Investigating the molecular dissolution process of binary solid dispersions by molecular dynamics simulations
State Key Laboratory of Quality Research in Chinese Medicine,Institute of Chinese Medical Sciences,University of Macau,Macau,China
1.Introduction
Solubilization of poorly soluble drugs attracts high attentions from formulation scientists in the pharmaceutical industry[1].Amorphous solid dispersion is one of important drug delivery techniques to enhance the solubility and dissolution rate for crystalline drugs with low solubility[2].In the system of solid dispersions,the crystalline structure of drugs was changed to amorphous state and resulted in higher bioavailability[3].The first solid dispersion(SD)was reported by Sekiguchi and Obi in 1961[4].Several products with solid dispersion techniqueshave been commercialized in the pharmaceutical market[5].In recent two decades,publications in solid dispersion area increase sharply.However,there are still four key problems about solid dispersions to be answered:the solid state structure of solid dispersions;the mechanism of dissolution enhancement;the aging issue during storage period;and the understanding of thein vitro/in vivocorrelation[6].Recent review has raised three possible assumptions of how polymeric amorphous solid dispersions(PASD)dissolve in aqueous solution:(a)PASD nano-clusters dissolve quickly into the solution;(b)the PASD particles gradually dissolve and the drug molecules remain amorphous state in the undissolved particles;(c)the PASD particles gradually dissolve,but the recrystallization of drugs may happen at the surface of the undissolved particles[7].However,there is still lack of direct and clear evidence for dissolution molecular mechanism of solid dispersions in aqueous environment.
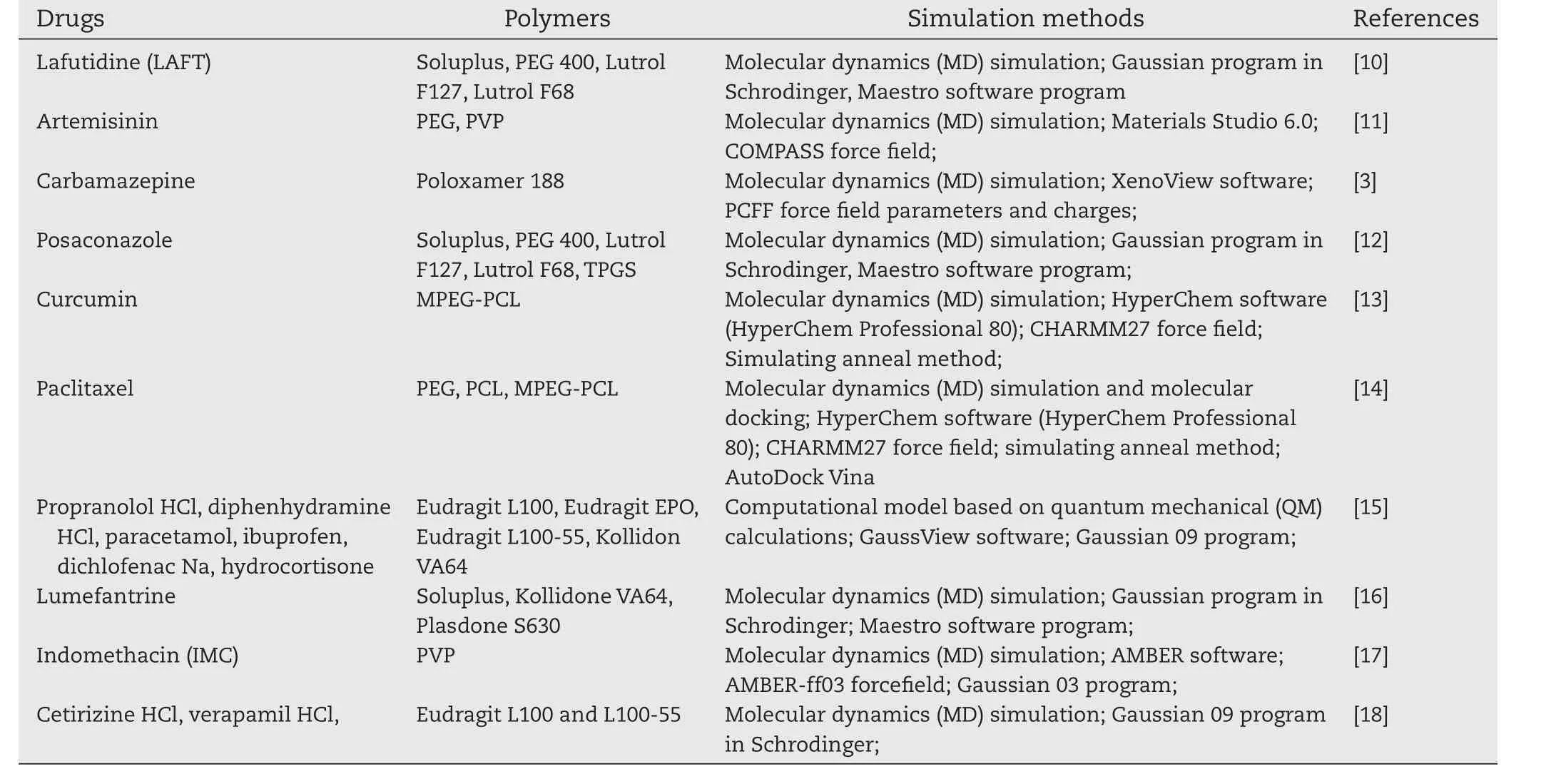
Table 1–Recent progress of molecular modeling in solid dispersions.
Molecular dynamics(MD)simulation describes the molecular systems by mimicking the behavior of molecules at the atomistic level[8].Meanwhile,molecular simulation is able to calculate the physical properties of drug/excipient systems without costly experiments[9].Recent progress about molecular modeling studies for solid dispersions was summarized in Table 1.Our previous study used molecular modeling to investigate the solid state structure of ibuprofen/polymer SD by the simulated annealing method and provided anin silicoprotocol to the preparation of SD[19].Three polymers were selected as the carriers,including polyethylene glycol(PEG),poloxamer and polyvinylpyrrolidone(PVP).Simulation results showed linear polymer chains form the random coils under heat and the drug molecules stick on the surface of polymer coils.This research provided more reasonable molecular images of solid dispersions than the existing theory.
Following our previous study[19],the aim of our study was to further investigate the dissolution molecular mechanism of SD by MD approach.Ibuprofen(IBU),an anti-inflammatory drug for moderating pain and fever,was still chosen as the model drug,and PEG,PVP and poloxamer were selected as the polymeric carriers.
2.Material and simulation details
2.1.Model establishing
The model of the selected polymer and drug molecules were built by Discovery Studio Visualizer 3.1(http://accelrys.com/products/discovery-studio/).The molecular structures of the polymers(PEG,PVP,poloxamer 188)were same as our previous publication[19].The model of PEG contains 20 repeating units of ethylene oxide.The PVP model contains nine repeating units of vinylpyrrolidone.The model of poloxamer was composed of a central chain with three repeating units of propylene oxide, flanked by two chains with 8 repeating units of ethylene oxide.Ibuprofen was used as a model drug.The structure of ibuprofen lattice was downloaded from Cambridge Structural Database and crystal structure of racemate and enantiomer(S)-(+)-ibuprofen was selected[20].These SD models with the Packmol program were further optimized from previous manual-built models in our previous publication[19].Packmol program[21](http://www.ime.unicamp.br/~martinez/packmol/)was used to obtain random distribution of polymers with a tolerance distance of 3.0 ? in a limited boundary box.The details of all systems were shown in Table 2.
2.2.Preparation process modeling of solid dispersions by the simulated annealing method
The molecular dynamics(MD)simulations utilized theAMBER14 software package with the general AMBER force field(gaff)forall carriers and drugs.The models of all systems were built using the LEAP module in AmberTools 1.5.The simulated annealing method was similar to our previous publication[19].After minimization,2 ns annealing simulation was performed.Firstly,the system was gradually heated from 0 to 500 K in 200 ps,and then kept at a temperature of 500 K for 1000 ps to equilibrate the systems.Next,the system was quickly cooled down from 500 to 300 K in 100 ps and finally the systems were kept at a temperature of 300 K for 700 ps for equilibration.
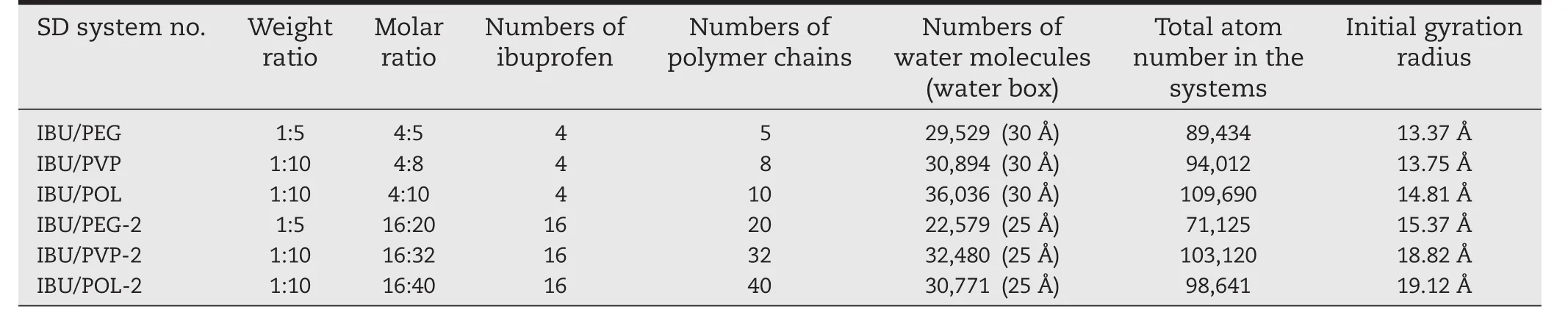
Table 2–The details of solid dispersion systems in the simulation process.
2.3.Dissolution process modeling of solid dispersions by MD simulations
After the simulated annealing process,the solid dispersion system was immersed in a water box with a solvation shell of 25 or 30 ? thickness using TIP3P model for water with the general AMBER force field(gaff)and the ff14SB force field.In the minimization procedure,the structures of drugs and polymers were subjected to 2000 steps of steepest descent minimization followed by 2000 steps of conjugate gradient minimization.The whole system was subjected to 2000 steps of conjugate gradient minimization followed by 3000 steps of conjugate gradient minimization.After minimization,the system was heated at 300 K while keeping the structure of the solid dispersion fixed with weak restraints.The non-bonded cutoff distance was 8.0 ?.Then,50 or 100 ns MD simulation at 300 K was performed in the solvated system to simulate the dissolution process of the solid dispersion.The Langevin Dynamics was used to control the temperature using a collision frequency of 1.0 ps?1.In these simulations,the parameter iwrap=1 of MD input files were used to wrap all molecules into a primary box.Constant pressure periodic boundary with an average pressure of 1 atm was also applied.Initial Gyration Radius gave the reference of the particle size for different SD systems after simulated anneal process.
3.Results and discussion
During simulated annealing process,the polymer chains formed random coils,the crystal structure of ibuprofen was broken and the drug molecules were stuck or inserted to the surface of the polymer coils to form an amorphous solid dispersion,the drugs molecules were molecularly dispersed in the SDs matrix.SD system of ibuprofen with polymers(e.g.PEG,poloxamer and PVP)also showed similar results.Various SD systems with polymers in different lengths and different weight ratios gained similar results(data not shown).The temperature change during the simulated annealing process was shown in Fig.1.It was not surprising that these results were similar to our previous report.
3.1.Simulation results of small particle systems
Fig.2,Fig.3 and Fig.4 indicated the dissolution process of small particles of solid dispersions with three polymers.In the dissolution process,the carboxylic group of ibuprofen molecules turned its direction from polymer molecules to external water molecules and ibuprofen molecules slowly left the surface of the polymer coils.At the same time,all three polymer coils including PEG,POL and PVP became quickly relaxed and the polymer chains were released in the solution,which was in agreement with the assumption(a)[7].Due to the less numbers of the polymer chain,the interaction between the chain–chain and chain–drug was weaker compare to larger particle system.When the particle was immersed in the aqueous condition,the polymer matrix had easily disentangled the random coils.At the same time,the water molecules quickly permeated into the carrier matrix,while the polymer and drug molecules rapidly dispersed and diffused into the water.
3.2.Simulation results of large particle systems
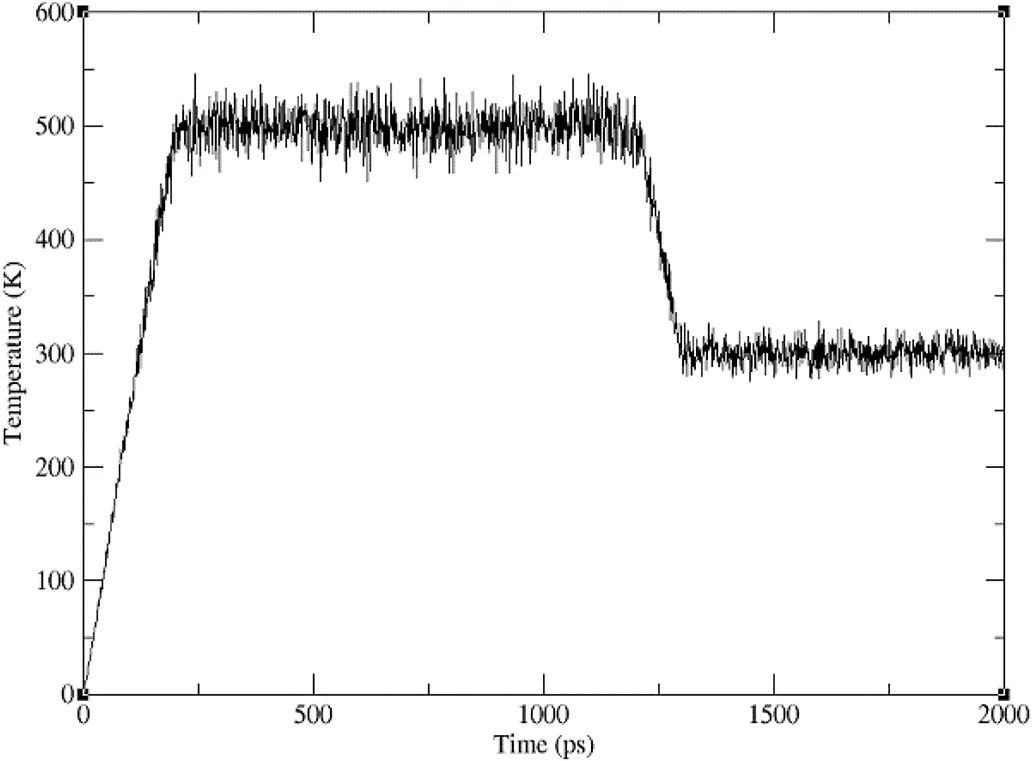
Fig.1–Temperature change vs time for the systems in the simulated annealing method.
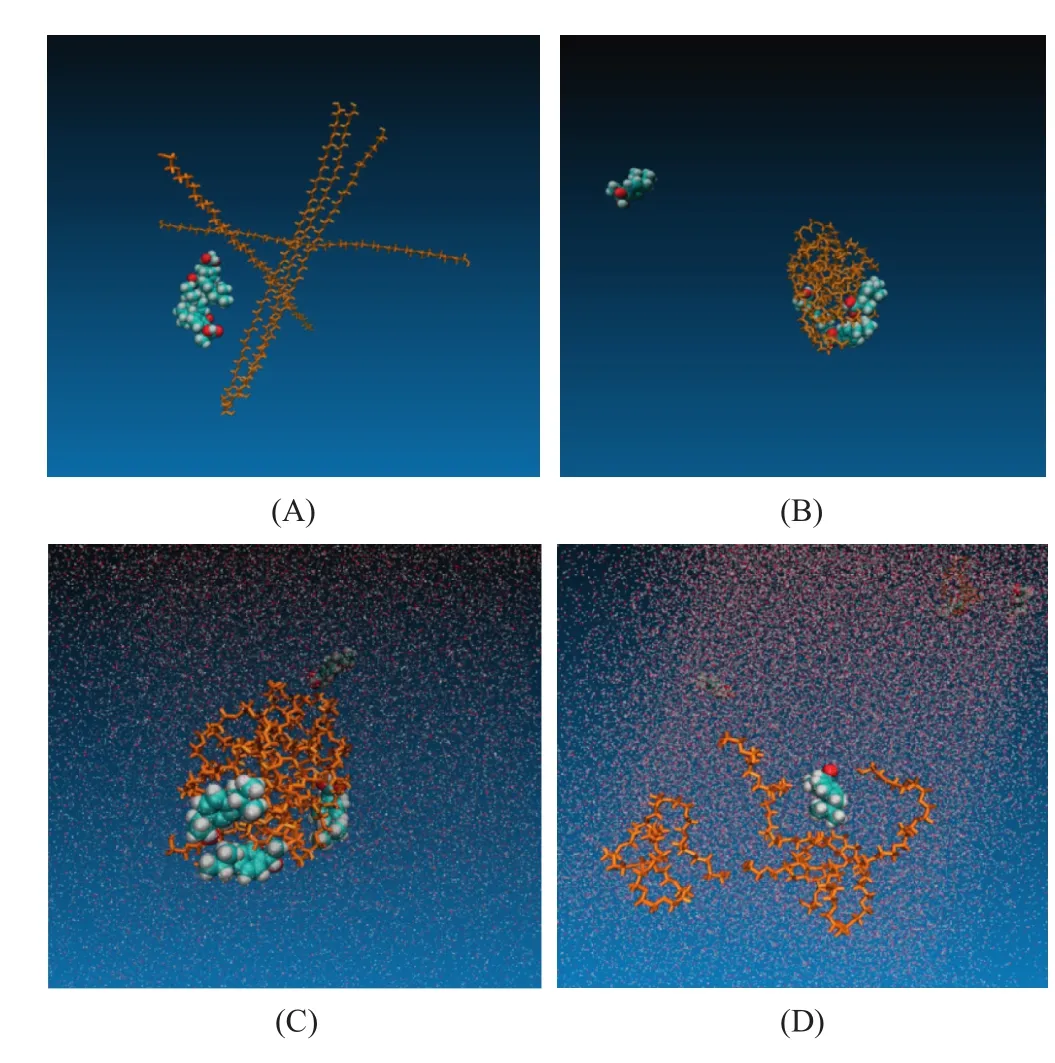
Fig.2–Simulation process of IBU-PEG small particle system(drug-polymer unit ratio 4:5,weight ratio 1:5).(A)Initial model of ibuprofen crystal and PEG system,(B)the solid dispersion system after simulated annealing process,(C)solid dispersion in aqueous phase at 0 ns,(D)solid dispersion in aqueous phase after 50 ns.
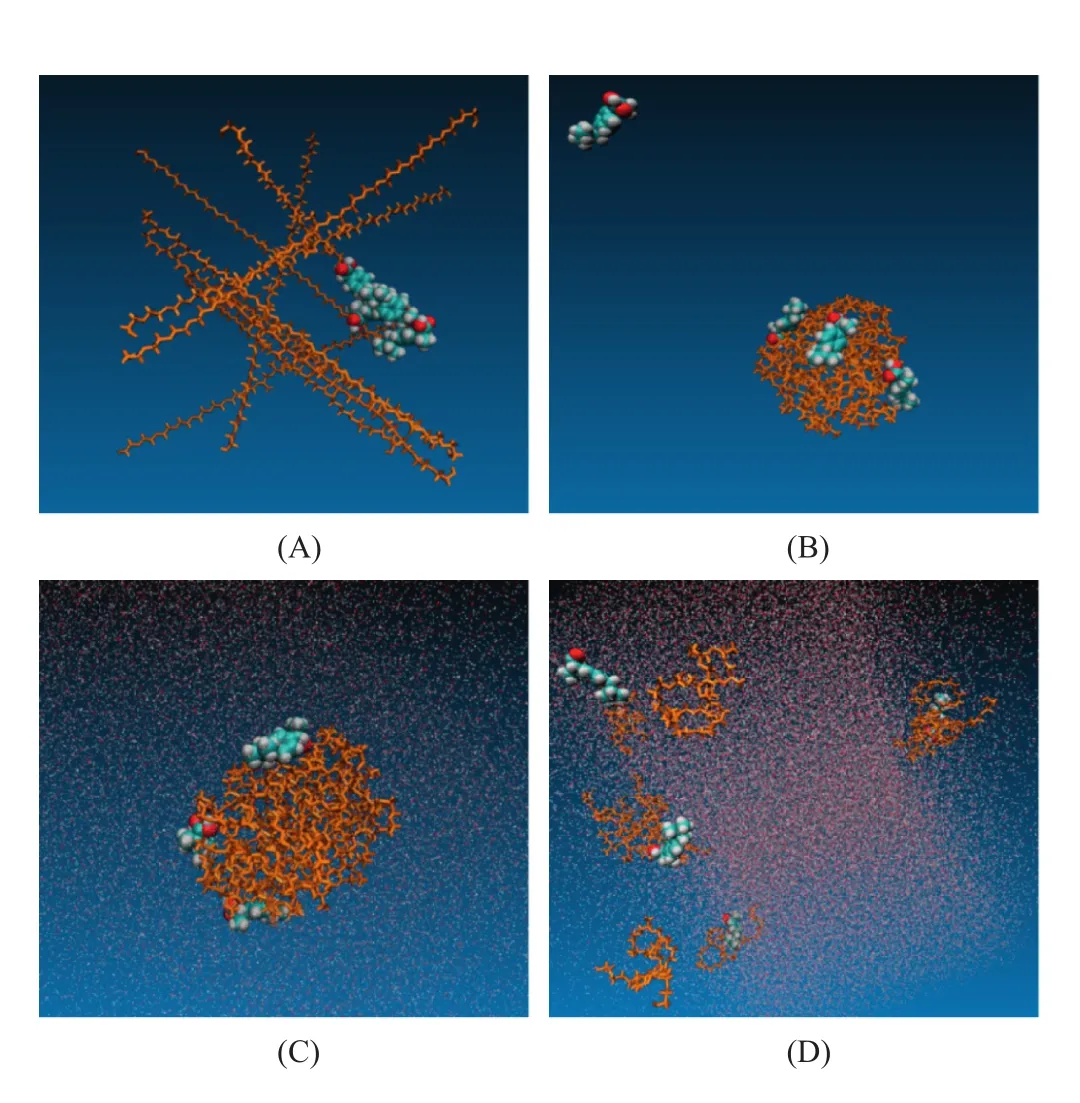
Fig.3–Simulation process of IBU-POL small particle system(drug-polymer unit ratio was 4:10,weight ratio 1:10).(A)Starting model of ibuprofen crystal and POL system,(B)the solid dispersion system after simulated annealing process,(C)solid dispersion in aqueous phase,(D)solid dispersion in aqueous phase after 50 ns.
Fig.5,Fig.6 and Fig.7 indicated the dissolution process of large particles of solid dispersion with three polymers.In the dissolution process,more and more ibuprofen molecules in the large particles were gradually released from solid dispersion systems and the polymer coils increased the relaxed state with the time.However,large SD particles with different polymers presented various dissolution behaviors,which was different from small SD particles.The larger particle system of IBUPEG and IBU-PVP showed the aggregation of drug molecules at the later stage of dissolution process,which may result in further recrystallization and low dissolution rate.In the preparation process of solid dispersions,it is clearly shown that the drug molecules were randomly dispersed on the surface of amorphous polymer coils by the simulated annealing method.However,the drug aggregation in the dissolution process indicated that the solid dispersion systems with PEG or PVP may have the recrystallization problem in the GI tract and then lead to the bioavailability issue.Previous studies investigated the reprecipitation inhibitory effect of three polymers(PEG 6000,PVP K30 and Eudragit)for patchouli alcohol supersaturated solutions[22].The results showed that the inhibitory effect of PEG 6000 and PVP K30 was less effective than that of Eudragit.
3.3.Discussion of two dissolution behaviors in large particle systems
PEGs are semi-crystalline polymers of ethylene oxide and widely used in solid dispersions.However,it is quite interesting that PEG promotes crystallization for some compounds(e.g.ibuprofen,fenofibrate),which is different from other polymers for crystallization hindrance[23].For PVP,recent research with ATR(Attenuated Total Reflection)–FTIR(Fourier-Transform-Infrared)spectroscopic imaging indicated that too fast dissolution of PVP/Aprepitant solid dispersion could negatively influence the bioavailability due to high local super-saturation and consequent recrystallization[24].The good water affinity of PVP solid dispersions makes the polymer fast dissolving,which leads to phase separation and local recrystallization of the drug molecules[25].Fig.8B also showed that the gyration radius of IBUPVP system increased much faster than another two systems after 20 ns,which also indicated that the PVP system dissolved more quickly than PEG and POL systems.Moreover,Fig.9B showed that the overall mean square displacement plot of PVP was clearly increased sharply compared to PEG and POL systems,which also indicated that the displacement of the whole atoms in PVP system moved faster.The simulation results gave a clear molecular image of the aggregation and/or recrystallization process in solid dispersion systems with PEG or PVP.
For large particles with poloxamer,the drug and polymer molecules of poloxamer SD particles were gradually released and the drug remains amorphous form in the undissolved particles in 100 ns simulations,which is different from the systems with PEG and PVP.The possible reason is that amphiphilic property of poloxamer,as a nonionic surfactant,could effectively hinder the aggregation of drug molecules in the dissolution process.This result was also observed in the experimental studies.For example,solubility and dissolution rate of nifedipine/poloxamer solid dispersion were better than those of the systems with PEGs or cyclodextrin complex[26].Furthermore,the increasing amount of Soluplus in the Aprepitant-Soluplus-PVP ternary solid dispersion systems could effectively delay the onset of recrystallization due to the amphiphilic nature of Soluplus[27].Another experimental study indicated that hypromellose acetate succinate(HPMCAS)with the amphiphilic nature showed better stabilization of solid dispersions than that of hypromellose systems[28].Thus,we can see that the amphiphilic polymer(e.g.poloxamer,Soluplus or HPMCAS)could effectively prevent the recrystallization in the dissolution process and may enhance the bioavailability of poorly soluble drugs in solid dispersions.

Fig.4–Simulation process of IBU-PVP small particle system(drug-polymer unit ratio was 4:8,weight ratio 1:10).(A)Starting model of ibuprofen crystal and PVP system,(B)the solid dispersion system after simulated annealing process,(C)solid dispersion in aqueous phase,(D)solid dispersion in aqueous phase after 50 ns.
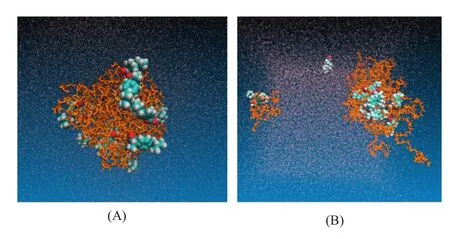
Fig.5–Simulated dissolution process of IBU-PEG large particle system(drug-polymer unit ratio was 16:20,weight ratio was 1:5).(A)IBU-PEG SD system in aqueous phase,(B)IBU-PEG SD system in aqueous phase after 100 ns.
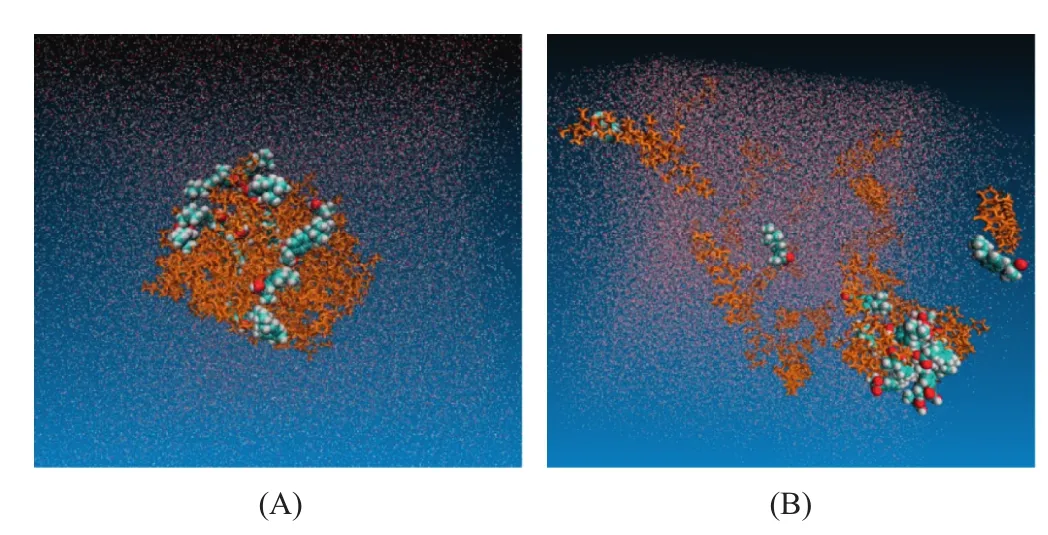
Fig.6–Simulated dissolution process of IBU-PVP large particle system(drug-polymer unit ratio was 16:32,weight ratio was 1:10).(A)IBU-PVP SD system in aqueous phase,(B)IBU-PVP SD system in aqueous phase after 100 ns.
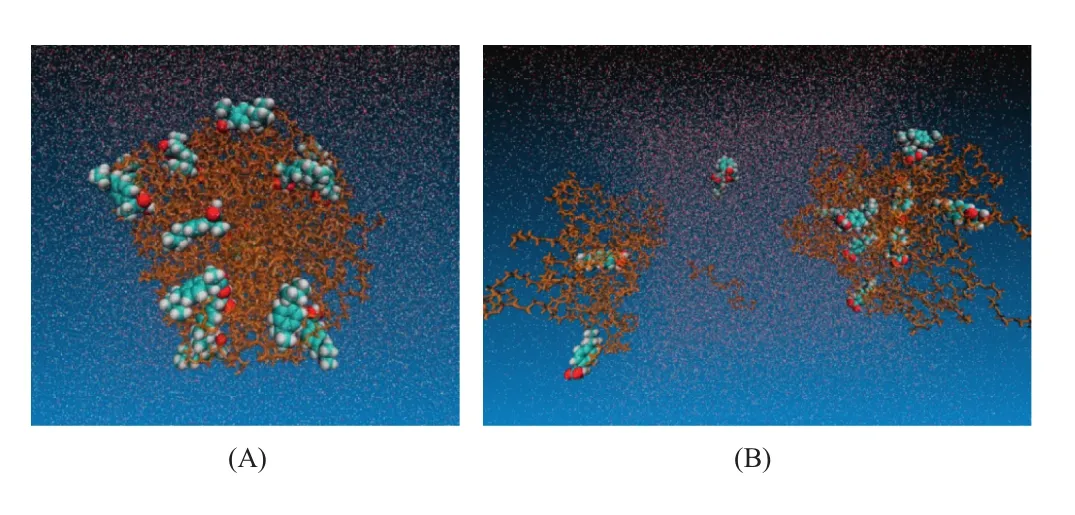
Fig.7–Simulated dissolution process of IBU-POL large particle system(drug-polymer unit ratio was 16:40,weight ratio was 1:10).(A)IBU-POL SD system in aqueous phase,(B)IBU-POL SD system in aqueous phase after 100 ns.
3.4.Combination of our simulation results and the three dissolution assumptions
Our simulation results indicated that the dissolution process of small SD particles agrees with the hypothesis(a),while large SD particles agreed with the hypotheses(b)and(c)[7].The hypothesis(a)assumed that the drug and polymer molecules rapidly dissolved and dispersed in the aqueous solution.However,large SD particles with different polymers showed different dissolution behaviors.In the dissolution process,the drug molecules in the large particles with PEG or PVP easily shift and aggregate together,which was in consistence with the hypothesis(c)of drug recrystallization in the dissolution process[24,25,29].The large SD particles with poloxamer could effectively hinder the aggregation of drug molecules in the dissolution process,indicated in hypothesis(b).Therefore,our simulations could effectively mimic the dissolution process of solid dispersion.
4.Conclusion
This research investigated the dissolution process of solid dispersion particles at molecular level.The results agreed with recent hypotheses of different dissolution mechanism of solid dispersions[7].Our research will provide useful clues for future formulation development of solid dispersions.
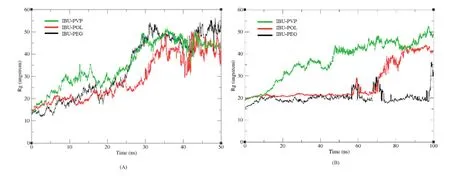
Fig.8–The mass-weighted radius of gyration of SD system in different size scale,with fluctuation as a function of time.(A)Small particle size system,(B)larger particle size system.
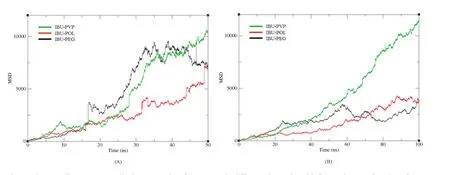
Fig.9–The overall mean square displacement plot of SD system in different size scale,with fluctuation as a function of time.(A)Small particle size system,(B)larger particle size system.
Conflict of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgment
University of Macau research grants(MYRG2016-00038-ICMSQRCM and MYRG2016-00040-ICMS-QRCM)are gratefully acknowledged for providing financial support.
R E F E R E N C E S
 Asian Journal of Pharmacentical Sciences2018年3期
Asian Journal of Pharmacentical Sciences2018年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- LAL test and RPT for endotoxin detection of CPT-11/DSPE-mPEGnanoformulation:What if traditional methods are not applicable?
- Activity of Brucea javanica oil emulsion against gastric ulcers in rodents
- Model evaluation for the prediction of solubility of active pharmaceutical ingredients(APIs)to guide solid–liquid separator design
- Systematically optimized topical delivery system for Loperamide hydrochloride:Formulation design,in vitro and in vivo biopharmaceutical evaluation
- Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier
- Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis
