to March 20,2019) to identify RCTs that evaluated the outcomes associated with TAVR compared to SAVR in patients with severe aortic stenosis who were deemed low surgical risk with a Society of Thoracic Surgeons predicted risk of mortality(STS PROM) score of < 4%. Relevant randomised articles were identified using the terms ‘transcatheter aortic valve replacement’, ‘TAVR’, ‘surgical aortic valve replacement’, ‘SAVR’, ‘TAVI’, ‘SAVI’ and ‘low risk’. The search strategy is explained in Supplemental Table 1S. The reference lists of published original articles were manually screened for articles that may have been missed. Two investigators (AA and SZ) independently reviewed the lists.Throughout this process, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(PRISMA) guidelines for all stages of the design and implementation (Supplemental Table 2S).
2.2 Study selection
In our meta-analysis, we included studies that met the following a priori inclusion criteria; (1) a randomised controlled design, (2) compared TAVR treatment strategy with SAVR, (3) involved patients with severe aortic stenosis, (4)STS PROM of < 4%, and (5) reported outcomes of at-least 1-year follow-up. Studies were excluded when; (1) Outcome event data were not available, and (2) studies with less than 1-year outcome data. Reviews were excluded as part of our search strategy. If more than one study was published involving the same data, then only the study that reported 1-year outcome was included in the analysis, although allpost-hocarticles were reviewed to supplement missing data where applicable.
2.3 Data extraction and quality assessment
The following data were extracted from each article: author information, year of publication, sample size, demographic data, and clinical data. Our outcomes of interest were all-cause mortality, stroke, cardiovascular mortality,atrial fibrillation, stroke, moderate-severe paravalvular leak,pacemaker implantation, myocardial infarction, major or life-threatening bleeding, and major vascular complications.Two independent investigators (AM and SZ) used a standardised data form independently to extract all the data, and any disagreements were resolved by mutual discussion. We evaluated suitable trials for completeness according to the intention to treat principle.
2.4 Data synthesis and analysis
Summary effects with 95% confidence intervals (CI) for all the clinical endpoints were calculated with a random effects model using DerSimonian and Laird estimator.[20]We usedI2statistic to quantify the proportion of observed inconsistency across study results not explained by chance.[21]Systemic bias, including publication bias, was assessed by examining the funnel plot asymmetry and quantified by using Egger’s regression test to calculate two tailed p- values[22]and its implications for our results were assessed by the fail-safe[23]and the trim-and-fill method.[24]All analyses were performed with R software.[25]Since we utilized already published data, our study was exempt from institutional board review and principles outlined in the Declaration of Helsinki have been followed. For all analysis,Pvalues were 2-sided, withP-values of less than 0.05 considered to indicate statistical significance.
3 Results
Our initial search identified 336 publications that were narrowed down to 213 potentially relevant unique articles after removing duplicates and non-relevant titles. The search of conference proceedings and bibliographies did not identify any additional articles. After the abstract screening, we excluded a further 199 articles for various reasons, as mentioned in the PRISMA diagram (Figure 1). We excluded further 10 articles on full-text review and came up with a final list of four trials for our analysis of 1-year outcomes.[26-29]
Supplemental Tables 3 and 4 summarise the characteristics of the included studies and its participants. A total of 2887 randomised patients with severe aortic stenosis from three RCTs and onepost hocanalysis of an RCT with a low surgical risk (STS PROM < 4) status had the relevant data available. All the studies were of high quality in the study assessment due to their randomization.
The meta-analysis results shows TAVR seem significantly better than SAVR at 1-year for the individual outcomes of all-cause mortality (RR = 0.61, 95% CI:0.39-0.96;P =0.03 andI2= 0%), cardiovascular mortality (RR = 0.55,95% CI:0.30-0.90;P =0.02 andI2= 0), and atrial fibrillation (RR = 0.27, 95% CI: 0.20- 0.35;P< 0.01 andI2=63%), whereas there was a significantly higher risk of paravalvular risk (RR = 5.70, 95% CI:1.73-18.79;P< 0.01 andI2= 34%), and a greater number of pacemaker implantation associated with TAVR compared to SAVR at 1-year (RR =3.96, 95% CI: 1.65-9.54;P< 0.01 andI2= 86%; Figures 2-4).As shown, there was no difference between TAVR and SAVR for the outcomes of myocardial infarction, stroke, major vessel complications and major or life-threatening bleeding.
Figure 1. PRISMA diagram for the search strategy. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses;RCTs: randomised controlled trials; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
No funnel plot asymmetry was visualised but not reported here for all the outcomes due to a low number of studies. However, we performed a linear regression of the funnel plot to find the eggers regression test, which was non-significant for all the outcomes suggesting a lack of publication bias (allP> 0.05).
4 Discussion
For low surgical risk patients with severe AS who undergo aortic valve replacement, the novel findings of this analysis are as follows: (1) TAVR is associated with a significantly reduced risk of all-cause mortality and cardiovascular mortality, (2) there is an increased risk of moderatesevere paravalvular leaks with TAVR along with a greater need of PPM implantation compared to SAVR, and (3) AF occurs in a significantly lower number of patients who undergo TAVR compared to SVR.
Our findings have significant implications. In addition to absolute risk reduction of 2.6% in stroke or all-cause mortality over 1 year follow-up, the increased utilization of TAVR comes with potential cost savings due to a lower length of hospital stay (3vs. 7 days) and lower postoperative complications including post-procedural bleeding and atrial fibrillation, despite a significantly higher cost of a transcatheter valve than a surgical bioprosthesis. The costeffectiveness was demonstrated in the Placement of AoRTic TraNscathetER Valve Trial (PARTNER) 2A study where TAVR, compared to SAVR in intermediate risk AS patients,was expected to be cost-saving by an average of $8000-10000 and to increase quality-adjusted survival by 0.15 to 0.27 years.[30]
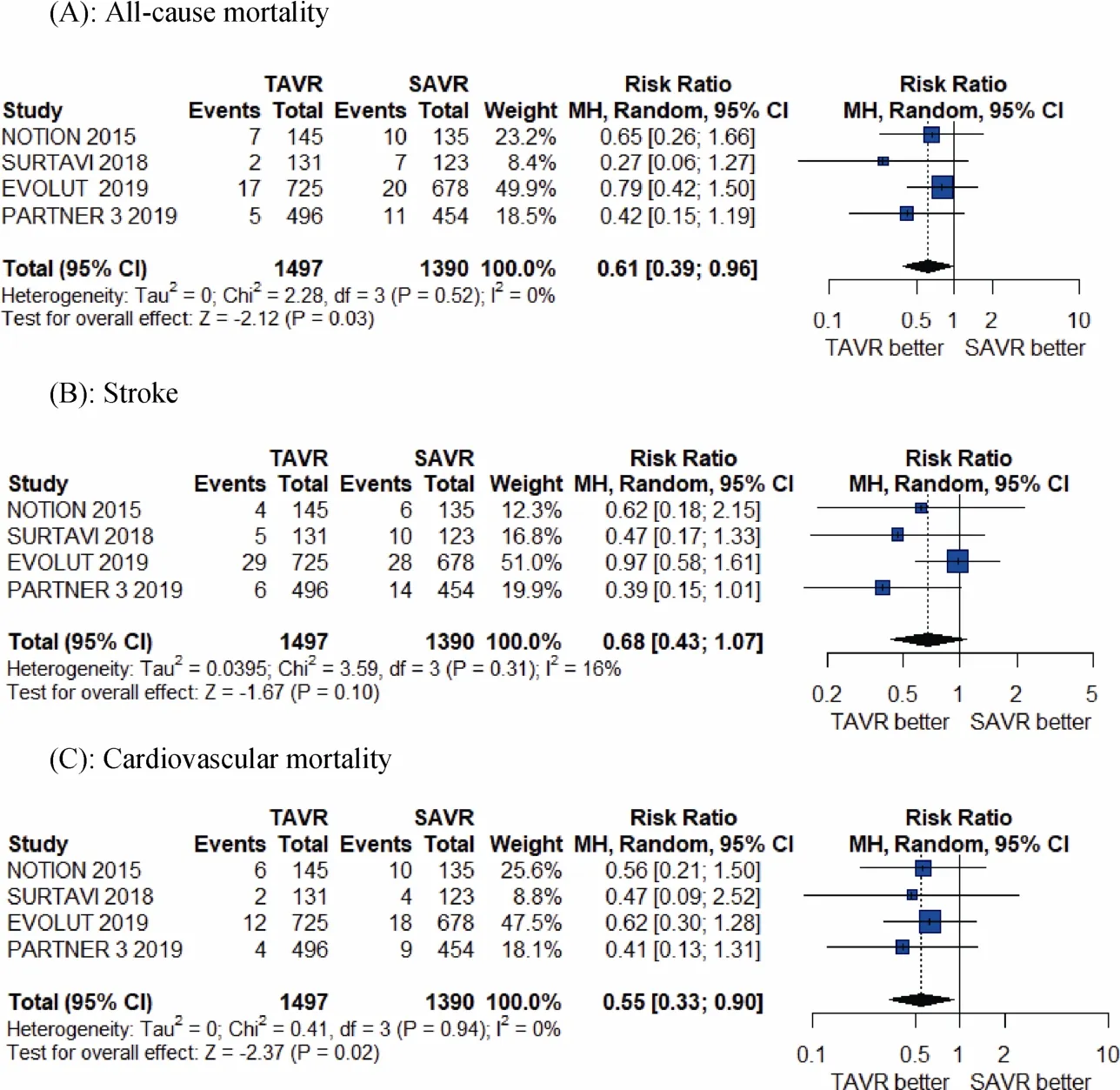
Figure 2. Pooled results of TAVR vs. SAVR in low-surgical risk patients. (A): All-cause mortality; (B): stroke; (C): cardiovascular mortality. TAVR: transcatheter aortic valve replacement; SAVR: surgical aortic valve replacement.
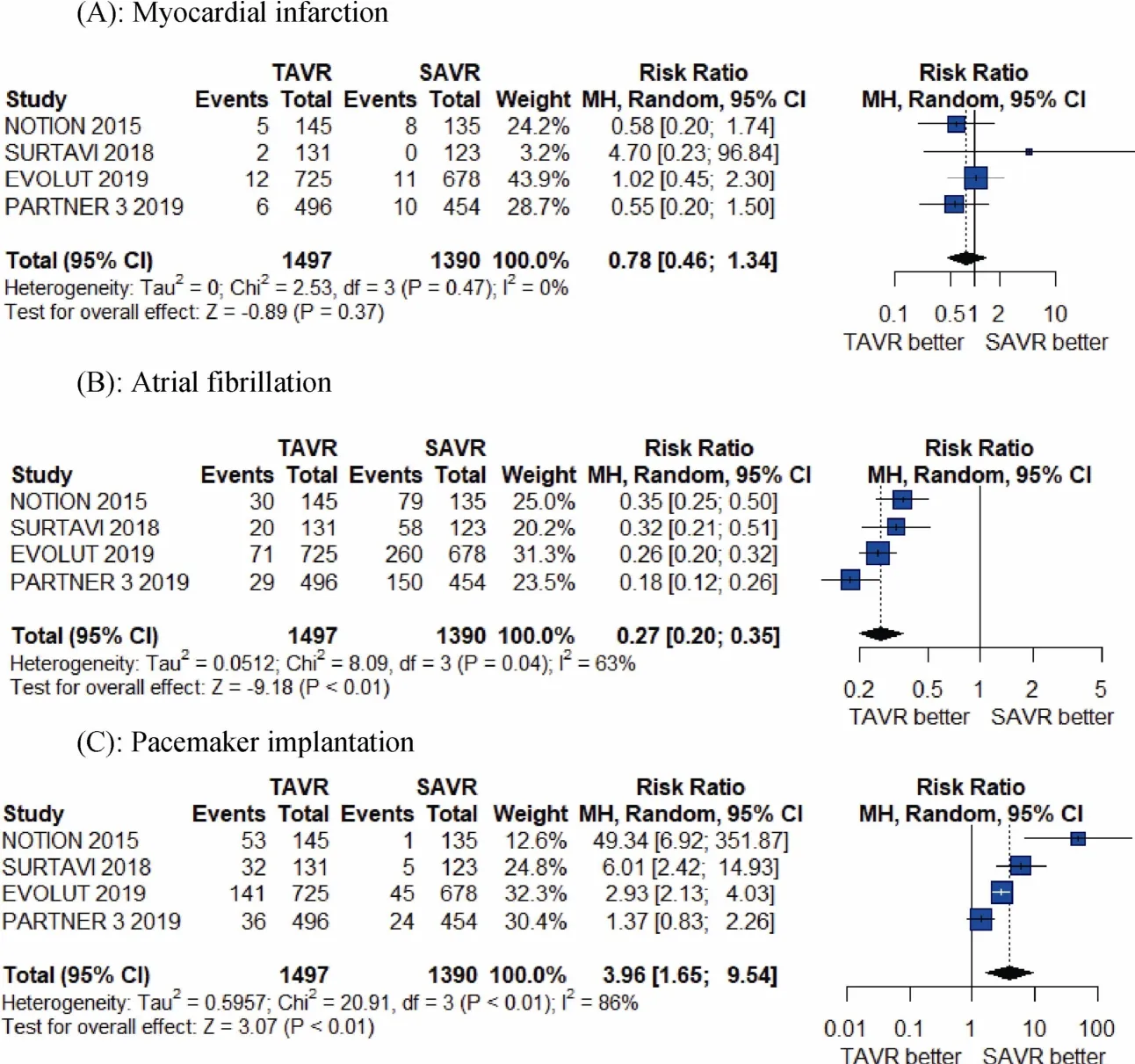
Figure 3. Pooled results of TAVR vs. SAVR in low-surgical risk patients. (A): Myocardial infarction; (B): atrial fibrillation; (C): pacemaker implantation. TAVR: transcatheter aortic valve replacement; SAVR: surgical aortic valve replacement.
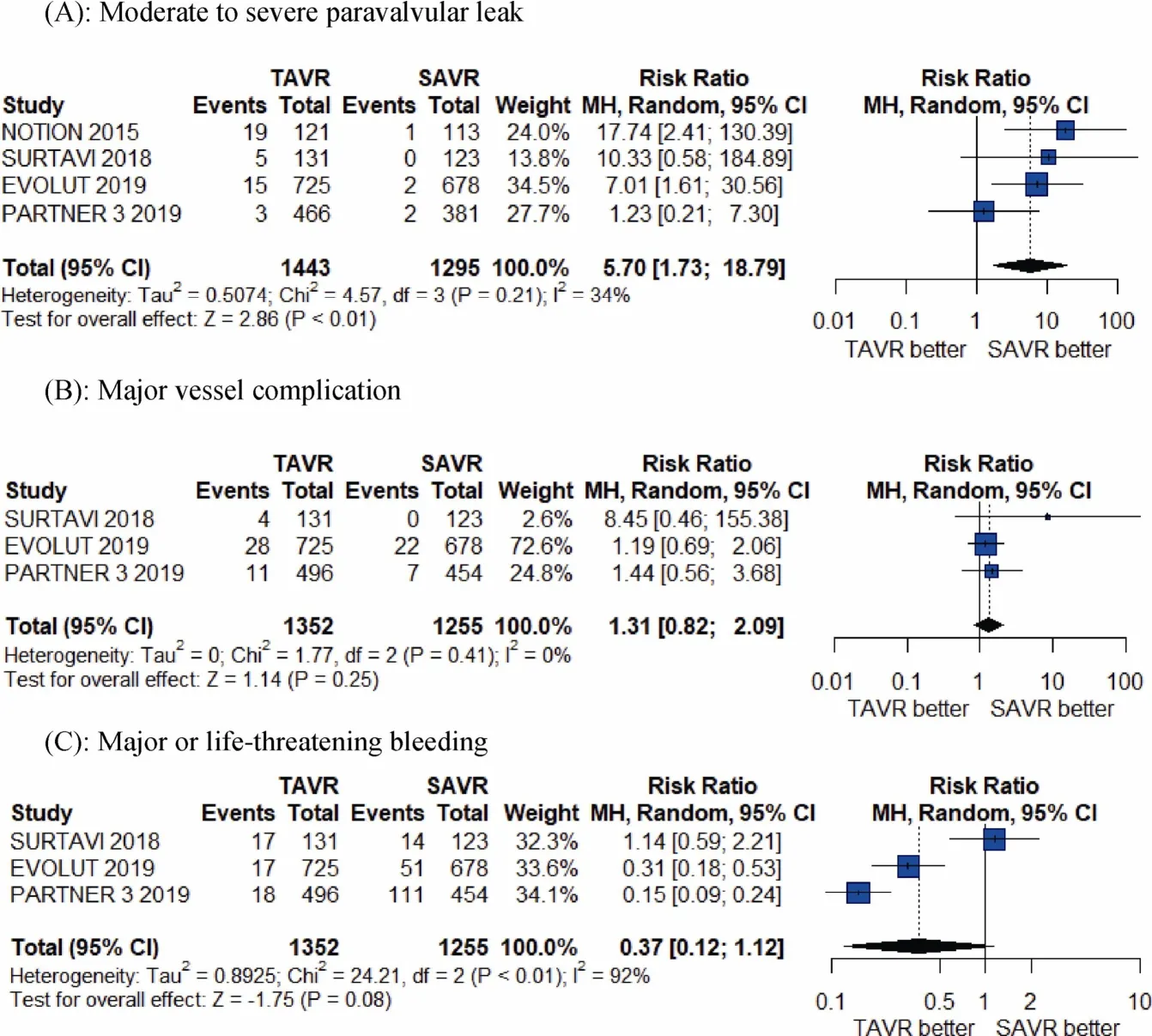
Figure 4. Pooled results of TAVR vs. SAVR in low-surgical risk patients. (A): Moderate to severe paravalvular leak; (B): major vessel complication; (C): major or life-threatening bleeding. TAVR: transcatheter aortic valve replacement; SAVR: surgical aortic valve replacement.
Despite the positive and encouraging results in our meta-analysis, we do need long-term data to confirm the durability of the observed early clinical benefits of TAVR versus the longer-term impact of associated adverse events,such as valve degeneration, paravalvular leak, new persistent left bundle branch block (LBBB) and permanent pacemaker (PPM) implantation. The benefits of TAVR seen in this analysis are somewhat diminished by an increased number of a moderate or higher paravalvular leak and pacemaker implantation, which was an expected finding.[31]These factors are even more critical for these low-risk patients who are presumably younger and have a longer life expectancy. Although, a recent 1-year follow up of Low-Risk TAVR (LRT) trial showed the incidence of 1.5%moderate-severe paravalvular leak and 7.5% PPM implantation, which is amongst the lowest of all the TAVR trials.[32]We can expect improved results with improved valve designs, operator expertise, and further technical modifications.[33]The incidence of new LBBB and the need for PPM implantation has been unexpectedly higher with TAVR as it has been associated with significant increases in morbidity and mortality.[34-36]A recent report from the STS/ACC TVT registry confirms the increased burden and clinical implications of these conduction abnormalities after TAVR, and these were particularly noted in patients with a higher body mass index and those who undergo valve-in-valve procedures.[37]Further research is needed to identify the predictors of worse outcomes that will help in identifying a suitable group of patients who can undergo TAVR with lower complications.
The success and expansion of TAVR indications for these lower risk population are expected to facilitate a significantly large population with TAVR valves that are relatively healthier. The number of patients presenting with acute coronary syndrome with TAVR values is expected to increase, which will pose a significant challenge to the interventional community, especially in community hospitals.[38,39]Furthermore, the latest data from 551 Sapien 3 TAVRs with post-deployment aortograms suggests that repeat TAVR(TAV-in-TAV) may not be possible in > 21% of patients as it would risk obstruction of the coronary artery.[40]
Regarding long-term outcomes, it is worth noting that there is wide variation in the reported structural valve degeneration (SVD) and the notion that surgical bioprosthetic valves last a long time is not correct.[41]Furthermore, the initial reports of long-term data with TAVR are very encouraging with the 6-year follow-up outcomes from the Nordic Aortic Valve Intervention (NOTION) Trial, showing no difference in all-cause mortality, stroke or myocardial infarction with TAVR versus SAVR (42.5%vs. 37.7%;P=0.58). Importantly, the moderate to severe SVD, as defined by a combination of real SVD and patient-prosthesis mismatch (with a mean gradient of ≥ 20 mmHg), was significantly less in patients who underwent TAVR (4.8%vs. 24%;P< 0.001).[42]Though the SVD definition may have biased against SAVR, the 6-year data in NOTION, as well as the 5-year data in PARTNER and US CoreValve pivotal trials,showed at least mid-term durability of TAVR devices. Similar findings were recently noted in the UK TAVI registry.[43]
4.1 Limitations
A significant limitation of our analysis is the lack of long-term follow-up and a small number of included trials.Another trial in younger low surgical risk patients (NOTION-2 NCT02825134) is expected to report its finding next year. The results of that study will further add to the evidence of TAVR outcomes in younger and low surgical risk patients. Additionally, we performed a trial-level metaanalysis due to the lack of availability of patient-level data.Moderate heterogeneity was noted for the atrial fibrillation,and paravalvular leak, whereas high heterogeneity was noted for the outcome of pacemaker implantation. We performed influential analyses for the outcomes with moderate to high heterogeneity to explore the source, as shown in Supplemental Figure 1S. The differences were partially explained by the use of a balloon-expandable valve in PARTNER 3 compared to self-expandable ones in other trials. However, due to a small number of trials, we were unable to do subgroup analysis to explore that. Finally, we appreciate that NOTION trial used older generation Core-Valves, but this only suggests that the superiority of the TAVR even with older valves as every trial showed a trend to improved outcomes with TAVR. Similarly, the original Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial was of intermediate risk population, and the sub-studypost-hocanalysis of low-risk patients from SURTAVI might have residual confounding.However, these unknown risk factors should be similar in both groups of TAVR and SAVR, which would bias the association of confounders towards the null. Furthermore,our results are significant on influential analyses as shown in the supplement.
4.2 Conclusion
In conclusion, TAVR seems to result in superior 1-year outcomes, including all-cause mortality and cardiovascular mortality, compared to SAVR in symptomatic severe AS patients with low surgical risk. Further advances are needed to improve the associated higher rates of a paravalvular leak and PPM implantation with a self-expanding valve. Finally,long-term data is warranted to determine the impact of TAVR in this patient population.
4.3 Impact on daily practice
The results of this meta-analysis provide support for widespread utilisation of TAVR for symptomatic aortic stenosis patients with a lower surgical risk. Although these findings are promising, and they depict TAVR as a preferable option for all patients owing to reduced peri-operative complications and a shorter recovery, a longer follow up is required.
Acknowledgements
Part of this manuscript was presented as moderated poster at TCT 2019 in San Francisco. Malik A, Zaid S,Ahmad H, Goldberg J, Dutta T, Undemir C, Cohen M,Aronow W, Lansman S, Tang G. TCT-734 Transcatheter Compared to Surgical Aortic Valve Replacement in Patients With Low Surgical Risk: A Meta-Analysis of All Randomized Controlled Trials. Journal of the American College of Cardiology. 2019 Oct 1; 74(13 Supplement): B720.http://www.onlinejacc.org/content/74/13_Supplement/B720.abstract.All authors have no conflicts of interest to declare.

Figure 1S.
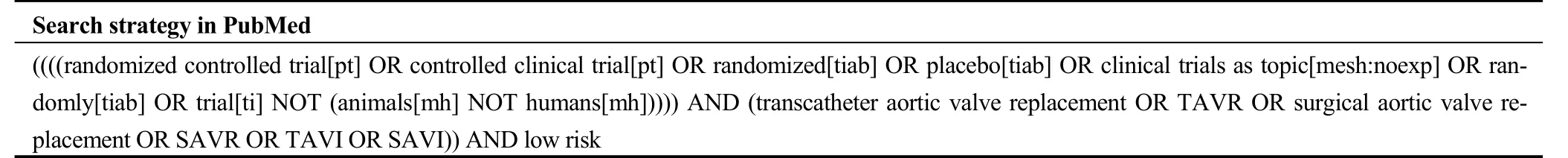
Table 1S. Search strategy in PubMed
Table 2S. PRISMA checklist

Table 3S. Baseline characteristics of trials and population.
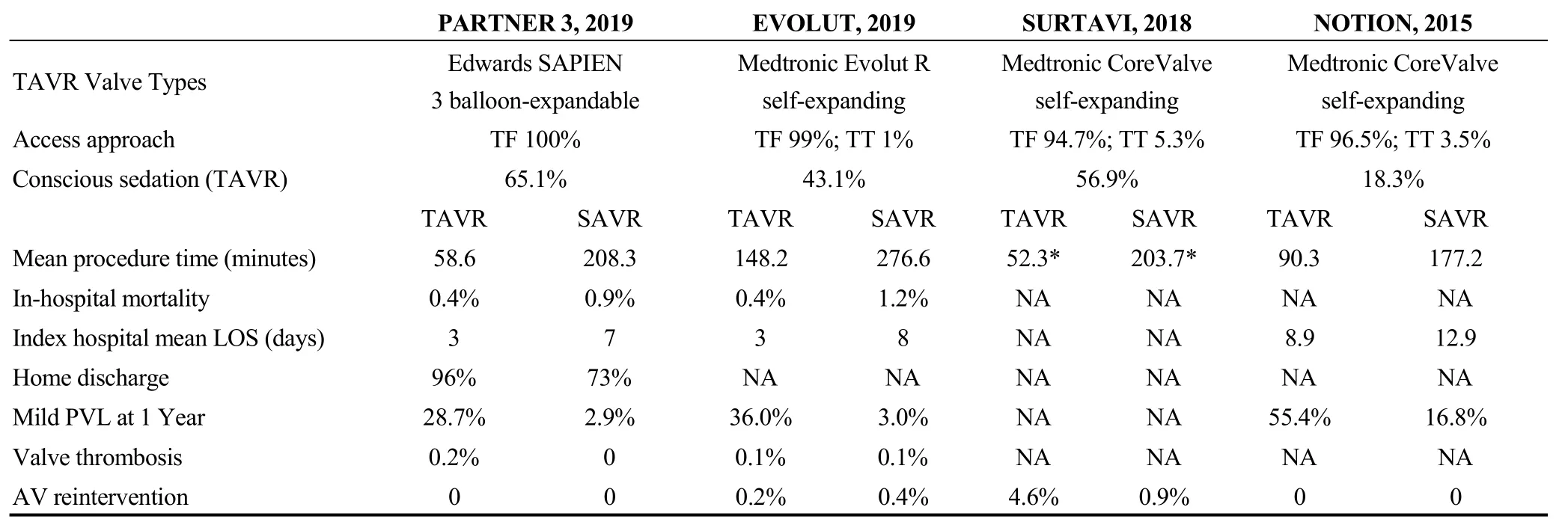
Table 4S. Procedural and in-hospital characteristics of the included trials.
 Journal of Geriatric Cardiology2020年1期
Journal of Geriatric Cardiology2020年1期
- Journal of Geriatric Cardiology的其它文章
- Polymorphic ventricular tachycardia during phase II cardiac rehabilitation in a patient with heart failure: a case report
- “One-man” bailout technique for high-speed rotational atherectomy—assisted percutaneous coronary intervention in an octogenarian
- Aspergillus infection of pacemaker in an immunocompetent host: a case report
- Is dual therapy the correct strategy in frail elderly patients with atrial fibrillation and acute coronary syndrome?
- Anemia in patients with high-risk acute coronary syndromes admitted to Intensive Cardiac Care Units
- Prognostic factors in heart failure patients with cardiac cachexia
