Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis
Xiuping Zhang,Yang Shi,Zhiyue Zhang,Zhenlei Yang,Guihua Huang*
Department of Pharmaceutical Science,Shandong University,44 Wenhua Xi Road,Ji’nan 250012,China
1.Introduction
Osteoarthritis(OA)is the most common chronic joint disorder caused by degeneration or loss of joint cartilage and a leading cause of disability in the USA,Europe and Japan[1].It affects approximately 60%of men and 70%of women aged sixty- five years or older[2].These numbers are still increasing due to the aging population,the rising prevalence of obesity and the lack of definitive treatments to prevent disease progression[3].In addition to articular cartilage degeneration,the irreversible changes in subchondral bone and the induction of a severe secondary synovial inflammation make the disease even more difficult to treat[4].In the past decades,researchers have attempted to treat OA using various strategies,including non-pharmacologic treatment,pharmacologic treatment and surgical treatment(such as osteotomy)[2].However,all of these treatments failed to fully reverse the cartilage destruction during OA progression.Most efforts right now have concentrated on developing systemic pharmacologic treatment(e.g.oral administration of nonsteroidal anti-inflammatory drugs(NSAIDs))to relieve the pain and improve the joint function of patients bearing OA[5–7].However,such drugs bear considerable risk of systemic adverse effects as the cardiovascular and gastrointestinal side events observed for most NSAIDs and cyclooxygenase COX-2 inhibitors[8].Therefore,new OA candidate drugs are still in urgent need.
Tetramethylpyrazine(TMP),a major active ingredient extracted from Chinese traditional herbsLigusticum chuanxiong hort,has achieved a good clinical efficacy in ischemic cardiovascular and cerebrovascular diseases[9–11].Recently,TMP is also demonstrated to be an effective candidate for treating OA[12].Its pharmacologic effects may be related to multiple mechanisms as follows:(1)TMP attenuates the IL-1-induced cartilage and chondrocyte destruction,thus decreasing the degradation of glycosaminoglycan,increasing the chondrocyte activity and inhibiting chondrocytes apoptosis[13,14];(2)TMP promotes the proliferation of chondrocyte via pushing the progression of cell cycle[15];and(3)TMP scavenges cytotoxic oxygen free radicals and mitigates inflammation,which may also play a role in treating OA[16].Compared to NSAIDs and COX-2 inhibitor,this Chinese traditional herb extract shows a relatively safer systemic pro file when administered orally.
In addition to oral administration,other administration routes are developed for drugs to treat OA.Unlike other diseases,OA is locally restricted to one or a few joints.This feature provides a unique opportunity for local intra-articular(IA)injection of drugs.On one hand,IA injection allows the therapeutic agents to be delivered directly to the joint at extremely high concentrations;on the other hand,it reduces systemic toxicity due to limited uptake into circulation.Thus,a similar therapeutic effect can be achieved with a reduced dose compared to the systemic route[17].IA injection of intermediate molecular weight hyaluronic acid or glucocorticosteroids has been exploited to relieve knee pain associated with OA[18–20].Despite the promising advantages of IA injection,challenges do exist that limit the clinical application.One major limitation of IA injection is the rapid clearance of drugs from the joint space.Studies have shown that small molecular drugs(MW<10 kDa)can be cleared from synovial fluid by lymph drainage within 5 h of injection[21].Multiple IA injections are also required for TMP(once a week)to meet the therapeutic effect.This may lead to inflammatory injury and infection in the local tissues.Reducing the number of injections is critical to improving the patient compliance.
To solve this problem,several sustained release formulations(i.e.,gels[22],liposomes[23,24],nanoparticles[25–27]and microspheres[28,29])have been designed to prolong the retention of drugs within the articular spaces.In particular,microspheres(MS)with sufficient size and higher drug loading are considered to be the most popular IA drug delivery system.Biological materials,such as gelatin and albumin,have been used as microsphere cargos to prolong the retention of drugs for up to several weeks[30,31].In the recent decades,synthetic materials have gradually become a substitute of the biological materials for microsphere fabrication due to their ease of large-scale synthesis and relatively low immunogenicity.PLGA is a synthetic biocompatible polymer approved by US Food and Drug Administration(FDA)[32].On account of the sustained release properties and good safety of PLGA-MS,we postulated that it could be employed as an ideal intra-articular delivery carrier.Drying is a requisite process to improve the stability of microspheres.Typically,freeze-drying is the most commonly used drying technology,but it has the drawbacks of low energy efficiency,high purchase and maintenance costs[33].It is necessary to find an alternative drying technology,such as vacuum-drying technology and to take into account the effect of drying techniques on thein vitroandin vivoproperties of microspheres.
In the current study,TMP was intra-articularly injected to treat OA.To enhance the retention of the drug in the articular cavity,we proposed to encapsulateTMP into a microsphere composed of PLGA,thereby to reduce the dose and number of injections.The TMP-MS was also dried by vacuum-drying and freeze-drying to study thein vitrorelease andin vivoretention properties.Pharmacodynamics was evaluated by joint swelling and histologic analysis in the osteoarthritis rat model induced by papain,and it was also investigated whether there is the same therapeutic effect after reducing the dose of TMP microspheres compared toTMP solution.We envision that this TMP-PLGA-MS would be a potent strategy to treat chronic OA in the clinical trials.
2.Materials and methods
2.1.Materials
Poly(lactic-co-glycolic acid)(PLGA)polymers(75:25,MW:25 kDa,Viscosity:0.27 dl/g(CHCl3/25°C))were purchased from Shandong Institute of Medical Instrument(Shandong,China).TMP was purchased from Beijing Yanjing Pharmaceutical Co.Ltd.(Beijing,China).Gelatin(G9382,from bovine skin,gel strength:~225 g Bloom)and dialysis bags with a molecular mass cutoff of 12,000 Da were obtained from Sigma Chemical Co.(Beijing,China).Papain was purchased from Merck.All solvents used were HPLC grade.All other chemicals were obtained commercially as analytical-grade reagents.
Experimental animals:Wister rats(age:6 weeks,weight:300±20 g)were provided by Drug Safety Assessment Center of Shandong Institute of Materia Medica.The animal experiment was approved by the Institutional Animal Care and Use Committee of Drug Safety Evaluation Center of Shandong Institute of Pharmaceutical Industry.
2.2.Preparation of TMP loaded PLGA microspheres
TMP-PLGA microspheres were prepared by an oil-in-water(O/W)emulsion solvent evaporation protocol as previously described[34].Briefly,80 mg PLGA and 8 mg TMP were dissolved in 1 ml of organic solvent(dichloromethane:ethyl acetate=1:4)and dispersed into 20 ml of 1%gelatin solution(saturated with TMP)to form an O/W emulsion.This mixture was then homogenized(DIAX 900,Heidolph&Co.,Germany)at 10,000 rpm for 60 sec and stirred using a propeller mixer(ETSD4,IKA,Germany)for 3 h to evaporate the organic solvent.Then the microspheres suspension was centrifuged(L-500,Xiangyi,China)at 4000 rpm for 10 min and washed three times with distilled water.A portion of the microspheres was vacuum dried at a room temperature in a vacuum chamber(ZDF-1,Lvdeng,China)for 72 h.The other portion was quickly frozen to ?80 °C and lyophilized in a freeze dryer(Virtis frozen mobile,Virtis Co.,USA)(?45 °C)for 12 h.And 2%mannose was added as a lyoprotectant.
2.3.Characterization of TMP loaded PLGA microspheres
The morphology of the TMP microspheres was observed by scanning electron microscopy(SEM)(Hitachi 4500,Japan).The mean particle size was determined by laser dynamic light scattering(Mastersizer X,Malvern,Worcestershire,UK,136 equipped with a 100-mm lens)using a Fraunhofer diffraction model.
The encapsulation efficiency ofTMP microspheres was calculated by the ratio of the actual amount of TMP incorporated in the microspheres to the total amount of TMP added in the preparation.The drug loading was calculated by the ratio of the weight of encapsulated TMP to the total weight of TMP microspheres.Brie fly,20 mg of freeze-dried or vacuum-dried microspheres were dissolved in 2 ml of acetonitrile and quantified by UV spectrophotometry at a detection wavelength of 279 nm.
2.4.In vitro release of TMP from microspheres
In vitrodrug release was carried out in phosphate buffer saline(PBS)medium(pH 7.4)containing 1%(v/v)sodium lauryl sulfate(SDS).Briefly,20 mg of vacuum-dried microspheres or 26 mg of freeze-dried microspheres(containing 2 mg of TMP)were suspended in 2 ml of PBS and placed in a dialysis bag(Molecular weight cut off:12,000)while theTMP solution containing the same dose was performed as a control.The dialysis bag was placed in a vial containing 28 ml of the release medium and shaken at 37°C in a constant temperature water bath.At predetermined time points,5 ml of supernatant was withdrawn and an equal volume of fresh medium was added.The TMP concentration of each sample was determined by UV spectrophotometry.
2.5.In vivo retention in the articular cavity
The retention of TMP in the articular cavity was investigated by measuring the drug concentration in the articular cavity of healthy rats at predetermined time points(0.25,3,24,48,72,96,168,336 h)after intra-articular administration[35].Brie fly,seventy-two femaleWister rats(age:6 weeks,weight:300±20 g)were randomly divided into three groups:TMP solution group,freeze-driedmicrospheresgroupandvacuum-dried microspheres group.Both dried microspheres were dispersed in normal saline(NS)and sterilized by60Co radiation prior to administration.0.1 ml of TMP microspheres or TMP solution was injected into the right knee joint of rats at a dosage of 2.1 mg of TMP.The synovial fluid and joint tissue were then collected at each time point.The synovial fluid was collected by injecting 1 ml of normal saline(NS)into the articular cavity and rinsing repeatedly.The right knee joint was removed and homogenized(DIAX 900,Heidolph&Co.,Germany)with 0.5 ml of NS.TMP was extracted for analysis according to previously reported method with modifications[36].Brie fly,a certain amount of acetonitrile and sodium hydroxide were added into the sample(0.5 ml),vortex-mixed for 3 min and followed by centrifugation at 10,000 rpm for 10 min.The concentrations of TMP retained in the joint tissue and synovial fluid were quantitatively detected by high performance liquid chromatography(HPLC)(LC-10AD pump and SIL-9A detector Shimadzu Corporation,Kyoto,Japan).Chromatographic separations were carried out on a Kromasil C18column(150 mm × 4.6 mm,5.0 μm).Mobile phase was methanol–water(0.1%acetic acid in water,45:55,v/v)at a flow rate of 1 ml/min.The injection volume was 20μl.
2.6.The biocompatibility of PLGA microspheres
Forty- five femaleWistar rats were randomly assigned to control group,blank microspheres group andTMP microspheres group.0.1 ml of normal saline,blank microspheres and TMP microspheres were injected into the right joint of rats,respectively then the outside diameter of the knee joint was measured with a caliper at 1 d,1 week,and 4 weeks after the injection.The swelling of the knee joint was evaluated by the difference between the knee diameter at different time points after administration and the diameter of the knee before the administration.The knee joint was removed and fixed with 10%formalin,decalcified in 10%(w/v)acetic acid,then embedded in paraffin and cut into sections at 3–5 μm.Sections were stained with hematoxylin and eosin(H&E)for histologic microscopic observation.
2.7.Pharmacodynamic evaluation
2.7.1.Experimental OA model and treatment regimens
Male Wistar rats(300±20 g)were randomly divided into four groups:healthy control group,OA model group,TMP solution treated group and TMP microspheres treated group.Except the healthy control group,the rats in the other three groups were intra-articularly injected with 20μl of 4%papain solution and 0.03 M cysteine on the 1st,4th,and 7th d for inducing the OA model[37].The OA model was considered to be established on the 9th d after the first injection of the papain solution,then the TMP solution or TMP microspheres were injected into the OA knee joint[35].In the TMP solution treated group,0.1 ml of TMP solution(containing TMP 2.1 mg)was injected intraarticularly once a week for 6 weeks.While the OA model rats in the TMP microspheres treated group were once treated with 0.1 ml of TMP freeze-dried microspheres suspensions(containing TMP 4.2 mg).
2.7.2.Measurement of joint swelling and joint range of motion
The swelling of the knee joint was evaluated by the difference between the knee diameter at different time points afterad ministrationand the diameter of the knee before the induction of OA.The outer diameter of the knee was measured with a caliper before induction of OA and on the 1st,5th,10th,20th and 30th d after administration.Joint range of motion refers to the maximum curvature that can be achieved during joint movement.The joint range of motion was measured with a protractor before or after the induction of OA and at the time points after administration.
2.7.3.Histologic examination
Three rats were randomly sacrificed at 2 weeks,3 weeks and 6 weeks after intra-articular administration and the knee joints were removed and fixed with 10%buffered formalin for 7 d,decalcified in 5%(w/v)nitric acid for 3 h and dehydrated.They were then embedded in paraf fin and cut into 5μm-thick serial sections.Sections were stained with hematoxylin and eosin(H&E)to observe the general morphology and stained with safranin O&fast green for proteoglycan analysis.The pathological changes in cartilage and synovium were observed by double-blind method under optical microscope.According to Mankin’s histologic grade of OA,cartilage damage was assessed by scoring of chondrocyte hyperplasia,cell arrangement,safranin O-staining and other indicators[38].The synovial inflammation was evaluated by the scoring of synovial cell proliferation,hypertrophy,inflammatory cell proliferation,in filtration and granulation tissue hyperplasia.The histologic score for each item ranges from 0 to 4,and 4 points represent the most severe degeneration in cartilage and synovium.
2.8.Statistical analysis
All data were presented as means±standard deviation(SD).The differences between two groups were con firmed by the unpaired Student’s t-test and considered to be statistically significant ifP<0.05.An analysis of variance(ANOVA)200 test was also used if necessary.Pharmacokinetic parameters were obtained using DAS 201 2.0(drug and statistics for windows)program.
3.Results and discussion
3.1.Characteristics of TMP-PLGA microspheres
As shown in the SEM photographs in Fig.1,both microspheres exhibited a smooth spherical appearance,and the vacuumdried microspheres exhibited some adhesion.
The mean particle size of the vacuum-dried microspheres and the freeze-dried microspheres were 10±2.8μm and 10±2.2μm,respectively.The encapsulation efficiencies and drug loading of vacuum-dried microspheres were 81.95±1.52%and 8.22±0.19%,and those of freeze-dried microspheres were 81.36±1.15%and 81.36±1.15%,respectively.
3.2.In vitro release
Thein vitrorelease profile ofTMP from PLGA microspheres was shown in Fig.2.The TMP solution was completely released in the first 5 h,while the drug release time in vacuum-dried microspheres and freeze-dried microspheres was up to 32 d,reflecting that the microspheres have excellent sustainedrelease properties.In addition,the vacuum-dried microspheres exhibited a burst release with a cumulative release rate of 49.23%in the first 5 h.And vacuum-dried microspheres released 54.56%of the drug on the 1st d,whereas the freezedried microspheres released only 16.57%.This burst release phenomenon of the vacuum-dried microspheres may be due to the uneven distribution of the drug during the drying process,an increase in the amount of the drug distributed on the surface of the polymer matrix,and the increase of the matrix porosity caused by the rapid evaporation of the residual organic solvent[39–42].
The mathematical model of thein vitrorelease behavior was fitted.Thein vitrorelease behavior of TMP solution was consistent with the first order kinetic model(r=0.9881),and that of the vacuum-dried microspheres was in accordance with the Ritger–Peppas equation,with the equation of lnQ=0.5843lnt+0.7532(r=0.9960).The release of TMP from the freeze-dried microspheres con firmed to the Higuchi equation,and the equation was Q=3.121t1/2+0.558(r=0.9960).
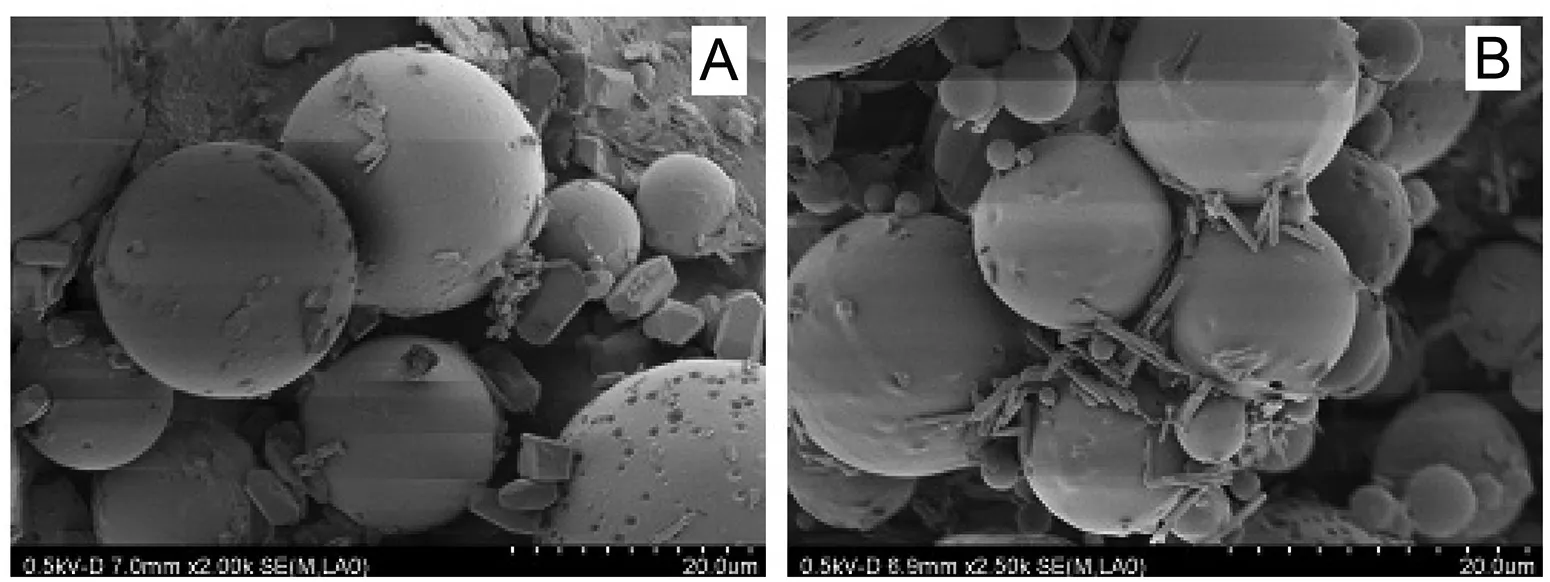
Fig.1–Scanning electron microscopy(SEM)photographs of(A)freeze-dried TMP-PLGA microspheres and(B)vacuum-dried TMP-PLGA microspheres.
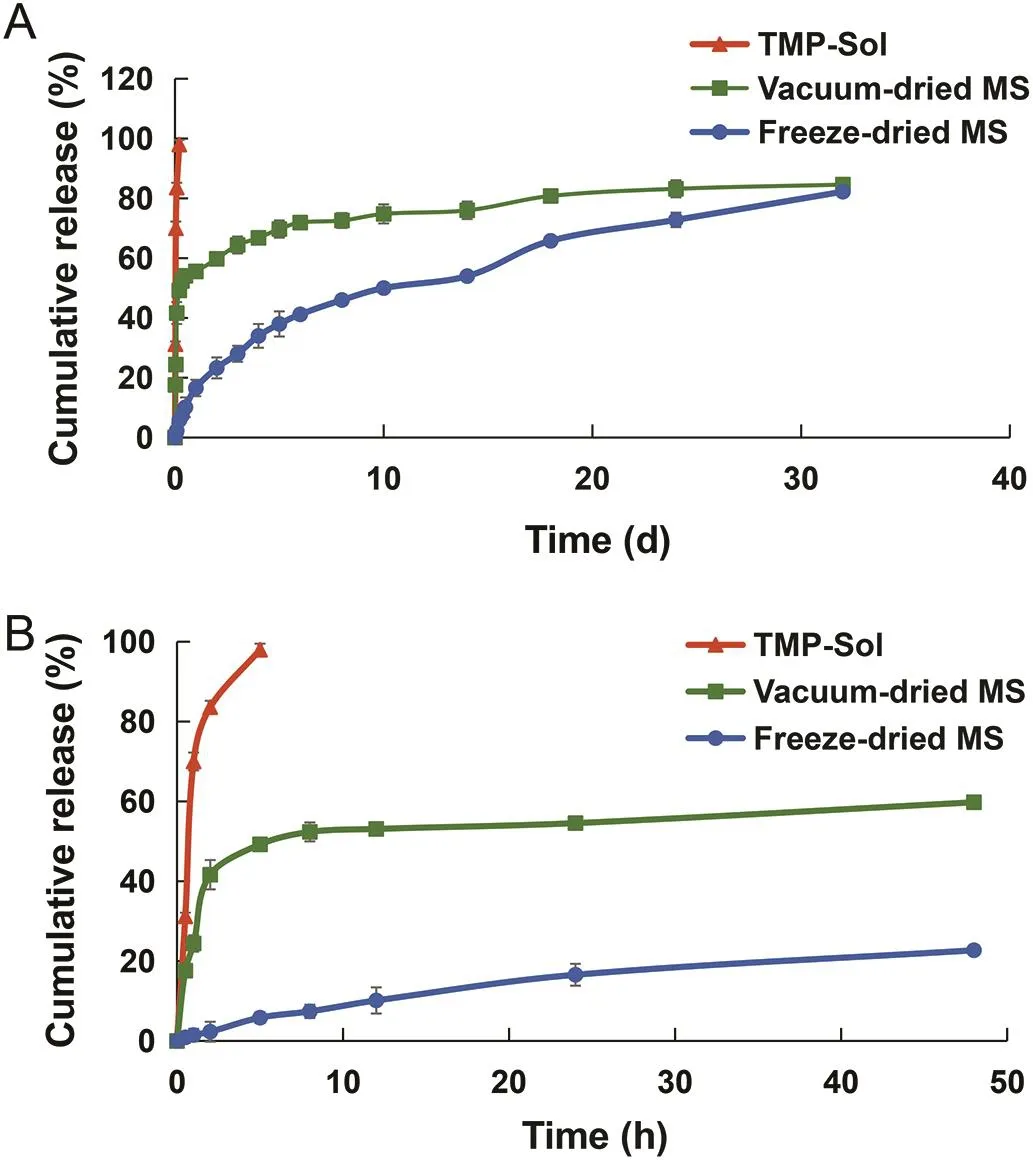
Fig.2–In vitrorelease pro file of TMP from microspheres:(A)the release curve for 32 d;(B)the release curve for the first 48 h.
3.3.In vivo retention in the articular cavity
A series of standard solutions of tetramethylpyrazine hydrochloride were prepared using water as a solvent,and a standard curve of TMP in joint tissues and synovial fluid was established.In the concentration range of 0.2-20μg/ml,the peak area of TMP presented a good linear relationship with the concentration.The correlation coefficient of the standard curve of joint tissue and synovial fluid was 0.9978 and 0.9992,respectively.The detection limit was 2 ng/ml.
The mean concentration-time curves ofTMP in joint tissue and synovial fluid after intra-articular injection of TMP solution,vacuum-dried microspheres and freeze-dried microspheres were shown in Fig.3.In the joint tissues,the concentration of TMP in the freeze-dried microspheres group was 65.02±8.94μg/g on the 2nd d after administration,which was significantly higher than that in the TMP solution group(23.3±2.28μg/g)(P<0.01).At this point,TMP in the vacuumdried microspheres group quickly reached a peak concentration of 74.05±5.18μg/g.On the 2nd d,the concentration of TMP in the vacuum-dried microspheres group decreased to 32.50±2.64μg/g,which was significantly lower than that of the freeze-dried microspheres(32.50±2.64μg/g)(P<0.01).Three days after IA injection of TMP solution,the concentration of the drug dropped below the limit of detection and the concentration of TMP retained in the joint tissue was too low to be detected.TheTMP concentration in the freeze-dried microspheres in the joint tissue reached a maximum of 119.61±9.4μg/g on the 10th d.Obviously,freeze-dried microspheres prolonged the retention time of TMP in the joint tissue to 30 d,whereas the TMP solution showed only 3 d of retention time.Similarly,as can be seen from the drug-time pro file in synovial fluid(Fig.3B),the freeze-dried microspheres also demonstrated high retention concentrations and extended retention times in synovial fluid.
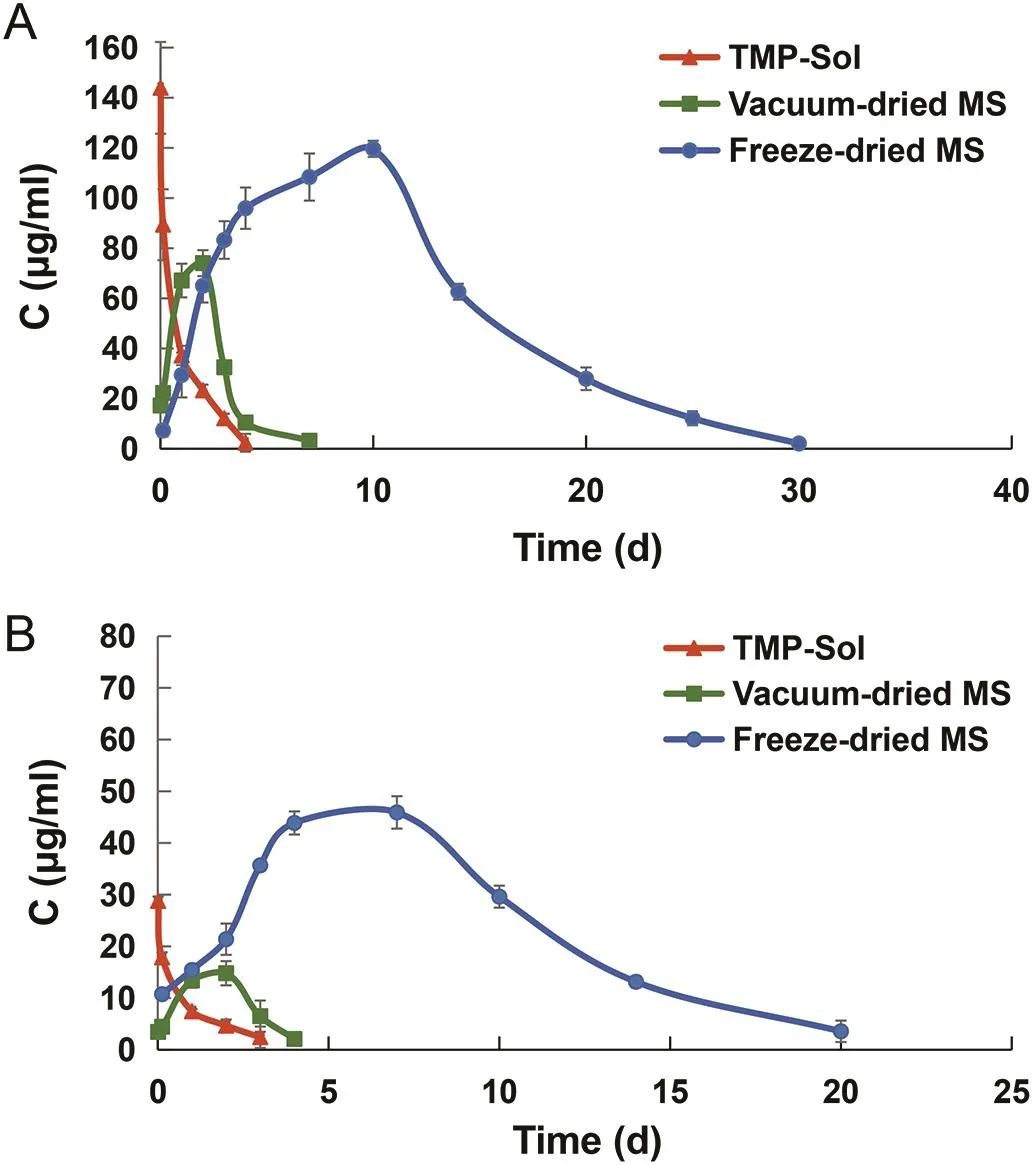
Fig.3–Mean concentration-time curves of TMP distributed in(A)joint tissue and(B)synovial fluid of TMP solution,vacuum-dried microspheres and freeze-dried microspheres.
The pharmacokinetic parameters of TMP in joint tissue and synovial fluid were listed in Table 1.The area under the curve(AUC)of freeze-dried microspheres in the joint tissue was extremely large,13.2 times that of the TMP solution and 7.9 times that of the vacuum-dried microspheres.The AUC of the freezedried microspheres in synovial fluid were 17.6 and 11.6 times larger than that of TMP solution and vacuum-dried microspheres,respectively.The mean residence time(MRT)of freeze-dried microspheres in joint tissue was 9.2 times longer than that of TMP solution and 4.6 times longer than that ofvacuum-dried microspheres.Thus,freeze-dried microspheres not only exhibited localized high concentrations in joint tissue,but also displayed a long retention time for one month.
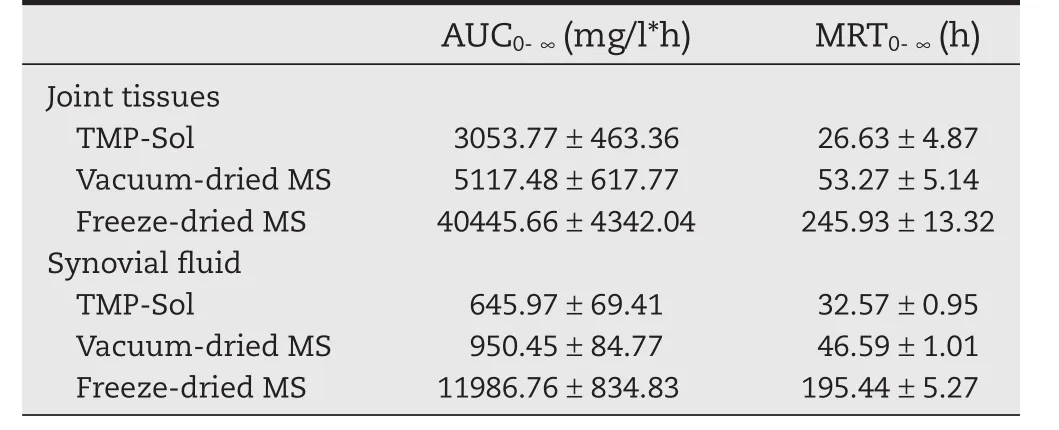
Table 1–The pharmacokinetic parameters of TMP in joint tissue and synovial fluid after intra-articular injection of TMP solution,vacuum-dried microspheres and freeze-dried microspheres.
The high retention properties of freeze-dried microspheres in joint tissues and synovial fluid were related to the sustained release properties of the PLGA microspheres.PLGA microspheres can prolong the drug retention time to several d even several weeks,depending on the nature of the drug and the proportion of PLGA composition.Studies have shown that lornoxicam encapsulated in PLGA microspheres can prolong the retention time to 96 h,whereas the drug itself has a short retention time(less than 48 h)[39].Another result is that the intra-articular retention of vacuum-dried microspheres was far below that of freeze-dried microspheres,regardless of intraarticular drug concentrationor retentiontime.This phenomenon may be attributed to the rapid release of TMP from the vacuum dried microspheres.Indeed,in vitrodrug release results were in line with our hypothesis and showed that the burst release of TMP in vacuum dried microspheres reached 49.23%in the first 5 h.Thus,~50%of TMP in the vacuumdried microspheres would very likely to be cleared into the blood circulation at the same rate as the TMP solution.
3.4.The biocompatibility of PLGA microspheres
The biocompatibility of PLGA microspheres in rat knee joint was evaluated primarily by histologic examination.The microscopic images after intra-articular injection of normal saline,blank PLGA microspheres and TMP-PLGA microspheres were shown in Fig.4.As can be seen from the figure,intra-articular injection of saline and blank PLGA microspheres essentially did not produce inflammatory symptoms.One day after intraarticular injection of TMP-PLGA microspheres,some inflammatory symptoms were generated in the joint cavity,such as synovial hyperplasia,inflammatory cell in filtration and fibrosis.After 1 week,the symptoms of inflammation were diminished,and no signs of articular inflammation were observed after 4 weeks of intra-articular injection.Thus,the local inflammatory symptoms would be healed within 4 weeks.In addition,no joint swelling was observed in the gross morphology observation.These results indicated that PLGA microspheres injected directly into the joint cavity are biocompatible and are considered to be histologically safe.The resulting injury may be caused by injection,as only a minimal and local mild inflammation occurred and it could be healed within a certain period of time.
3.5.Pharmacodynamics evaluation
3.5.1.Measurement of joint swelling and joint range of motion
The progress of osteoarthritis is often accompanied with joint pain,swelling,stiffness and activity difficulties.The joint swelling and range of joint activity could be used as indicators to evaluate the effect of the drug for treating OA.In the model group,the diameter of the knee joint after papain induction was 52.85±2.96 mm,which was significantly higher than that before induction(41.74±4.69 mm)(P<0.05),indicating that the papain-induced OA model was successfully established.As shown in Fig.5A,the joint swelling of the treated group showed a downward trend,while the untreated model joint remained swollen.On the 10th d,the joint swelling in the TMP solution treated group was significantly lower than that in the model group(P<0.01).On the 20th d,the joint swelling of TMP microspheres treated group was significantly decreased compared to the model group(P<0.05).The slower onset time of TMP microspheres may be related to its sustained release.Especially,under the condition of this administration regimen,the joint swelling both in the microspheres group and the solution group was significantly improved when compared to that in the model group on the 30th d after administration(P<0.01),indicating that the single-dose microspheres possessed a longacting effect.
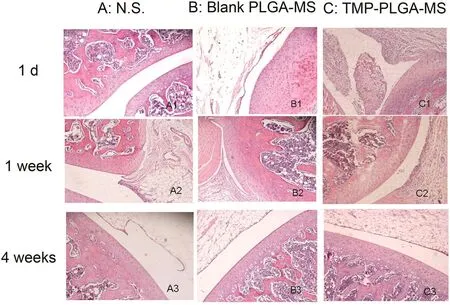
Fig.4–Histologic observation of articular cavity after intra-articular injection of(A)normal saline;(B)blank PLGA microspheres;(C)TMP-PLGA microspheres.
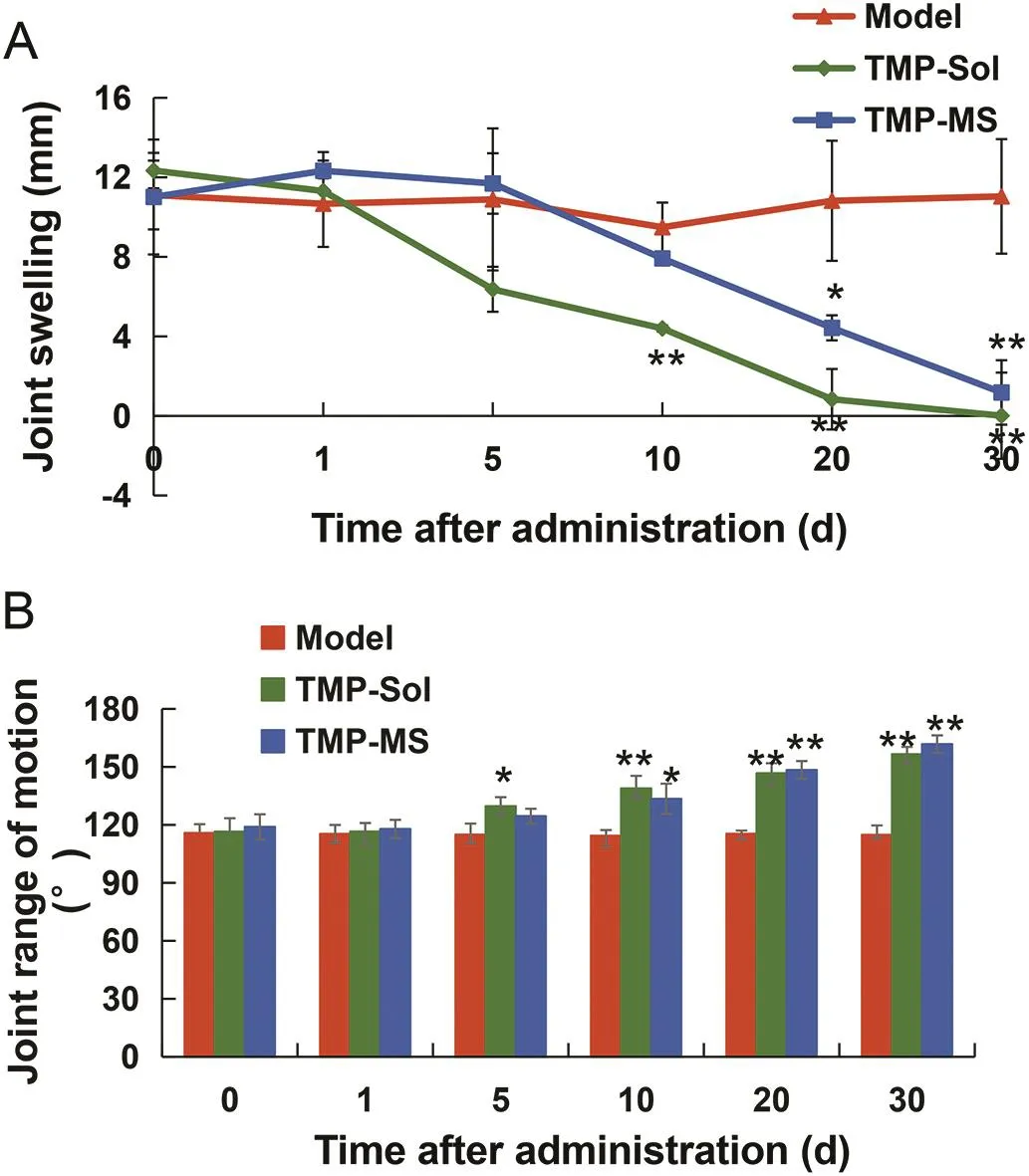
Fig.5–Mean joint swelling(A)and joint range of motion(B)in papain-induced OA rats at different time points after the treatment with TMP solution and TMP microspheres.
The joint range of motion was showed in Fig.5B.Before the induction of OA,the joint range of motion was 158.57 ± 7.61°,and it was significantly reduced to 115.78±4.56°after the induction of OA(P<0.01),indicating the successful establishment of the osteoarthritis model.The joint range of motion of the TMP-solution group was significantly higher than that of the model group on the 5th d after administration,and on the 10th d,the joint range of motion of the microsphere group began to improve significantly(P<0.05).After treated with TMP-solution and TMP-microspheres,the joint range of motion remained significantly improved on the 30th d(P<0.01).
3.5.2.Histologic evaluation
Histologic evaluation was performed by hematoxylin and eosin(H&E)staining and safranin O&fast green staining to observe the morphology of articular cartilage and synovium(Fig.6A)and the staining of cartilage matrix for proteoglycan analysis(Fig.6B).As shown in the figure,the surface of normal articular cartilage was intact and smooth,chondrocytes were arranged in a regular manner,and the cartilage matrix was well stained with safranin O dye.After 6 weeks of papain induction of OA,untreated cartilage was destroyed,local destruction deep to subchondral bone and replaced by fibrous connective tissue,and fibrous tissue exhibited hyperplasia.Besides,the cartilage matrix showed the loss of safranin O staining and the proteoglycan was depleted.In the treatment group,the cartilage damage was improved compared to the untreated OA model after 2 weeks:the cartilage layer recovered integrity and chondrocytes arranged in normal.However,inflammatory symptoms such as inflammatory cell in filtration, fibrillation and hyperemia were still present.From the 2nd and 3rd weeks of HE-stained joint cavity photos we can see that TMP microspheres had a better anti-inflammatory effect compared to theTMP solution group and the fibrous exudates were less than the solution group.These results may be due to high local drug concentration in the joint cavity of the microspheres.Six weeks after the first administration,it could be observed that the morphology of cartilage and synovium was normal and the density of the safranin O staining was enhanced,indicating that TMP microspheres and TMP solution have prominent therapeutic effect on papain-induced OA model rats.The histologic scores of the 4 groups sections are shown in Table 2.Each item of the scores had a high grade in the osteoarthritis model rats.After 3 weeks of treatment,the scores of various items of osteoarthritis showed a decreasing trend.And the scores were further reduced to a normal level after 6 weeks of treatment.Combined with the dosing regimen,one-third dosage of TMP microspheres achieved the similar or even better therapeutic effect on the treatment of OA compared to the TMP solution,and reduced the number of intraarticular injections to a single injection.Thus,TMP microspheres possess the powerful advantages in decreasing the dosage,reducing the number of injections and even improving the therapeutic effect.
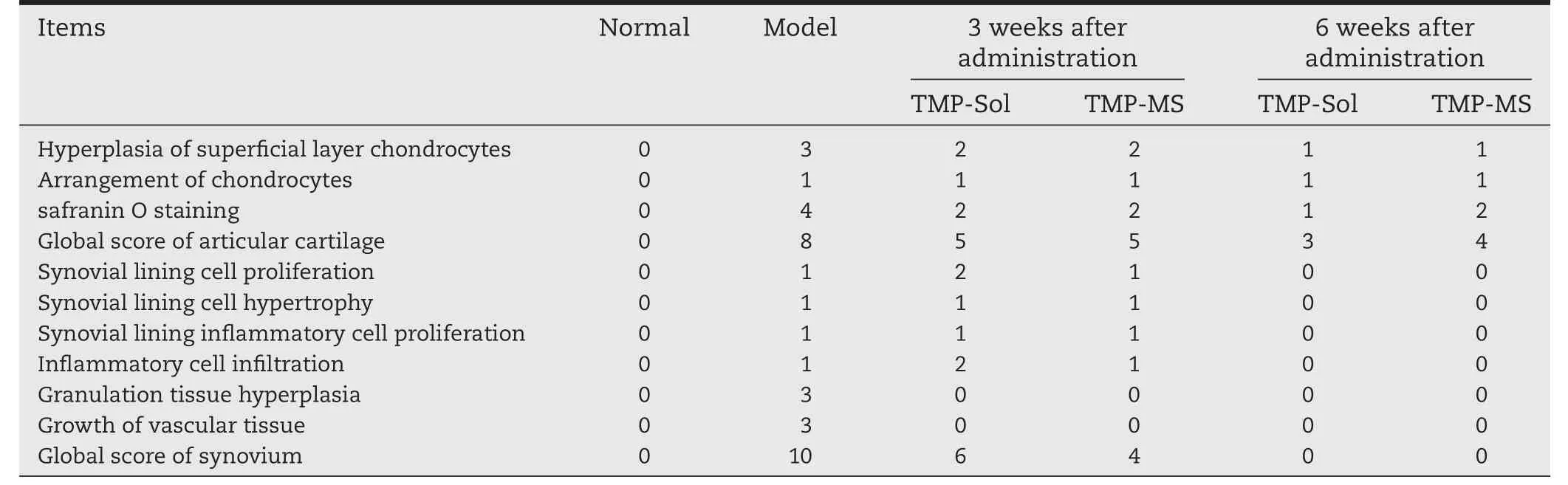
Table 2–Pathological score results of knee cartilage and synovial membrane in papain-induced osteoarthritis model rats.
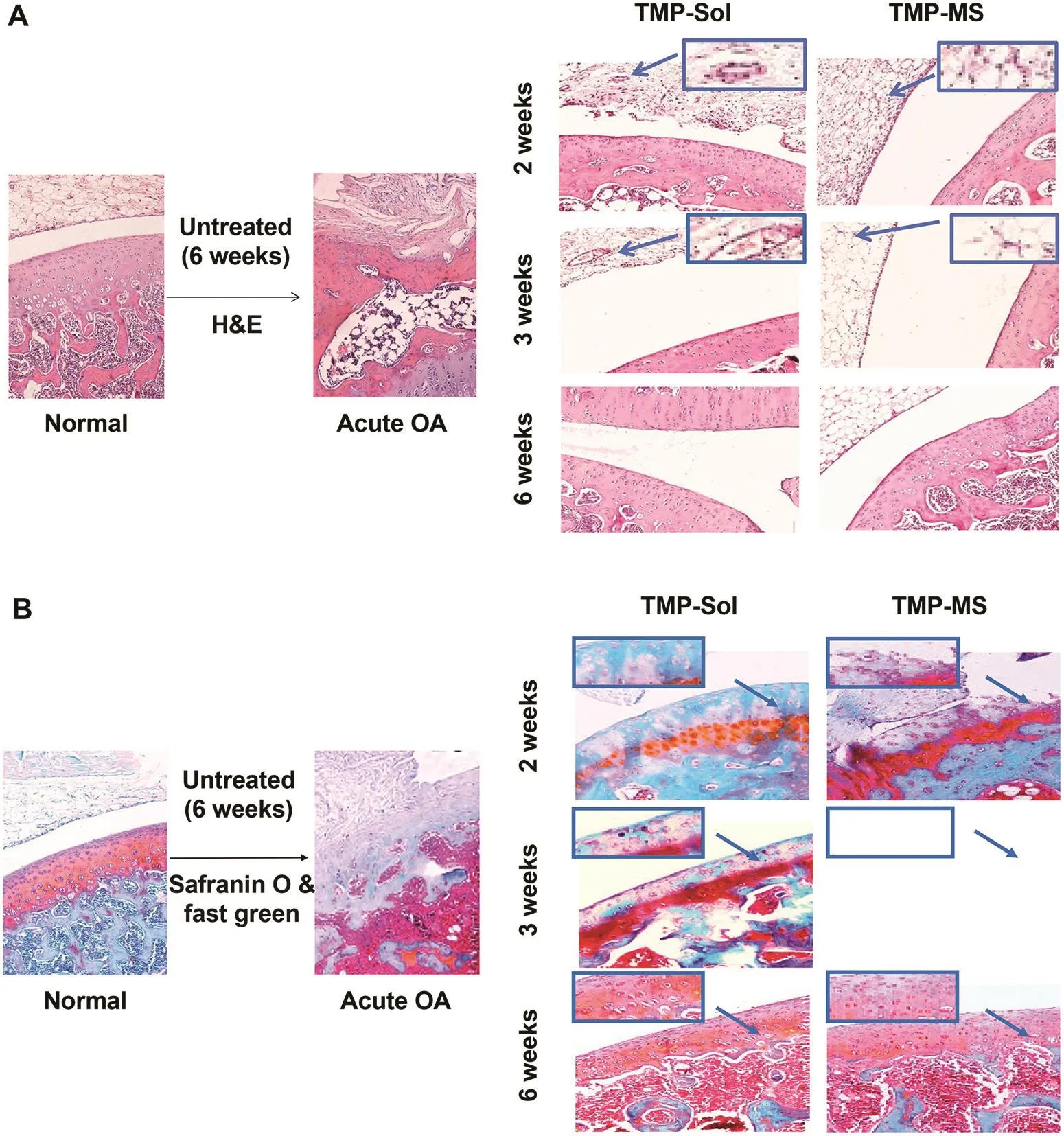
Fig.6–Histologic analysis of articular cavity:(A)stained with H&E for general morphology observation;(B)stained with safranin-O for proteoglycan analysis.
Pharmacodynamic assessment results showed that even though only one-third dose of TMP microspheres compared to the free drug solution,can effectively reduced the swelling of the knee joint,relieved inflammatory symptoms and proteoglycan loss,and achieved similar therapeutic effect with solution group after 30 d.Therefore,the TMP freeze-dried microspheres would be more popular in OA therapies.
4.Conclusion
In conclusion,TMP loaded PLGA microspheres are a promising candidate for the treatment of osteoarthritis,which attenuated inflammation,decreased the depletion of proteoglycan and protected the articular cartilage.The controlled release freeze-dried TMP-PLGA microspheres could prolong the retention time of the drug in the joint tissue to 30 d and the AUC in the join tissues is 13.2 times that of TMP solution and 7.9 times that of vacuum-dried microspheres.Compared with the TMP solution,TMP freeze-dried microspheres reduced the dose from 12.6 mg to 4.6 mg and decreased the number of injections,but achieved the similar effect.Further studies can focus on whether the freeze-dried PLGA microspheres can improve efficacy compared to the solution group in the same dosing regimen.More studies will be performed to determine whether TMP microspheres have any potential for clinical application.
Conflicts of interest
The authors declare that there are no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgements
We thank Prof.Guihua Huang for the guide of the experiment and thank Lei Miao for the revision of the language.We would like to acknowledge Professor Xianglan Cui and YanWang for the help of animal pharmacodynamic experiment.We also thank Professor Benjie Wang and Ruichen Guo from the Department of Pharmacology in Qilu Hospital for technical support.
Appendix
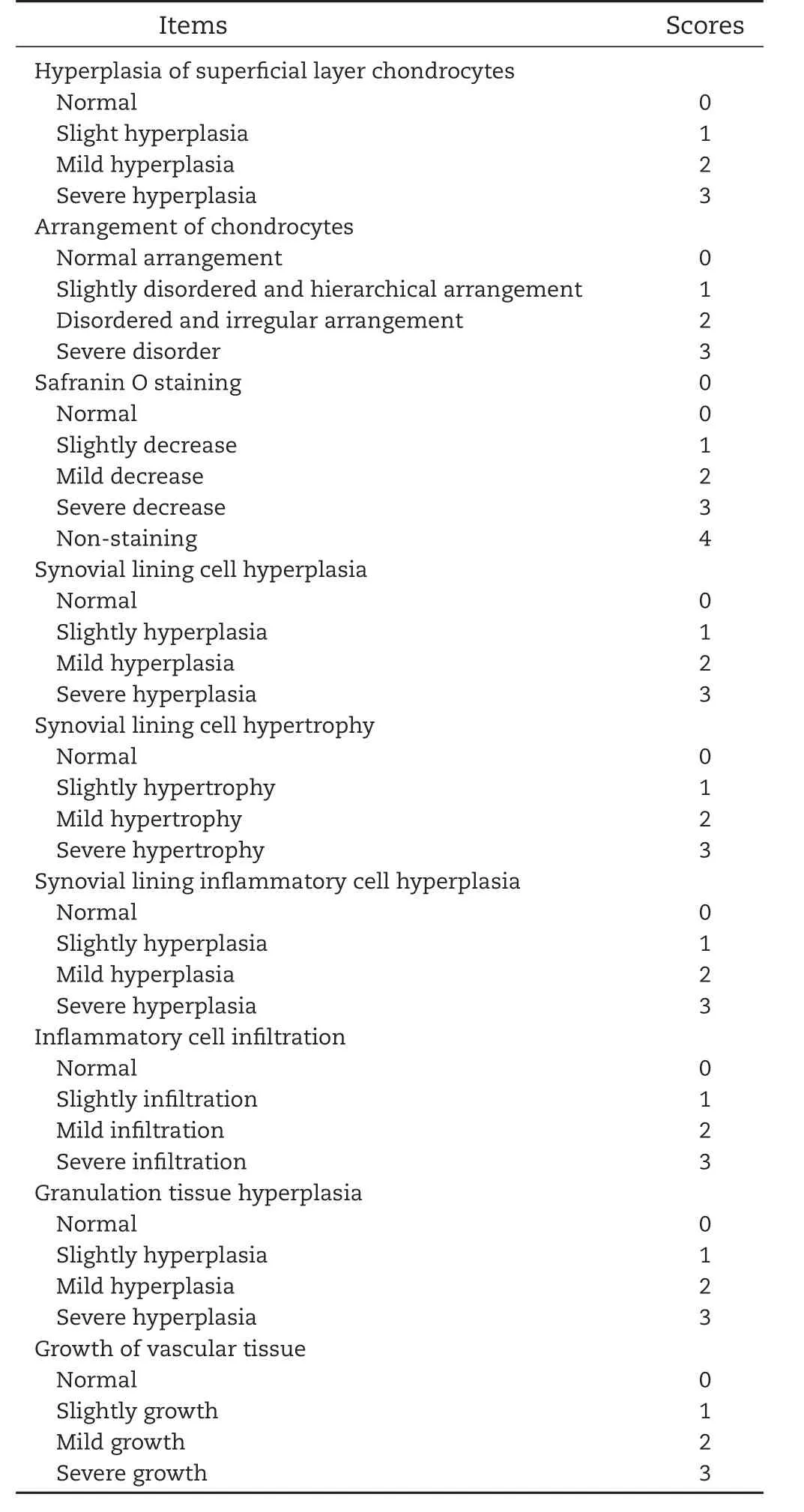
Table Pathological score of knee cartilage and synovial membrane in papain-induced osteoarthritis model rats.
 Asian Journal of Pharmacentical Sciences2018年3期
Asian Journal of Pharmacentical Sciences2018年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- LAL test and RPT for endotoxin detection of CPT-11/DSPE-mPEGnanoformulation:What if traditional methods are not applicable?
- Activity of Brucea javanica oil emulsion against gastric ulcers in rodents
- Model evaluation for the prediction of solubility of active pharmaceutical ingredients(APIs)to guide solid–liquid separator design
- Systematically optimized topical delivery system for Loperamide hydrochloride:Formulation design,in vitro and in vivo biopharmaceutical evaluation
- Investigating the molecular dissolution process of binary solid dispersions by molecular dynamics simulations
- Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier
