Adult immunization improvement in an underserved family medicine practice
Mohamad Sidani, Jaden Harris, Roger J. Zoorob
Adult immunization improvement in an underserved family medicine practice
Mohamad Sidani, Jaden Harris, Roger J. Zoorob
Objective:Vaccines prevent many cases of infectious disease, yet immunization campaigns are hindered by various barriers. This work presents the results of a quality improvement project addressing barriers to vaccine compliance in an under served teaching practice by reducing missed opportunities and increasing provider and patient compliance rates for pneumococcal, Tdap,influenza, and zoster vaccines in adults.
Methods:The study intervention aimed to address patient knowledge, provider knowledge and skills, proactive care coordination, and outreach and counselling of high-risk groups. Aggregate patient data from intervention at year-end were compared to the prior year. Outcome targets were as follows: improved vaccination rates by one-half of the difference between baseline and Healthy People 2020 goals; reduced patient refusals by 10%; and reduced missed opportunities by 50%.
Results:All of the vaccination rates improved, but with mixed results regarding the target outcomes. The rates of vaccine refusal were mixed in terms of the direction of the change, the significance, and achieving targets. Missed opportunities all improved, but the significance was mixed and none reached targets.
Conclusion:This project has helped to identify patient and provider knowledge of vaccination as a key to increasing compliance, and missed opportunities as the greatest challenge in achieving targets. The burden of documentation is significant on providers, and future work should focus on methods to improve the ease of documentation. Clinical outcomes and improvements were encouraging; however, it is clear that there remain challenges to reaching Healthy People 2020 goals within the study population and nationally.
Immunization; vaccine; practice improvement; pneumococcal; Tdap; influenza;zoster
Introduction
Vaccines are among the greatest advances in modern medicine, and have prevented many cases of infectious diseases, yet vaccination campaigns are hindered by various barriers and require consistent evaluation and innovation.The Healthy People 2020 (HP2020) program is an important US public health effort to promote health, reduce disparities, and advance research. Among the objectives of HP2020 is to: “Reduce, eliminate, or maintain elimination of cases of vaccine-preventable diseases [1].” The influenza ( flu) vaccine is an example of an effective, but underusedimmunization. Even though vaccines are widely available,affordable, and efficacious, influenza continues to take a dramatic toll on the unvaccinated each year, impacting high-risk populations and the elderly with hospitalization, death, and economic burden [2, 3].
Barriers to immunization compliance are numerous,including knowledge and attitudes of patients and providers,economic concerns, access to care [2, 3], and racial disparities[4]. Many practices face challenges to provider compliance with evidence-based guidelines, including lack of awareness or familiarity with guidelines, lack of agreement, lack of self-efficacy and outcome expectancy, and external barriers,including patient and environmental factors [4]. Situational constraints, such as presenting illness, also limit the ability of providers to administer vaccines during a given clinical encounter. Teaching practices face additional challenges, as medical residents may not assign high priority to adult immunizations because vaccines may be not highly valued or the use of vaccines closely monitored [3].
Other common reasons patients forego vaccination include the belief that healthy people do not need vaccinations, concerns over side effects, and reporting that their physician did not recommend vaccinations [5]. Indeed, only one-fourth of primary care physicians issued influenza vaccination reminders during the 2011–2012 influenza season [6]. Patient fear of vaccine risk is also a factor in declining recommended vaccines. For example, a common concern is that the influenza vaccine can cause the flu; however, inactivated influenza vaccine does not cause the flu, although injection site swelling,redness, and tenderness are possible [7]. Although the live-attenuated nasal spray flu vaccine contains live virus and has a greater potential for side effects, the live-attenuated nasal spray flu vaccine does not cause influenza either [8]. This common misconception may be partially attributable to the concurrent onset of other seasonal illnesses, perceived as flu, following vaccination [2]. Similarly, misconceptions about mercury toxicity in vaccine formulations are prevalent [2]. Concerns about egg allergies remain, but such myths have been diminished by recent studies [6]. Risk of adverse reactions is higher in allergic or immunocompromised patients, and also in young children. The Centers for Disease Control and Prevention (CDC)recommends screening and using evidence-based precautions to decrease such reactions [7]. One unresolved concern is that influenza vaccine may cause Guillain-Barré syndrome, and research into this association is ongoing [9].
The current study is the result of a quality improvement project designed to systematically address perceived barriers to adult vaccine compliance in an underserved teaching practice. This was accomplished by reducing missed opportunities and increasing both provider and patient knowledge and compliance. Similar to national rates, the baseline immunization rates at the study practice were low compared to HP2020 goals[1], and therefore represented an opportunity for improvement.The intervention aimed to improve immunization rates over the course of 1 year by enhancing provider and patient knowledge, soliciting and addressing patient concerns, and reducing missed clinical opportunities. Although the HP2020 goals cover a wide range of infectious diseases for all age groups,this work focused on rates of pneumococcal, Td or Tdap, influenza, and zoster vaccines in adults.
Methods
The study was a quality improvement project conducted in an underserved urban family medicine residency practice from July 2012 through June 2013. Age at the time of the visit determined inclusion in the appropriate vaccine age groups used. The influenza vaccine rate was calculated by administration of at least 1 dose during the influenza season of the 12-month assessment period, although pneumococcal polysaccharide (PPSV),herpes zoster, and tetanus (Td and Tdap) booster vaccines were limited to visits during the calendar year, and required investigation of broader appropriate timeframes for immunization history. The inclusion groups for each vaccine varied by appropriate guidelines and age groups defined by the funding source, which varied slightly from HP2020. The study population was adults >19 years of age assessed for influenza vaccine needs during the typical flu season. Adults >65 years of age were assessed for a single lifetime dose of PPSV on record.Adults 19–64 years of age were assessed for a recorded Td or Tdap immunization in the past 10 years. Finally, a single lifetime dose of zoster vaccine was assessed for adults >60 years of age. The study clinics (hereafter referred to as clinics) are designated as medically-underserved by the US Health Resources and Services Administration (HRSA). The specific patient population tends to be the most underserved members within these communities, predominantly uninsured, underinsured, or publicly insured, and primarily members of minority communities. Specifically, the rate of self-pay was 8.6%, publicly insured was 53.4%, and charity care offered by the affiliated hospital system was 10.3%. The racial distribution was approximately 53% African American, 30% Latino, and 17% Caucasian.These demographics owe in part to the geographic locations of clinics and acceptance of public insurance options, along with sliding-scale payment and charity fee schedules.
The intervention plan consisted of two goals. The first goal was to reduce the overall rate of missed opportunities to administer vaccines by one-half of the difference between the HP2020 goals and baseline. This goal was divided into two elements: teamwork and training. For the teamwork component, a team-based approach was used to optimize care coordination and case management strategies at point-of-service and through outreach to high-risk groups, including older adults and patients captured in clinical disease registries. A“vaccines task force” consisting of key operational stakeholders met monthly to discuss strategy and progress. This team included the department chair, medical director, nurse quality manager, project manager, and a resident physician trainee champion. Other members of the clinical team were present at clinical operations meetings and were invited to contribute feedback. The team developed patient outreach methods which combine annual reminder notices sent by mail, educational pamphlets and waiting room posters, limited annual appointment solicitation phone calls, and verbal counselling to high-risk groups. Proactive care coordination was implemented by the quality nurse and support staff to help ensure that patient charts were reviewed before or during each visit, opportunities for vaccination were identified, vaccines administered, necessary counselling provided, and accurate records maintained.Standing orders for nursing staff were put in place for influ-enza and PPSV vaccines. An additional training component introduced two new dedicated didactic sessions per year which aimed to increase faculty and resident awareness of evidence based guidelines, proper documentation processes, and patient counselling methods.
The second goal was to improve overall patient compliance by educating patients about the need for vaccines and addressing their fears and misconceptions. This goal was broken down into passive and active patient education components. The passive component included the development by the Vaccine Task Force of a set of educational pamphlets and waiting room posters to increase patient knowledge regarding the need for and safety of vaccines in adults, and mailing vaccine reminder letters to patients. The active component involved encouraging providers to counsel their patients on the importance of vaccinations, and to respond to patient fears and misconceptions. This encouragement was delivered in the form of discussion as a standing item in the weekly clinical management meetings via dedicated residency didactic sessions, and by the ongoing presence of program champions,including the medical director, nurse manager, and resident project leader.
Data was collected using a reporting tool (SAP Crystal Reports) to query a clinical electronic health record system.This reporting included all patient records queried from the Health Maintenance Table section of the patient record for inclusion groups seen in the clinics during the year previous to the study implementation. The rates of vaccinated adults>19 years of age with Tdap and influenza, PPSV for adults>65 years of age, and zoster for adults >60 years of age were used for baseline. Reports were compiled and analyzed by the Quality Assurance (QA) team to determine baseline rates of immunization and the difference between baseline rates and HP2020 goals. At the end of the study year, data for all adults seen in clinics during that year defined the ending rates.De-identified lists were used to aggregate all data points. Data analysis was conducted using a simple Z-test for proportions with significance set at a 95% confidence level.
Results
Outcome evaluations were based on several parameters, and split into administrative processes and clinical outcomes.Administrative process outcomes included the frequency of team meetings held, the number of didactic sessions held,and completion of educational materials and outreach to patients. These measures are perhaps the simplest to control,record, and improve, yet are essential in the overall project design. Each of these measures reached targets set, including monthly dedicated team meetings and a regular agenda item in clinical management meetings, successful integration of 2 yearly didactic sessions, completion and use of educational posters and pamphlets for patient waiting rooms, and a mass mailing of immunization reminders to high-risk patient groups.
Clinical outcome measures for this project were the rate of vaccine administration, rate of patient refusals, and rate of missed opportunities determined by the number of patients seen without any recorded status regarding the recommended vaccines. The outcome targets were to improve vaccine administration rates by one-half the difference between baseline and HP2020 goals, reduce patient refusals by 10% from baseline,and reduce clinical missed opportunities by 50% from baseline. An overview of clinical outcomes is shown in Table 1.
Vaccination rates improved, but with mixed results with respect to reaching the target of one-half the difference between baseline and HP2020 goals for each vaccine.Baseline influenza vaccine administration for adults >19 years of age during the flu season (2012–2013) was 24.4% and the post-intervention result was 35.2% (an overall improvement of 10.8%). Although this was a significant and encouraging improvement, it did fall far short of the target of 47.2%. The baseline PPSV for adults >65 years of age was 64.8% and the end result was 73.9% (an improvement of 9.1%). This finding was also significant, but fell short of the target of 77.4%. The baseline Td or Tdap for adults 19–64 years of age was 40.3%and the end result was 52.8% (an improvement of 12.5%). This finding was significant and considered another encouraging result, but we did not set a target for Td or Tdap because there was no HP2020 goal for Tdap. Finally, the baseline administration of zoster vaccine was 15.2% and the end result was 23.0% (an improvement of 7.8%). This result was also signifi-cant and slightly exceeded the target of 22.6%.
The rates of patient refusal for vaccines were mixed with respect to the direction of change, significance, and reaching the 10% reduction target. The baseline refusal rate for influ-enza was 14.5% and the end refusal rate post-intervention was 11.1%, which was a significant improvement of 3.4% and exceeded the 10% target of reduction of 13.1%. The baseline refusal rate for PPSV was 9.9% and the end result was 9.2%(a 0.7% improvement). This result was not significant, nor did it reach the target of 8.9%. The baseline refusal rate for Td or Tdap was 6.8% and the end result was 6.9%; this result was actually slightly worse than baseline, but the difference was not significant. The target was 6.1%. The baseline refusal rate for zoster was 20.9% and the end result was 15.4%, which wasa significant improvement of 5.5% and exceeded the target of 18.8%.
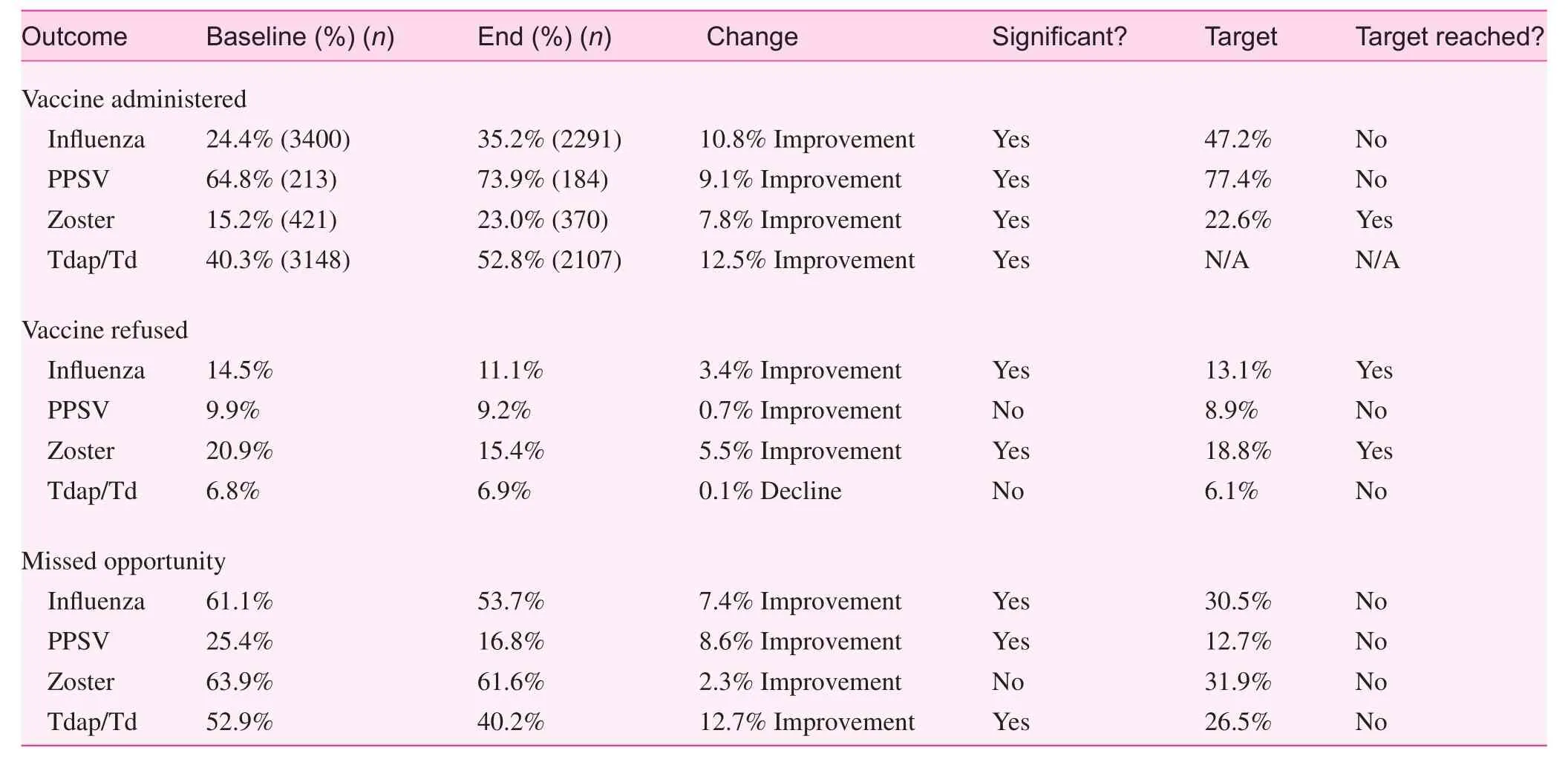
Table 1. Overview of clinical outcomes
Missed opportunities all improved, but significance was mixed, and none reached the target of a 50% reduction from baseline. The baseline missed opportunity rate for influenza was 61.1%, and the end result was 53.7%, which was a significant improvement of 7.4% (target=30.5%). The baseline missed opportunity rate for PPSV was 25.4%, and the end result was 16.8%, which was a significant improvement of 8.6%(target=12.7%). The baseline missed opportunity rate for Td or Tdap was 52.9%, and the end result was 40.2%, which was a significant improvement of 12.7% (target=26.5%). The baseline missed opportunity rate for zoster was 63.9%, and the end result was 61.6%, which was an improvement of 2.3%. This result was neither significant nor reached the target of 31.9%.
Our study showed that the pre-visit review by the QA nurse had significant changes in refusals and missed opportunities regarding the influenza vaccination (Table 2). It has been shown that standing orders for nurse recommendations are a significant patient motivator, further enhanced by physician follow-up [10, 11].
This study was conducted as a retrospective review of outcomes from a clinical and training quality improvement project. As such, the study was granted exemption from human subjects research requirements by the Baylor College of Medicine Institutional Review Board.
Discussion
Nationally, influenza vaccination among adults is estimated to be less than 40% in the 2010–2011 and 2011–2012 flu seasons, although the HP2020 goal is 70% [1]. Similarly, the rates of other common adult immunizations are suboptimal. For example, PPSV coverage among non-institutionalized adults>65 years of age is 60% overall, although the HP2020 goal is 90% [1]. The shingles (herpes zoster) vaccine for adults>60 years of age has a national administration rate of 6.7%and a HP2020 target of 30%. In 2012, the percentage of adults>19 years of age who received Tdap in the previous 7 years was approximately 14.2%; however, there was no associated HP2020 goal for Tdap vaccination [3].
Chatterjee and O’Keefe [12] suggested that because of the successes of immunizations in recent decades and fading memory of the incidence of some diseases, there appears to be a shift away from the fear of disease to a fear of vaccines. This is evidenced by the public controversies surrounding parents and even heal th care providers opting not to vaccinate children and themselves based on common misconceptions [12, 13].The intervention in this study was aimed in part to address this phenomenon.
Our results were generally encouraging, indicating the value of a dual-pronged approach to adherence, thus addressing both provider and patient needs. Barriers to vaccine administration prevalent in the literature were also factors in this situation, and attempts to address the barriers produced modest gains. An overall evaluation of clinical outcomes yielded mixed results in each aspect of our assessment, as vaccination, refusal, and missed opportunity rates improved overall, but varied within each vaccine group and many fell short of targets.
This study had several limitations. The program addressed multiple interventions, including physician and patient education, standing orders for influenza and pneumococcal vaccines, better record keeping, and targeting missed opportunities. Although each factor worked toward the ultimate successful outcomes seen in the project, it is difficult to evaluate the effect of each factor independently. Moreover, the study was limited to urban underserved practices, and this may limit generalization of the results to other settings. It is also worth noting that the study was conducted over a single year in a residency training practice. Another limitation was the use ofde-identified aggregate data, thus the impact of interventions on individual compliance was not measurable. In addition and also identified as a major barrier to addressing missed opportunities, was the burden of documentation itself. Providers must record vaccine status in the electronic health record note with proper codes or manually enter vaccine status into the Health Maintenance Table section of the patient record to ensure the vaccine status is reflected properly in the study data and enable scheduled reminders to be functional. Therefore, if vaccine administration was recorded in the wrong section of the chart note, it was reflected as a missed opportunity in the study. The burden of documentation is significant on providers, and future study should focus on methods to ease documentation. Additionally, even though outreach to high-risk groups was conducted, tracking of PPSV for high-risk groups was not possible because of difficulties with data extraction from the medical record, and thus was not reported separately.
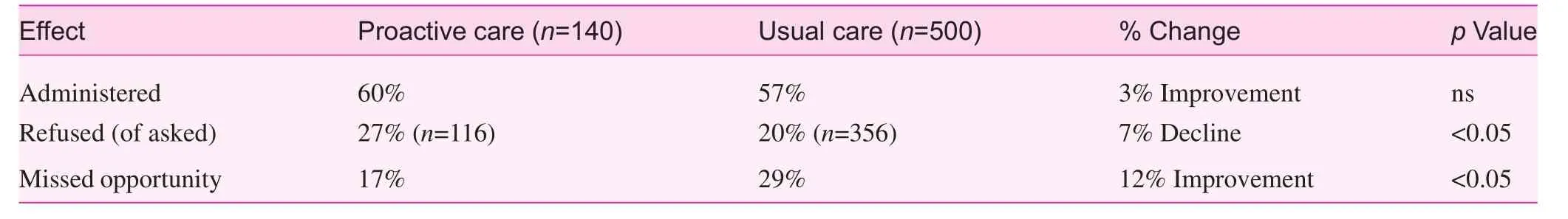
Table 2. Effect of proactive quality assurance on influenza vaccination rates
Subjective feedback from providers was positive, with anecdotal reports of increased resident provider confidence in counselling patients, improved documentation practices, and greater patient compliance. In this study, vaccination rates all improved significantly, and other outcomes were encouraging,although it is clear that there remains a significant challenge to reaching HP2020 goals within the study population and nationally.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was funded by the 2013 American Academy of Family Physicians Foundation P fizer Immunization System Implementation Award.
1. Immunization and Infectious Diseases. [homepage on the internet]. Healthy People 2020. [Updated 2015, Feb 04, Cited 2015,Feb 04] Available from: http://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives.
2. Golovyan DM, Mossad SB. Prevention and treatment of influenza in the primary care of fice. Cleve Clin J Med 2014;81(3):189–99.
3. Jacobson JA. Residents’ role in immunizing adults: rationale, opportunity, obstacles, and strategies. Virtual Mentor 2012;14(1):23–9.
4. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH,Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? J Am Med Assoc 1999;282(15):1458.
5. Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med 2008;121(7 Suppl 2):S28–35.
6. Maurer J, Harris KM. Issuance of patient reminders for influenza vaccination by US-based primary care physicians during the first year of universal influenza vaccination recommendations. Am J Public Health 2014;104(6):e60–2.
7. Seasonal influenza Vaccine Safety: A Summary for Clinicians& Health Professionals. [homepage on the internet]. Centers for Disease Control and Prevention. [updated 2012 Dec. 12; cited 2015 Jan 22] Available from: http://www.cdc.gov/ flu/professionals/vaccination/vaccine_safety.htm.
8. Live Attenuated influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). [homepage on the internet] Centers for Disease Control and Prevention. [updated 2014 Sept. 9; cited 2015 Jan 22] Available from: http://www.cdc.gov/ flu/about/qa/nasalspray.htm.
9. Israeli E, Agmon-Levin N, Blank M, Chapman J, Shoenfeld Y.Guillain-Barré syndrome–a classical autoimmune disease triggered by infection or vaccination. Clin Rev Allergy Immunol 2012;42(2):121–30.
10. Daniels NA, Gouveia S, Null D, Gildengorin GL, Winston CA.Acceptance of pneumococcal vaccine under standing orders by race and ethnicity. J Natl Med Assoc 2006;98(7):1089–94.
11. Zimmerman RK, Nowalk MP, Tabbarah M, Hart JA, Fox DE,Raymund M. Understanding adult vaccination in urban, lower-socioeconomic settings: influence of physician and prevention systems. Ann Fam Med 2009;7(6):534–41.
12. Chatterjee A, O’Keefe C. Current controversies in the USA regarding vaccine safety. Expert Rev Vaccines 2010;9(5):497–502.
13. Domínguez A, Godoy P, Castilla J, María Mayoral J, Soldevila N,Torner N, et al. Knowledge of and attitudes to influenza in un vaccinated primary care physicians and nurses. Hum Vaccin Immunother 2014;10(8):2378–86.
Department of Family and Community Medicine, Baylor College of Medicine, Houston,TX, USA
Roger J. Zoorob, MD, MPH
Department of Family and Community Medicine, 3701 Kirby Drive, Suite 600, Houston, TX 77098, USA
Tel.: +713-798-2333
E-mail: Roger.Zoorob@bcm.edu
25 March 2015;
20 April 2015
 Family Medicine and Community Health2015年2期
Family Medicine and Community Health2015年2期
- Family Medicine and Community Health的其它文章
- Evaluation of obstetrics procedure competency of family medicine residents
- Student self-assessment of strengths and needed improvements during a family medicine clerkship
- Depression and race affect hospitalization costs of heart failure patients
- Rural congestive heart failure mortality among US elderly,1999–2013: Identifying counties with promising outcomes and opportunities for implementation research
- Exploring point-of-care transformation in diabetic care: A quality improvement approach
- Hospitalizations and healthcare costs associated with serious,non-lethal firearm-related violence and injuries in the United States,1998–2011
