Bevacizumab Combined with Icotinib Overcomes Osimertinib Resistance in a Patient of Non-Small Cell Lung Cancer
Ling Zhang,Lei Sun,Xiaoyan Mu,Youxin Ji
1Department of Oncology,Qingdao Cancer Hospital,Qingdao,Shandong 266042,China
2 Department of Oncology,the Affiliated Qingdao Central Hospital,Qingdao University,Qingdao,Shandong 266042,China
Key words:Epidermal growth factor receptor-tyrosine kinase inhibitor;resistant mutation;nonsmall cell lung cancer;bevacizumab
Abstract A 61-year-old Chinese woman was diagnosed as primary pulmonary adenocarcinoma of left superior lobe with epidermal growth factor receptor (EGFR) 19 del mutation positive.Treatment with icotinib was given,but her disease progressed after 6 months remission.CT-guide needle biopsy for the new lesion in inferior lobe of left lung demonstrated intrapulmonary metastasis,and EGFR gene panel by Amplification Refractory Mutation System Polymerase Chain Reaction (ARMS-PCR) confirmed EGFR T790M mutation.Treatment with osimertinib was initiated.After 2 months remission,the disease progressed.Re-biopsy was performed for the tumor in the inferior lobe of left lung,and ARMS-PCR demonstrated no other gene mutation except EGFR 19 del.Icotinib was re-challenged,but disease progressed continuously.Bevacizumab was added,and partial response was achieved after 2-cycle of combination therapy.The non-small cell lung cancer (NSCLC) in this case maintained EGFR activating mutation and lost EGFR T790M mutation was a genetic change after osimertinib treatment.This case suggests the re-challenge of the first-generation EGFR-TKIs combined with bevacizumab may overcome the tumor resistance and prolong survival of NSCLC patient.
EPIDERMAL growth factor receptor (EGFR)C797S/G point mutation or loss of EGFR T790M was the most common genetic change in patients with non-small cell lung cancer (NSCLC) harboring EGFR T790M mutation after resistance to osimertinib.1,2Anti-EGFR antibody combined with EAI045 or Brigatinib could overcome osimertinib resistancein vitroorin vivoif it was caused by EGFR L858R/C797S/T790M or EGFR del 19/C797S/T790M.3,4But their clinical effects are largely unclear.There has been no standard care for patients who lose EGFR T790M mutation after osimertinib resistance;re-challenge with first generation EGFR-tyrosine kinase inhibitor (TKI) or systemic chemotherapy might be the optimal method.5About 10%-30% Caucasian or 50% eastern Asian patients with NSCLC harbor EGFR mutation,and 60% of them will acquire EGFR T790M mutation after the first generation EGFR-TKI treatment with 10-13 months remission.6,7Acquired resistance would happen after a median PFS of 9.6 months in patients receiving osimertinib treatment (an irreversible third-generation EGFR-TKI).8The resistance mechanism of Osimertinib is complex.To date,several mechanisms about resistance to Osimertinib have been identified,such as secondary EGFR mutation,EGFR amplification,and loss,low expression.9-11Among them,EGFR C797S/G mutation happened in 20%-60%patients.12So Re-biopsy to study the resistance mechanisms at the time of disease progression is necessary to direct therapeutic regimens for patients with osimertinib resistance.13,14
Currently,no drug or therapeutic strategies has been approved for the treatment of osimertinib resistant patients,especially for patients who are T790M negative or lost.Many strategies aremainly limitedin vitroorin vivo,3in lack of clinical data.It has been reported cytotoxic chemotherapy or protracted EGFR-TKI treatment might be applied after disease progressed,but only a short survival time benefit was reached.15-17We report a patient of NSCLC,who lost EGFR T790M mutation after osimertinib treatment,was responsive to icotinib plus bevacizumab therapy.
CASE DESCRIPTION
A 61-year-old Chinese woman complained cough and fever for 2 weeks.She was admitted into hospital.CT scan showed a 10.1 cm X 6.1 cm mass in the left lung (Figure 1A),with multiple metastatic nodulars of bilateral lungs,mediastinal lymph nodes enlargement,left pleural effusion,and small patchy lesions in the right lung.Core needle biopsy for the mass of left lung demonstrated pulmonary adenocarcinoma (Figure 1B).EGFR test of tumor tissue was conducted by direct sequencing and EGFR 19 del was found.Diagnosis of primary lung adenocarcinoma with metastasis harboring EGFR activating mutation positive was established.
The patient was treated with icotinib (Betta Pharmaceuticals,Hangzhou,China) orally at a dose of 125 mg three times a day for 2.5 months and partial response was reached (Figure 1C).However,after 6 months remission,follow up CT scan found that the primary tumor enlarged and new lesion appeared in the inferior lobe of left lung.CT-guide needle biopsy was performed for the new lesion and intrapulmonary metastasis of lung adenocarcinoma was confirmed by pathologists (Figure 1D-F).We performed EGFR gene panel test for biopsied specimen by Amplification Refractory Mutation System Polymerase Chain Reaction(ARMS-PCR),and T790M mutation was found.
Subsequently,osimertinib (Tagrisso,AZD9291,AstraZeneca) at a dose of 80 mg orally once daily was administered.On the follow up CT examination,the primary tumor of the left lung enlarged.Two months later,the patient experienced short of breath and pain of the right chest.CT scan showed right pleural effusion,and the metastatic lesion in the inferior lobe of left lung enlarged to 1.6 cm×1.8 cm (Figure 1G,H).We performed thoracocentesis and cytopathology found cancer cells in right pleural effusion.
Re-biopsy was performed for the enlarged lesion in the inferior lobe of left lung,and ARMS-PCR of the biopsy specimen revealed EGFR 19 del positive still,but EGFR T790M mutation was lost.Treatment of this patient was back to icotinib at a dose of 125 mg three times each day orally.However,after 2 months,her disease progressed (Figure 1I,1J).Bevacizumab(Avastin,Roche,Switzerland) was added intravenously at a dose of 7.5 mg/kg on day 0,every 21 days a cycle.After 2 cycles of icotinib combined with bevacizumab treatment,the primary tumor and metastatic lesions shrank significantly,the disease reached remarkable response,and maintained remission till the last follow-up in 4 months (Figure 1K,1L).Adverse effects in this patient included Grade 1 nausea one month after treatment,Grade 1 hypertension and rash 4 months after treatment.No grade 3-4 adverse event was observed.
DISCUSSION
EGFR mutation accounts for 50% NSCLC patients in the East Asia.18,19The exon 19 deletion and the exon 21 L858R mutation of the epidermal growth factor are activating mutations,which enhance the sensitivity of the NSCLC cells to the first-,second-or third-generation EGFR-TKIs,such as gefitinib,afatinib or osimertinib.For the first-line therapy with first-generation EGFR-TKIs in patients with EGFR mutations,the objective response rates (ORR) are 50%-80% and progression-free survivals (PFS) are 9-12 months.6,7EGFR T790M mutation of exon 20 is the most common acquired resistance mechanism,which accounts for 50%-60% in patients resistant to the first-generation EGFR-TKIs.20
Osimertinib has high activity in advanced NSCLC with EGFR T790M mutation,but resistance may happen eventually,with a median PFS of 9.6 months.8The resistant mechanism was considered very complicated,while several resistant mechanisms to osimertinib had been identified.The C797S/G mutation appears to be a leading resistant mechanism to the third-generation EGFR-TKIs.12The structure of EGFR T790M mutation and C797S/G mutation happened more in cis than in trans.12,21-23MET amplification,EGFR T790M loss,HER-2 or alternative kinase activation,SCLC or squamous cell transformation,and EML4-ALK rearrangement were also reported after resistance to osimertinib.24
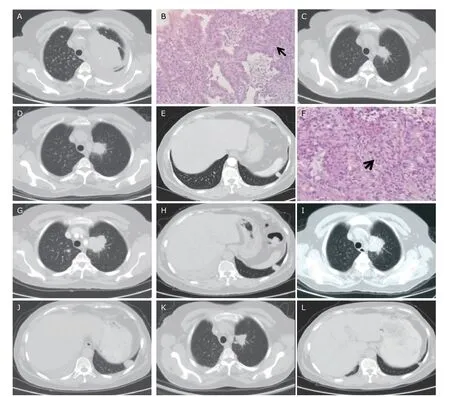
Figure 1.CT and pathological images showing the therapeutic responses of a 61-year-old woman with left pulmonary adenocarcinoma.(A) A 10.1 cm X 6.1cm mass in the superior lobe of left lung,with multiple metastatic nodules in bilateral lungs.(B) Microscopically nests of adenocarcinoma cells in the fibrous connective tissue arranged in an alveolar pattern (arrow) demonstrate pulmonary adenocarcinoma (HE,X10),EGFR 19 del mutation positive.(C) After 2.5 months treatment of icotinib,the primary tumor decreased to 3.2 cm X 2.2 cm,partial response (PR).(D) After 6 months remission,the primary tumor enlarged to 3.1cm X 3.0cm.(E) A new soft-tissue nodule found in the inferior lobe of left lung.(F) Biopsy of the new nodule revealed intrapulmonary metastasis pathologically (HE,X40),disease progressed.EGFR T790M mutation was found.After 2 months of osimertinib treatment,the primary tumor (G) and the metastatic lesion (H) enlarged.After 2 months of icotinib re-challenged treatment,the primary tumor (I) and the metastatic lesion (J) enlarged continuously;re-biopsy for the lesion in left inferior lobe showed EGFR 19 del mutation positive,but EGFR T790M mutation was lost.After 2-cycle of icotinib plus bevacizumab therapy,the primary tumor (K) and intrapulmonary metastatic lesion (L) shrank significantly.
Currently most studies focus on overcoming EGFR C797S/G mutation and alternative kinase activation by using combination therapy.EGFR 19 del/T790M/C797S would resist to all three generation EGFR-TKIs,but EGFR L858R/T790M/C797S might be sensitive to EGFR monoclonal antibody;and EGFR T790M mutation with C797S in trans might be sensitive to the third-generation EGFR-TKIs.25All above studies werein vitroorin vivo,the clinical outcomes were unclear.
At present,there is no standard care for patients who maintain EGFR activating mutation but loss EGFR T790M mutation after osimertinib treatment.The exact mechanism in osimertinib resistance of the loss of EGFR T790M is not fully understood.In our previous study,only one of three patients maintained stable disease for three months after first-generation EGFR-TKI re-challenged.For this patient,disease progressed with 2 months of icotinib treatment,but achieved partial response after combined with bevacizumab therapy.Further studies are needed for the combination of icotinib and bevacizumab in the treatment of patients with osimertinib resistance who loss EGFR T790M mutations.
In conclusion,the mechanism of losing EGFR T790M mutation and maintaining EGFR activating mutation in NSCLC have not been fully understood;re-challenge with the first-generation EGFR-TKIs combined with bevacizumab may overcome the tumor resistance to it,but this need further study in future.
Conflict of interest statement
All authors disclosed no conflicting interests.
Consent
Writen informed consent was acquired from the presented patient in this article.
REFERENCE
1.Ou SI,Cui J,Schrock AB,et al.Emergence of novel and dominant acquired EGFR solvent-front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M)NSCLC patient who progressed on osimertinib.Lung Cancer 2017;108(12):228-31.doi:10.1016/j.lungcan.2017.04.003.
2.Choo JR,Tan CS,Soo RA.Treatment of EGFR T790M-positive non-small cell lung cancer.Targeted oncology 2018;13(2):141-56.doi:10.1007/s11523-018-0554-5.
3.Wang S,Song Y,Liu D.EAI045:the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance.Cancer Letters 2017;385:51-4.doi:10.1016/j.canlet.2016.11.008.
4.Uchibori K,Inase N,Araki M,et al.Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer.Nature Communi 2017;8:14768.doi:10.1038/ncomms14768.
5.Suda K,Onozato R,Yatabe Y,et al.EGFR T790M mutation:a double role in lung cancer cell survival?J Thorac Oncol 2009;4(1):1-4.doi:10.1097/JTO.0b013e3181913c9f.
6.Mok TS,Wu YL,Thongprasert S,et al.Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma.N Engl J Med 2009;361(10):947-57.doi:10.1056/NEJMoa0810699.
7.Zhou C,Wu YL,Chen G,et al.BEYOND:A randomized,double-blind,placebo-controlled,multicenter,phase III study of first-line Carboplatin/Paclitaxel plus Bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer.J Clin Oncol 2015;33(19):2197-204.doi:10.1200/JCO.2014.59.4424.
8.Goss G,Tsai CM,Shepherd FA,et al.Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2):a multicentre,open-label,single-arm,phase 2 study.Lancet Oncol 2016;17(12):1643-52.doi:10.1016/S1470-2045(16)30508-3.
9.Ercan D,Choi HG,Yun CH,et al.EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors.Clin Cancer Res 2015;21(17):3913-23.doi:10.1158/1078-0432.CCR-14-2789.
10.Wang S,Song Y,Yan F,et al.Mechanisms of resistance to third-generation EGFR tyrosine kinase inhibitors.Fronti Med 2016;10(4):383-8.doi:10.1007/s11684-016-0488-1.
11.Russo A,Franchina T,Ricciardi GRR,et al.Third generation EGFR TKIs in EGFR-mutated NSCLC:Where are we now and where are we going.Crit Rev Oncol Hematol 2017;117:38-47.doi:10.1016/j.critrevonc.2017.07.003.
12.Nie K,Jiang H,Zhang C,et al.Mutational profiling of non-small-cell lung cancer resistant to osimertinib using next-generation sequencing in Chinese patients.Biomed Res Int 2018;2018:9010353.doi:10.1155/2018/9010353.
13.Tan CS,Cho BC,Soo RA.Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer.Lung Cancer 2016;93:59-68.doi:10.1016/j.lungcan.2016.01.003.
14.Ortiz-Cuaran S,Scheffler M,Plenker D,et al.Heterogeneous mechanisms of primary and acquired resistance to third-generation EGFR inhibitors.Clin Ca Res 2016;22(19):4837-47.doi:10.1158/1078-0432.CCR-15-1915.
15.Ding T,Zhou F,Chen X,et al.Continuation of gefitinib plus chemotherapy prolongs progression-free survival in advanced non-small cell lung cancer patients who get acquired resistance to gefitinib without T790M mutations.J Thorac Dis 2017;9(9):2923-34.doi:10.21037/jtd.2017.07.107.
16.Jia Y,Yun CH,Park E,et al.Overcoming EGFR(T790M)and EGFR(C797S) resistance with mutant-selective allosteric inhibitors.Nature 2016;534(7605):129-32.doi:10.1038/nature17960.
17.Kanda S,Horinouchi H,Fujiwara Y,et al.Cytotoxic chemotherapy may overcome the development of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) therapy.Lung cancer 2015;89(3):287-93.doi:10.1016/j.lungcan.2015.06.016.
18.Bell DW,Brannigan BW,Matsuo K,et al.Increased prevalence of EGFR-mutant lung cancer in women and in East Asian populations:analysis of estrogen-related polymorphisms.Clin Ca Res 2008;14(13):4079-84.doi:10.1158/1078-0432.CCR-07-5030.
19.Bae NC,Chae MH,Lee MH,et al.EGFR,ERBB2,and KRAS mutations in Korean non-small cell lung cancer patients.Cancer Genet Cytogenet 2007;173(2):107-13.doi:10.1016/j.cancergencyto.2006.10.007
20.Soejima K,Yasuda H,Hirano T.Osimertinib for EGFR T790M mutation-positive non-small cell lung cancer.Expert Rev Clin Pharmacol 2017;10(1):31-8.doi:10.1080/17512433.2017.1265446.
21.Hidaka N,Iwama E,Kubo N,et al.Most T790M mutations are present on the same EGFR allele as activating mutations in patients with non-small cell lung cancer.Lung cancer 2017;108:75-82.doi:10.1016/j.lungcan.2017.02.019.
22.Niederst MJ,Hu H,Mulvey HE,et al.The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies.Clin Cancer Res 2015;21(17):3924-33.doi:10.1158/1078-0432.CCR-15-0560.
23.Jakobsen KR,Demuth C,Madsen AT,et al.MET amplification and epithelial-to-mesenchymal transition exist as parallel resistance mechanisms in erlotinib-resistant,EGFR-mutated,NSCLC HCC827 cells.Oncogenesis 2017;6(4):e307.doi:10.1038/oncsis.2017.17.
24.Ham JS,Kim S,Kim HK,et al.Two cases of small cell lung cancer transformation from EGFR mutant adenocarcinoma during AZD9291 treatment.J Thorac Oncol 2016;11(1):e1-4.doi:10.1016/j.jtho.2015.09.013.
25.Lim SM,Syn NL,Cho BC,et al.Acquired resistance to EGFR targeted therapy in non-small cell lung cancer:Mechanisms and therapeutic strategies.Cancer Treat Rev 2018;65:1-10.doi:10.1016/j.ctrv.2018.02.006.
 Chinese Medical Sciences Journal2019年4期
Chinese Medical Sciences Journal2019年4期
- Chinese Medical Sciences Journal的其它文章
- An Optimized Protocol of Azoxymethane-Dextran Sodium Sulfate Induced Colorectal Tumor Model in Mice
- Ontology:Footstone for Strong Artificial Intelligence
- Antagonistic Effects of N-acetylcysteine on Mitogenactivated Protein Kinase Pathway Activation,Oxidative Stress and Inflammatory Responses in Rats with PM2.5Induced Lung Injuries
- Physiological Variables Associated with the Development of Acute Mountain Sickness
- A Single-center Retrospective Cohort Study on Cesarean Section under General Anesthesia
- Expression of PD1 and BTLA on the CD8+ T Cell and γδT Cell Subsets in Peripheral Blood of Non-Small Cell Lung Cancer Patients
