LAL test and RPT for endotoxin detection of CPT-11/DSPE-mPEGnanoformulation:What if traditional methods are not applicable?
Yanan Jin,Juanjuan Jia,Chan Li,Jianqi Xu,Jiai Sun,Kaiyuan Wang,Yaling Gan,Jing Xu,*,Yaqin Shi,**,Xingji Lianga,,f,***
aCollege of Chemistry&Environmental Science,Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education,Hebei University,Baoding 071002,China
bNational Institutes for Food and Drug Control,No.5,Huatuo Rd.,Daxing District,Beijing 102629,China
cLaboratory of Controllable Nanopharmaceuticals,Chinese Academy of Sciences(CAS)Center for Excellence in Nanoscience,No.11,First North Road Zhongguancun,Beijing 100190,China
dCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety,National Center for Nanoscience and Technology of China,No.11,First North Road Zhongguancun,Beijing 100190,China
eLaboratory of Molecular Iron Metabolism,College of Life Science,Hebei Normal University,Shijiazhuang 050024,China
fUniversity of Chinese Academy of Sciences,Beijing 100049,China
1.Introduction
Endotoxin refers to contaminants such as lipopolysaccharide(LPS)found in the gram-negative bacterial cell wall[1,2].As a powerful immunostimulant,endotoxin can activate a variety of mammalian immune cells once in the body especially the circulatory system and trigger excessive secretion of a wide range of cytokines.This can cause multiple organ failure or septic shock and even mortality for patients.Endotoxin contamination is a significant problem all over the world which affects hundreds of thousands of patients every year[3].In addition,endotoxin is extremely stable and can endure extreme pH,high temperature and even autoclaving.Accurate detection and strict control of endotoxin is therefore a critical step in both fabrication and quality control of medical products[4].
Endotoxin can be measured mainly by two methods:the rabbit pyrogen test(RPT)and limulus amebocyte lysate(LAL)assay[5,6].The RPT was first developed and is anin vivoassay based on the fact that endotoxin induces elevated body temperature in rabbits and the detection limit can be as low as 0.5 EU/ml(1 EU=1 Endotoxin Unit).Although requirement for animals,the experiment design and the judgment for positive results are slightly different from country to country,the goal of the test is the same.Today RPT is still required for some medical products in China and other countries.LAL test was discovered in 1970s and has now been adopted by most countries for detection of endotoxin[7].It is anin vitrotest based on the fact that protein extracts from horseshoe crab(Limulus polyphemus)amebocytes can clot in contact with endotoxin.Since its discovery,the LAL test has grown rapidly and has now replaced RPT as the main method for endotoxin detection for parental products in China.The LAL test is carried out in three different formats including the gel-clot assay,chromogenic test and turbidity test,and the gel-clot assay is the most common method.
In recent years,nanotechnology has been widely pursued as a new route to formulate small molecule chemotherapeutic drugs to lower the systematic toxicity,improve the pharmacokinetic pro files and enhance the therapeutic efficacy especially in cancer treatment[8–10].With more and more nanomaterials applied to medical products,it has become increasingly necessary to consider the influence of nanomaterials on detection of endotoxin[11,12].Multiple physical and chemical properties of nanomaterials such as the composition,size,shape,surface charge and targeting ligand,etc.can play a role in interacting with LPS and interfering with the test,generating false positive or negative results[13–17].Given the application of nanomaterials in nanomedicine,nanosafety assessment,in particular,the safety problems of nanodrugs before clinical application,is becoming increasingly important.At present,most of the detections of endotoxin are focused on empty nanocarriers or pure drugs,and there is limited endotoxin detections of drug-load nanoparticles.In addition,some researchers suggested that pyrogen test could be used when LAL detection was not applicable[18].However,due to the complexity of the nanoformulation,if there is interference in the detection of LAL test,pyrogen test may not be able to reflect the real results.Herein we demonstrate the issues with endotoxin detection of an irinotecan hydrochloride(nano)micelle(CPT-11/DSPE-mPEG2000micelle)formulation which was developed by our group for the treatment of advanced colorectal cancer.Our research has guiding significance for the clinical application of nanomedicines and also provides a reference for the study of micellar drugs of class PEG.Results indicated that for the CPT-11/DSPE-mPEG2000(nano)micelle,both LAL and RPT have their limitations and it is necessary for new methods to be developed to overcome these issues so that accurate evaluation of endotoxin content can be achieved.
2.Materials and methods
2.1.Materials and animals
Samples and reagents:irinotecan hydrochloride(nano)micelles,40 mg of C33H38N4O6?HCl(CPT-11),Lot numbers:20150325DM,20150427DM,20150624DM.Limulus reagents are manufactured by three manufacturers:Fuzhou New North Biochemical Industry Co.,Ltd.(Specifications:0.1 ml,Batch number:13121112,Sensitivity:0.25 EU/ml);ZhanjiangA&C Biological Co.,Ltd.(Specifications:0.1 ml,Batch number:1402261,Sensitivity:0.25 EU/ml and 0.1 ml,Batch number:1506152,Sensitivity:0.06 EU/ml);and Xiamen Limulus Reagent Experimental Factory Co.,Ltd.(Specifications:0.1 ml,Batch number:150208,Sensitivity:0.06 EU/ml).Bacterial endotoxin working standards are manufactured by National Institutes for Food and Drug Control(Specifications:80 EU/each,Batch number:150601–201580).Bacteria and endotoxin free water are manufactured by Zhanjiang A&C Biological Co.,Ltd.(Specifications:30 ml/bottle,Batch number:1501280)and Zhanjiang Bokang Marine Biological Co.,Ltd.(Specifications:50 ml/bottle,Batch number:1411150).Animals used in this experiment are Japanese white rabbits(body weight 2-3 kg).
2.2.LAL assay
2.2.1.Preparation of samples
According to the clinical instructions,irinotecan hydrochloride(nano)micelle is given intravenously to the patient and can be used either individually or combined with other drugs.
Combination therapy:irinotecan hydrochloride(nano)micelle is administered to patients at 180 mg/m2through intravenous infusion during 30 to 90 min the first day;leucovorin 400 mg/m2should be given immediately after the irinotecan hydrochloride(nano)micellar infusion within the same period.After that,5- fluorouracil can be given through intravenous injection.According to the chinese pharmacopoeia 2015 edition(1143 instruction for bacterial endotoxin test),bacterial endotoxin limit can be calculated as follows:L=K/M,where K is the maximum dose of endotoxin per kg of body weight per h,for injections K=5 EU/(kg h);M is the maximum dose of drug per kg of body weight per h.Average human body weight is 60 kg,the average body surface area is 1.62 m2,M=180 mg/m2×1.62 m2/60 kg=4.86 mg/(kg h).Therefore,the endotoxin limit L=5 EU/(kg h)÷4.86 mg/(kg h)=1.02 EU/mg.If the irinotecan hydrochloride(nano)micellar infusion time is 30 min,the infusion of calcium folinate with the same time,it should be 30 min,then irinotecan hydrochloride(nano)micelle and calcium folinate should be intravenously administered within 1 h.To ensure the safety of clinical medication for combination therapy,the bacterial endotoxin limit may take half of the above calculated value,that is,less than 0.5 EU/mg.
Monotherapy:The maximum dose of irinotecan hydrochloride(nano)micelles is 350 mg/m2,intravenously administered for more than 90 min,then the maximum dose per kg of body weight per h is M=350 mg/m2×1.62 m2÷60 kg÷1.5 h=6.3 mg/(kg h).Therefore,bacterial endotoxin limit for monotherapy treatment is L=5 EU/(kg h)÷6.3 mg/(kg h)=0.794 EU/mg.In order to better ensure the safety of clinical drug use,the bacterial endotoxin limit of this product should be less than 0.5 EU/mg in combination with the results of the bacterial endotoxin limit in the combination therapy.The Maximum Valid Dilution(MVD)is determined by the following calculation:MVD=CL/λ=10 mg/ml×0.5 EU/mg÷0.06 EU/ml=83.
Two bottles of irinotecan hydrochloride(nano)micelles(Batch numbers:20150325DM,20150427DM)were resuspended in 4 ml of endotoxin free water to obtain a drug concentration of 10 mg/ml,then to 0.125 mg/ml(diluted 80 times)respectively.
2.2.2.Procedure
The gel-clot LAL procedure follows the Chinese Pharmacopoeia(2015 edition,1143 instruction for bacterial endotoxin test).Brie fly,the test includes three tests:the confirmation test of the labeled lysate sensitivity,the interfering test,and the endotoxin assessment test.
2.3.RPT test
The RPT test was performed according to following procedures in the Chinese Pharmacopoeia 2015 edition(1142 instruction for Rabbit Pyrogen Test).
Brie fly,the average temperature of the first two body temperatures was taken as the normal body temperature of rabbits.The sample solution was injected within 15 min after the normal body temperature was measured.Then the body temperature was measured every 30 min,and measured 6 times.The rising temperature of the rabbit body temperature is that the highest body temperature in 6 times minus the normal body temperature.
Result judgment:In three rabbits,the body temperature rise was lower than 0.6°C,and the total temperature rise of three rabbits was less than 1.3°C.
3.Results and discussion
The fabrication and characterization of the nanoformulation have been reported previously[19].Brie fly,the nanoparticles showed a hydrodynamic diameter of 15.1±0.8 nm based on dynamic light scattering and slightly negative surface charge(ζ potential= ?4.6 ± 1.3 mV).The encapsulation efficiency of irinotecan hydrochloride of three batches of CPT-11/DSPE-mPEG2000micelle was assessed to be 90.0%±1.0%.
3.1.LAL assay
3.1.1.Confirmation test of labeled lysate sensitivity
The results showed in Table A1 demonstrated that 4 batches of Limulus reagent sensitivity determination values are between 0.5λ-2λ,which are eligible for bacterial endotoxin test.
3.1.2.Interference test
The interference preliminary test(SI)showed that a solution containing 10 mg/ml CPT-11/DSPE-mPEG2000micelle should be diluted at least 80-fold to a final drug concentration of 0.125 mg/ml to avoid interference with the detection of the endotoxin in the sample.
In the interference test,three groups of samples were tested.First is the endotoxin dissolved in endotoxin free water at different concentrations and the endotoxin free water is the negative control.The second and third groups are 20150325DM and 20150427DM sample solutions at a drug concentration of 0.125 mg/ml spiked with different amounts of endotoxin,respectively.The negative controls for the second and the third groups are 20150325DM and 20150427DM solutions at a drug concentration of 0.125 mg/ml.
The results are listed in Table 1.For both LAL reagents(manufactured by Zhanjiang A&C and Xiamen Limulus Reagent Company),the endotoxin free water showed negative results and the endotoxin positive controls(Group 1)showed the sensitivity of detection ES=λ,indicating that LAL results are valid.However,for samples 20150325DM and 20150427DM,the two kinds of LAL reagents behaved significantly differently.The results from LAL reagent manufactured by Zhanjiang A&C Biological Co.,Ltd.showed that with 20150427DM,the sensitivity of detection Etis not between 0.25λ-2λ,which indicated that 0.125 mg/ml sample concentration causes interference for the reaction between LAL reagents and endotoxin.On the other hand,the LAL reagent manufactured by Xiamen Limulus Reagent Experimental Factory Co.,Ltd.generated all positive results,including negative controls of samples 20150325DM and 20150427DM.These results demonstrated that at a drug concentration of 0.125 mg/ml,the irinotecan hydrochloride(nano)micelle interferes with the LAL test.
The sensitivity of LAL used in the current test is 0.06 EU/ml.If we further dilute the sample to 0.0625 mg/ml,the only choice would be the LAL reagent with a sensitivity of 0.03 EU/ml,which is the most sensitive LAL reagent available in China.Considering the risk of different test results due to the different batches of LAL reagents from different manufacturers or different batches of samples at a concentration of 0.0625 mg/ml,we concluded that LAL method is not suitable for bacterial endotoxin detection of this product.It has been reported in literature that some materials such as detergents or proteins may enhance or inhibit the interaction of LPS with the LAL reagent,causing false positive or negative results[13].The amphiphilic DSPE-mPEG2000can also be regarded as a detergent and may interfere with the test results.
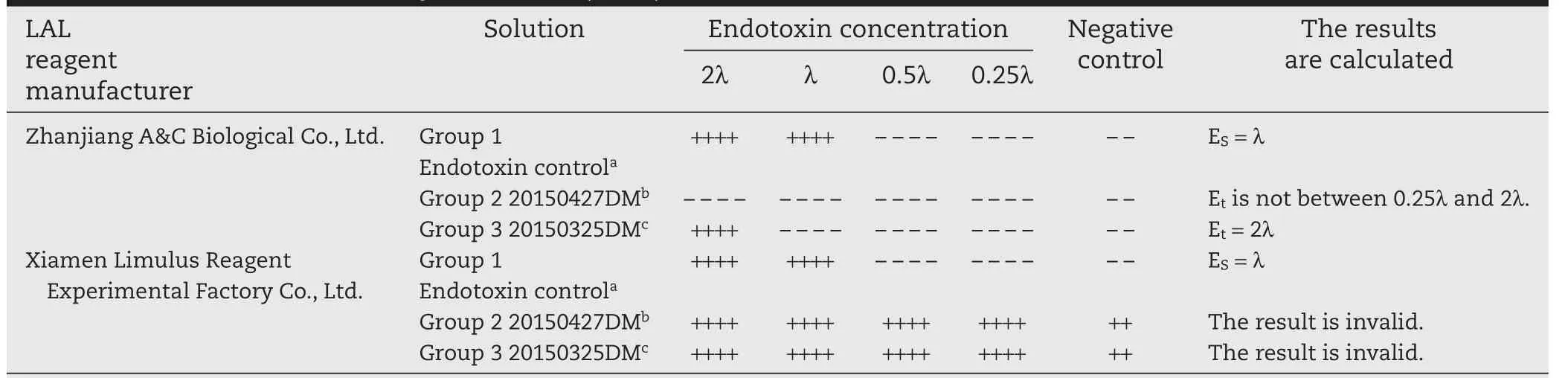
Table 1–Results of irinotecan hydrochloride(nano)micelle interference test.
3.2.RPT test
To test the interference of empty micelle to body temperature increase of rabbits,we performed a preliminary experiment.Empty micelle(batch 20150323EM)was diluted with 5%glucose injection solution to prepare a micelle solution of 12.0 mg/ml,which corresponds to a drug concentration of 2.8 mg/ml(maximum injection concentration)in drug-containing micelles.Each rabbit was injected with 10 ml of the solution per kg of body weight.All the procedures in this test followed the Chinese Pharmacopoeia(2015 edition,fourth part,1142 pyrogen test method).The results are listed in Table 2.In three rabbits,the body temperature rise was lower than 0.6°C,and the total temperature rise of three rabbits was less than 1.3°C.It showed that the empty micelles did not increase the body temperature of rabbits,and proved that the excipients used in the formulation did not interfere with the pyrogen test.
We then conducted the test with three batches of irinotecan hydrochloride(nano)micelles 20150325DM,20150427DM,and 20150624DM and the commercially available irinotecan hydrochloride injection as a control(trade name Campto,batch HR83C).The above samples were dissolved in a 5%glucose solution at a drug concentration of 2.8 mg/ml and 10 ml/kg body weight,and then injected into the rabbits.The results in Tables 3–6 showed that both the commercial irinotecan hydrochloride injection and irinotecan hydrochloride(nano)micelles did not cause the rabbit body temperature to rise.On the contrary,we observed that three batches of irinotecan hydrochloride(nano)micelles decreased the body temperatureof rabbits by 2.85,2.69 and 2.16°C,respectively(the sum of temperature decrease).A similar trend was observed with the commercial irinotecan hydrochloride injection during the test period in the rabbits(4.12°C).These results taken together suggested that irinotecan hydrochloride the drug itself at a dosage of 28 mg/kg can lead to a decrease in body temperature in rabbits.This not only causes discomfort in the animals involved in the test,but also may neutralize the warming effect of pyrogen,rendering the conclusions ambiguous in determination of the endotoxin content and safety of the products.The irinotecan hydrochloride has already been reported to induce acute hypothermia effect in mice which gradually recover subsequently and this effect on body temperature is irrelevant of sex of the animals[20].In addition,other drugs including amifostine and breviscapin have also been reported to exhibit similar effect of reducing animal body temperature during pyrogen test and potentially interfering with the accuracy of the results[21,22].In the two cases mentioned above,LAL test was used instead of the pyrogen test to avoid these issues.
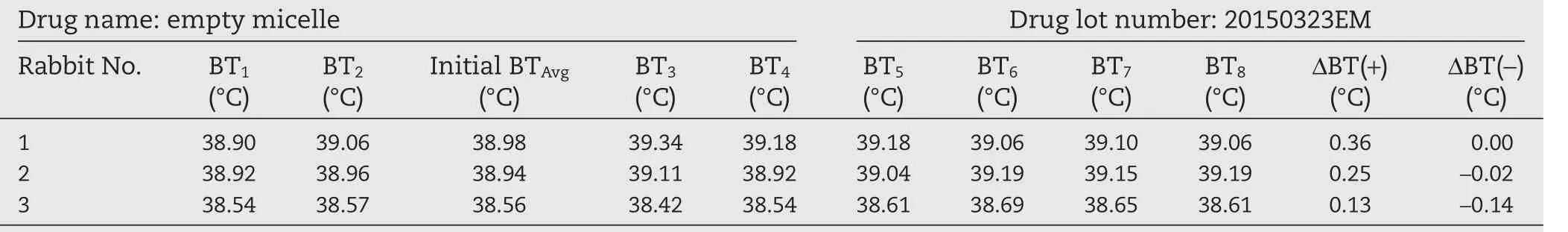
Table 2–Results of empty micellar pyrogen test.
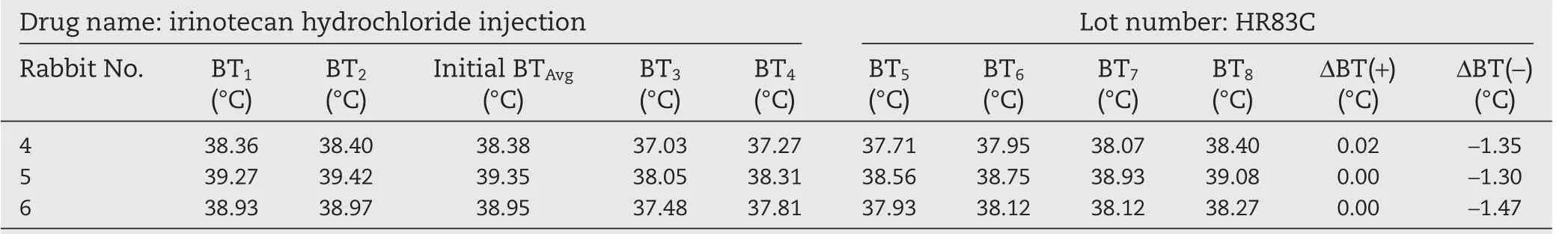
Table 3–Pyrogen test results of irinotecan hydrochloride(nano)micelles(high dose).
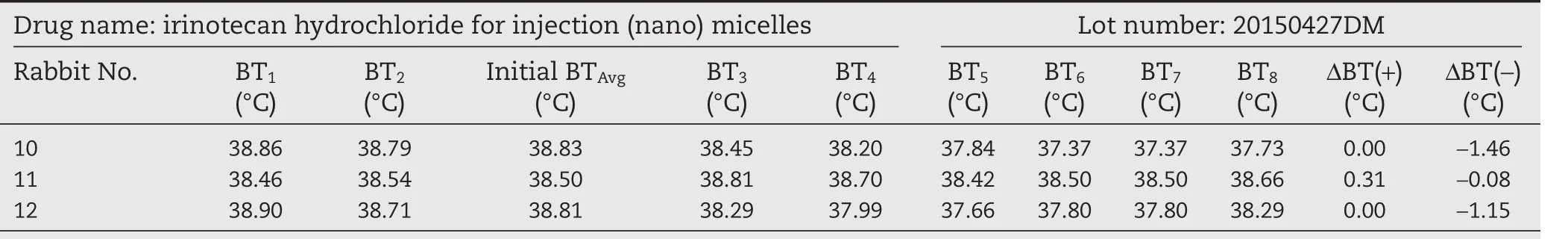
Table 5–Pyrogen test results of irinotecan hydrochloride(nano)micelles(high dose).
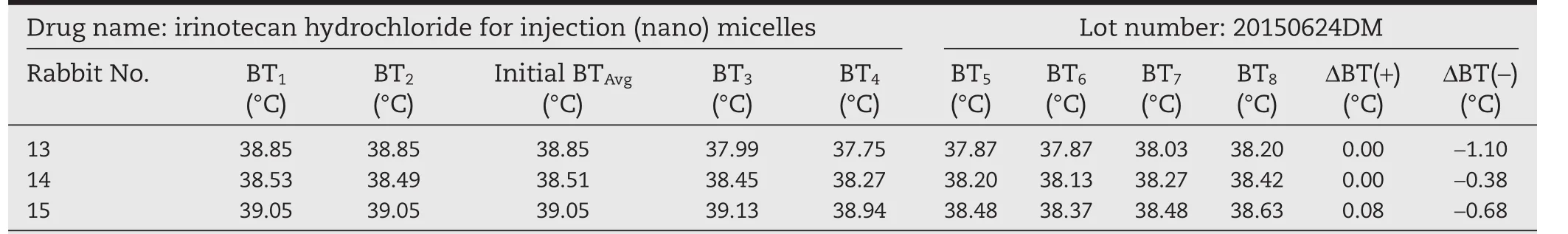
Table 6–Pyrogen test results of irinotecan hydrochloride(nano)micelles(high dose).
To overcome the body temperature decreasing effect of irinotecan hydrochloride,we reduced the injection dose.The samples were then prepared at a drug concentration of 11 mg/ml and the rabbits were injected with 1 ml per kg of body weight.The results are shown in Tables 7–9.At this injection dosage,three batches of irinotecan hydrochloride(nano)micelles including 20150325DM,20150427DM and 20150624DM did not raise the temperature of the rabbits more than the chinese pharmacopoeia required and the result of the pyrogen test can be regarded as passed.
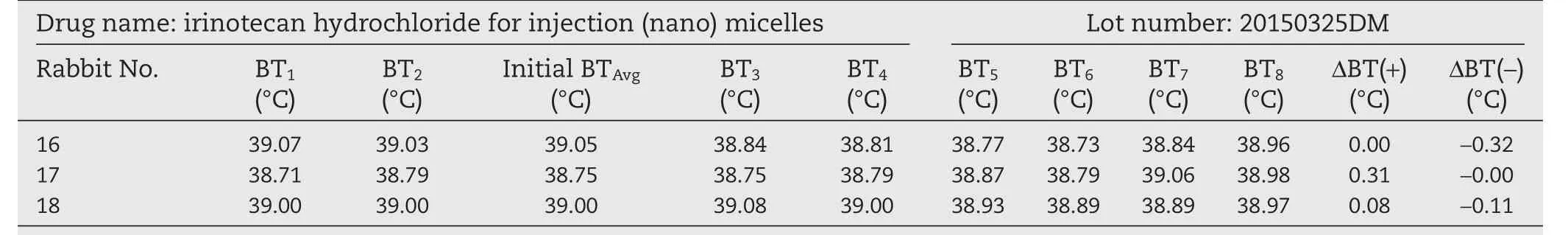
Table 7–Pyrogen test results of irinotecan hydrochloride(nano)micelles(low dose).
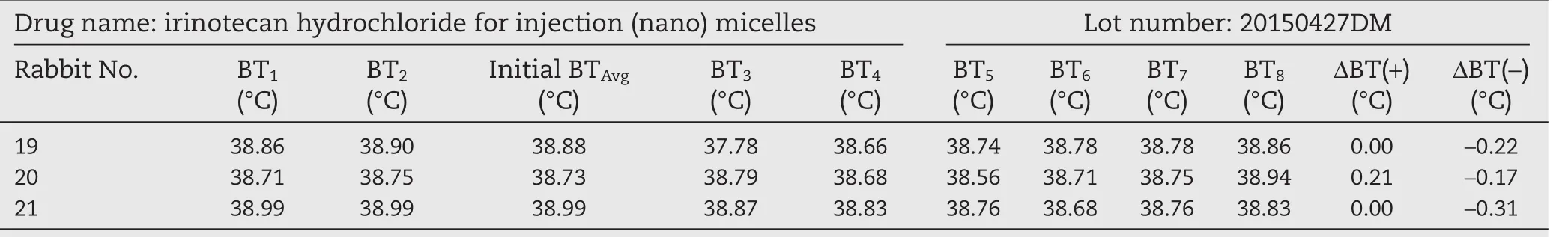
Table 8–Pyrogen test results of irinotecan hydrochloride(nano)micelles(low dose).
It is mentioned in literature that 3-10 times the injection dosage used in the clinics based on weight is recommended for pyrogen test,as long as the drug does not interfere with the physical wellness of the rabbit[23].Based on this,the dosage should be 33.6-112 mg/kg.In the first test,we used the highest concentration of the drug used in the clinics(2.8 mg/ml)and injected the rabbit with the largest volume of drug solution(10 ml),to ensure the safety of the nanoformulation.Since there is no clear direction on the dosage given to the animals in pyrogen test in the chinese pharmacopoeia,reducing the dosage to 11 mg/kg which is equal to the injection dosage used in the clinics based on weight,to avoid the hypothermia effect of the irinotecan hydrochloride might be acceptable.However,these issues call into question the relevance of RPT and drug safety in humans and may result in complex situation where the drug passes RPT,but causes fever in patients.
Meanwhile,drug-loaded nanoparticles are more complex than pure drugs,so it is necessary to detect the endotoxin of the preparations before clinical practice.This work will provide reference for experimental and clinical research of nanomedicines.
4.Conclusion
Pyrogenicity/endotoxin detection is an important step in characterization of nanomaterials for preclinical study.With more and more nanoformulation entering clinical study,the necessity to accurately detect the pyrogen is increasing.Here we presented the endotoxin detection results from the characterization of a CPT-11/DSPE-mPEG2000(nano)micelle formulation which has been declared clinically.
Firstly,formulation of drugs into nanosized particles introduces components that have never been used in pharmaceutics and may render the current LAL method not applicable.In this study,we found that the CPT-11/DSPE-mPEG2000(nano)micelle causes interference with the detection of the endotoxin using gel-clot format LAL assay.Secondly,the irinotecan hydrochloride drug itself at a dose of 28 mg/kg causes a hypothermia effect in the rabbits which forces us to reduce the dose to 11 mg/kg.So it is necessary to develop new methods to overcome these issues that may interfere with accurate evaluation of endotoxin content.New biosensors such as electrochemical biosensors,optical biosensors and mass-based biosensors may be considered as promising strategies for endotoxin detection.Because of their miniaturization,low cost,and high sensitivity,their release into market will be obvious success.
Conflicts of interest
The authors declare that there are no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China[Grant No.31600812];Strategic Priority Research Program of the Chinese Academy of Sciences[Grant No.XDA09030301];Natural Science Foundation Key Project[Grant No.31630027,31430031];and the National Distinguished Young Scholars Grant[Grant No.31225009].
Supporting information
1)
LAL reagent sensitivity confirmation test:According to Chinese Pharmacopoeia(2015 edition,fourth part,1143 bacterial endotoxin test method),the 0.25 EU/ml and 0.06 EU/ml Limulus reagent are tested for sensitivity respectively,the results are as follows.
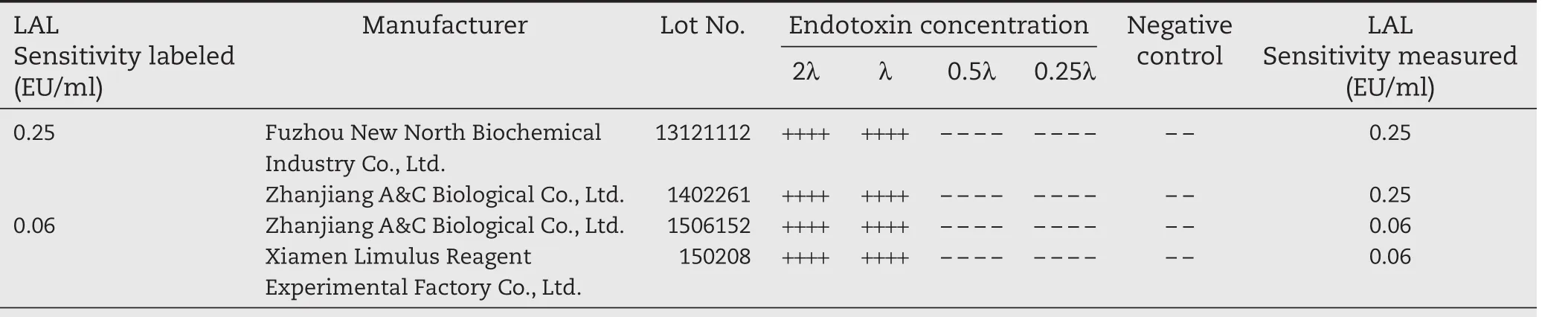
Table A1–Results of Limulus reagent sensitivity confirmation test.
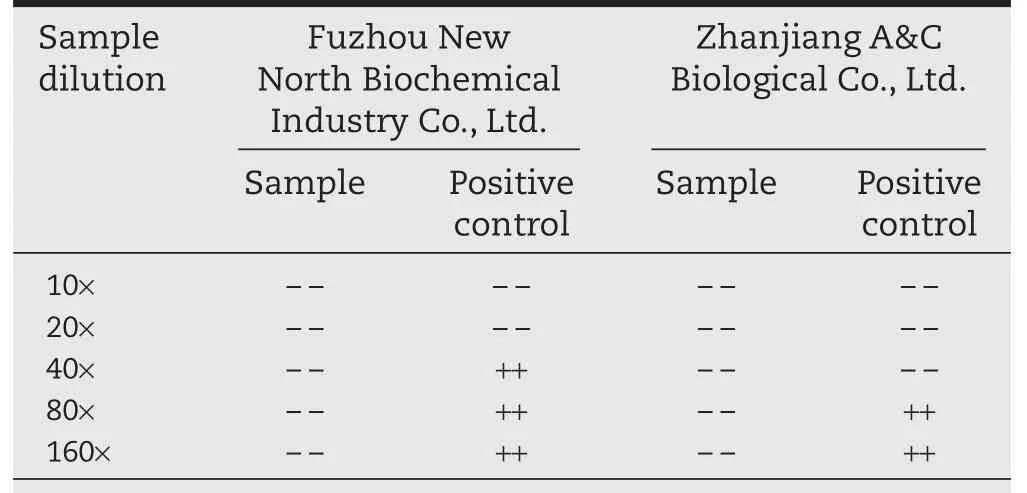
Table A2–Results of irinotecan hydrochloride(nano)micellar interference pretest.
2)
Interference test pretest:To determine the maximum noninterfering concentration of irinotecan hydrochloride(nano)micelle and provide information for the formal interference experiment,the interference pre-test was carried out.At present,the detection limit of the most sensitive Limulus reagent available is 0.03 EU/ml.If 4 ml of bacterial endotoxin free water was used to dissolve a bottle of irinotecan hydrochloride(nano)micelle sample to achieve a drug concentration of 10 mg/ml,then the maximum dilution factor of the solution is MVD=CL/λ=10 mg/ml×0.5 EU/mg÷0.03 EU/ml=166 times.One bottle of the 20150427DM sample was dissolved in 4 ml of bacterial endotoxin free water(Zhanjiang A&C Biological Co.,Ltd.production,Batch number:1501280)and then diluted 5,10,20,40,80 and 160 times with bacterial endotoxin free water.The above solutions were mixed with equal volume of 1 EU/ml of endotoxin standard solution to obtain a positive control solution containing 0.5 EU/ml of bacterial endotoxin.
The results in Table A2 showed that when irinotecan hydrochloride(nano)micelle 10 mg/ml solution was diluted 80-fold or higher(drug concentration 0.125 mg/ml),the sample did not interfere with the test.At lower concentrations,the sample showed interference to the detection of the endotoxin in positive control solution.
R E F E R E N C E S
 Asian Journal of Pharmacentical Sciences2018年3期
Asian Journal of Pharmacentical Sciences2018年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- Activity of Brucea javanica oil emulsion against gastric ulcers in rodents
- Model evaluation for the prediction of solubility of active pharmaceutical ingredients(APIs)to guide solid–liquid separator design
- Systematically optimized topical delivery system for Loperamide hydrochloride:Formulation design,in vitro and in vivo biopharmaceutical evaluation
- Investigating the molecular dissolution process of binary solid dispersions by molecular dynamics simulations
- Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier
- Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis
