阿苯達(dá)唑?qū)δ晚樸K肺癌細(xì)胞糖酵解?細(xì)胞周期及細(xì)胞凋亡的影響
何迎盈,羅治彬,李少林△
(1.重慶醫(yī)科大學(xué)基礎(chǔ)醫(yī)學(xué)院核醫(yī)學(xué)教研室 400016;2.重慶市合川區(qū)人民醫(yī)院腫瘤科 401520)
阿苯達(dá)唑?qū)δ晚樸K肺癌細(xì)胞糖酵解?細(xì)胞周期及細(xì)胞凋亡的影響
何迎盈1,羅治彬2,李少林1△
(1.重慶醫(yī)科大學(xué)基礎(chǔ)醫(yī)學(xué)院核醫(yī)學(xué)教研室 400016;2.重慶市合川區(qū)人民醫(yī)院腫瘤科 401520)
目的研究阿苯達(dá)唑(ABZ)對(duì)人耐順鉑肺癌細(xì)胞(A549/DDP細(xì)胞)糖酵解?細(xì)胞周期及細(xì)胞凋亡的影響?方法 用MTT比色法檢測(cè)ABZ對(duì)A549/DDP細(xì)胞增殖的影響,求得抑制率為0%?25%?50%?75%所對(duì)應(yīng)的ABZ濃度,并按抑制率將實(shí)驗(yàn)分為對(duì)照組,25%抑制濃度(IC25)組?半數(shù)抑制濃度(IC50)組和75%抑制濃度(IC75)組,每組給予相應(yīng)濃度的ABZ處理,并按作用時(shí)間再將每組分為12?24?36h亞組?按照分組,在設(shè)定時(shí)間點(diǎn),用比色法測(cè)定己糖激酶(HK)和丙酮酸激酶(PK)活性,酶標(biāo)儀法測(cè)定乳酸脫氫酶(LDH)活性,RT-PCR法測(cè)定Akt和Myc mRNA表達(dá),流式細(xì)胞儀檢測(cè)細(xì)胞周期和凋亡?結(jié)果ABZ抑制了A549/DDP細(xì)胞增殖,呈劑量依賴性,抑制率為0%?25%?50%?75%所對(duì)應(yīng)的 ABZ濃度分別為(0.00±0.00)μmol/L?(0.99±0.11)μmol/L?(5.73±0.65)μmol/L?(33.15±3.94)μmol/L?ABZ明顯降低了 A549/DDP細(xì)胞 HK?PK?LDH 的活性,且下調(diào)了Akt和Myc mRNA表達(dá),細(xì)胞周期阻滯,凋亡明顯?結(jié)論ABZ能抑制A549/DDP細(xì)胞糖酵解酶活性,下調(diào)糖酵解相關(guān)基因表達(dá),阻滯細(xì)胞周期,誘導(dǎo)細(xì)胞凋亡?
阿苯達(dá)唑;糖酵解;細(xì)胞周期;細(xì)胞凋亡;腫瘤細(xì)胞,培養(yǎng)的
腫瘤細(xì)胞主要靠糖酵解獲取能量,其活躍的糖酵解與糖酵解酶的 活化[1-2]?Akt及 Myc基 因 的 激 活 有 關(guān)[3-4]? 阿 苯 達(dá) 唑是臨床常用的抗寄生蟲藥物,通過抑制寄生蟲攝取葡萄糖,降低丙酮酸激酶(prruvate kinase,PK)?乳酸脫氫酶(lactate dehydrogenase,LDH)等活性,阻止三磷腺苷產(chǎn)生,耗竭糖原而殺滅寄生蟲?國(guó)外學(xué)者發(fā)現(xiàn),阿苯達(dá)唑(albendazole,ABZ)能阻止腫瘤細(xì)胞微管蛋白聚合誘導(dǎo)凋亡[5],還能抑制腫瘤血管形成和乏氧誘導(dǎo)因子-1表達(dá)[6-7]?本實(shí)驗(yàn)研究ABZ對(duì)人耐順鉑肺癌細(xì)胞(A549/DDP細(xì)胞)糖酵解?細(xì)胞周期和生存的影響?
1 材料與方法
1.1 材料
1.1.1 細(xì)胞系 A549/DDP細(xì)胞株購(gòu)自中國(guó)醫(yī)學(xué)科學(xué)院腫瘤細(xì)胞庫(kù)?
1.1.2 主要試劑 胎牛血清和RMPI 1640培養(yǎng)基購(gòu)自Gibco公司;ABZ購(gòu)自AccuStandard公司,純度為97.5%;MTT和二甲基亞砜(DMSO)購(gòu)自Sigma公司;BCA蛋白濃度測(cè)定試劑盒購(gòu)自碧云天公司;己糖激酶(hexokinase,HK)?PK 和LDH活性測(cè)定試劑盒購(gòu)自南京建成公司;RNA抽提試劑盒?引物?逆轉(zhuǎn)錄試劑盒?β-actin?Taq酶均購(gòu)自Takara公司;Annexin V/PI試劑盒購(gòu)自BD公司?
1.2 方法
1.2.1 實(shí)驗(yàn)分組 A549/DDP細(xì)胞接種于96孔板,加入含ABZ終濃度為1?2?4?8?16?32?64?128μmol/L的培養(yǎng)液?每個(gè)濃度設(shè)3個(gè)復(fù)孔?培養(yǎng)24h后加入5mg/mL MTT 20μL,4h后加入150μL DMSO,酶標(biāo)儀490nm處測(cè)定吸光OD值,計(jì)算細(xì)胞增殖抑制率?增殖抑制率(%)=[1―(實(shí)驗(yàn)孔OD值―空白對(duì)照孔OD值)/(陰性對(duì)照孔OD值―空白對(duì)照孔OD值)]×100%?按照實(shí)驗(yàn)結(jié)果,將實(shí)驗(yàn)分為對(duì)照組?25%抑制濃度(IC25)組?半數(shù)抑制濃度(IC50)組和75%抑制濃度(IC75)組?
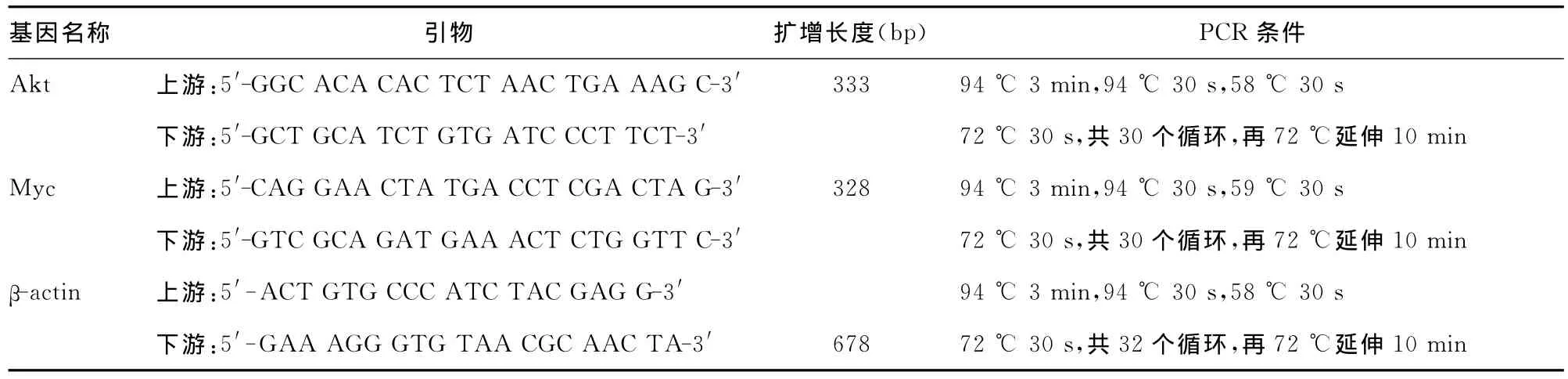
表1 引物序列及PCR反應(yīng)條件
1.2.2 HK?PK?LDH 活性檢測(cè) A549/DDP細(xì)胞接種于6孔板,按組加入含 ABZ終濃度為0.0?1.0?5.5?35.0μmol/L的培養(yǎng)液(以下細(xì)胞接種和ABZ處理均同此處)?12?24?36 h,用0.25%胰蛋白酶消化并收集細(xì)胞,生理鹽水漂洗2次后制成0.5mL細(xì)胞懸液移至2mL玻璃勻漿管冰上勻漿3min?樣本-20℃保存?按BCA蛋白濃度測(cè)定試劑盒說明加樣,酶標(biāo)儀562nm處測(cè)定吸光度,繪制標(biāo)準(zhǔn)曲線,計(jì)算各樣本的蛋白濃度?按酶活性測(cè)試盒說明加樣和處理,分光光度計(jì)340nm處測(cè)HK和PK的A1和A2值,酶標(biāo)儀450nm處測(cè)LDH吸光值?按說明書計(jì)算酶活性,將各組測(cè)得的活性與對(duì)照組比較,求得相對(duì)活性?
1.2.3 半定量 RT-PCR檢測(cè) Akt?Myc mRNA表達(dá) ABZ作用12?24?36h,離心棄去培養(yǎng)液,加入磷酸鹽緩沖液(phosphate buffer,PBS)輕洗離心兩次?用TRIzol法提取總RNA?經(jīng)Thermo Nanodrop儀檢測(cè),提示所獲樣品無(wú)降解和蛋白污染?每個(gè)標(biāo)本以1μg總RNA為模板按逆轉(zhuǎn)錄試劑盒說明逆轉(zhuǎn)錄合成cDNA?引物序列及PCR反應(yīng)條件見表1?2%瓊脂糖凝膠電泳檢測(cè)PCR產(chǎn)物,在凝膠成像系統(tǒng)下成像,Quanlity One軟件分析灰度值,對(duì)比相應(yīng)內(nèi)參求出相對(duì)灰度值?
1.2.4 細(xì)胞周期和細(xì)胞凋亡率的流式細(xì)胞儀測(cè)定 ABZ作用12?24?36h,轉(zhuǎn)移孔內(nèi)培養(yǎng)基至離心管,PBS輕洗2次孔板并收集清洗液至離心管,胰蛋白酶消化細(xì)胞至離心管?室溫下,1 000r/min離心3min,棄去上清液?
1.2.4.1 細(xì)胞周期檢測(cè) 加75%乙醇1mL吹打均勻,4℃過夜后離心收集細(xì)胞,1mL PBS洗后,加入500μL含50μg/mL的PI?100μg/mL RNase A?0.2%Trion X-100的 PBS溶液,4℃避光孵育30min后上機(jī)檢測(cè),Modfit軟件擬合數(shù)據(jù)?
1.2.4.2 細(xì)胞凋亡率檢測(cè) 加緩沖液配成1×106/mL的細(xì)胞懸液,取100μL至5mL培養(yǎng)管,加PI?Annexin各5μL,避光染色15min,再加緩沖液至400μL后上機(jī)檢測(cè),CellQuest軟件擬合數(shù)據(jù)?
1.3 統(tǒng)計(jì)學(xué)處理
所得數(shù)據(jù)用SPSS18.0軟件分析,結(jié)果用x±s表示,樣本均數(shù)的多重比較用單因素方差分析?以P0.05為差異有統(tǒng)計(jì)學(xué)意義?
2 結(jié) 果
2.1 分組實(shí)驗(yàn)結(jié)果 細(xì)胞增殖抑制率0%?25%?50%?75%所對(duì)應(yīng)的 ABZ濃度分別為:(0.00±0.00)μmol/L?(0.99±0.11)μmol/L?(5.73±0.65)μmol/L?(33.15±3.94)μmol/L?因此對(duì)照組?IC25組?IC50組和IC75組所對(duì)應(yīng)的ABZ作用濃度分別為:0.0?1.0?5.5?35.0μmol/L,再按 ABZ作用時(shí)間將各組分為12?24?36h3個(gè)亞組?
2.2 HK?PK?LDH活性檢測(cè)結(jié)果 HK?PK?LDH 活性檢測(cè)結(jié)果見表2?ABZ抑制HK活性有濃度依賴性和時(shí)間依賴性?IC75組ABZ抑制PK活性有時(shí)間依賴性?ABZ抑制LDH活性呈濃度和時(shí)間依賴性?
表2 各組HK?PK?LDH相對(duì)活性檢測(cè)結(jié)果(±s)
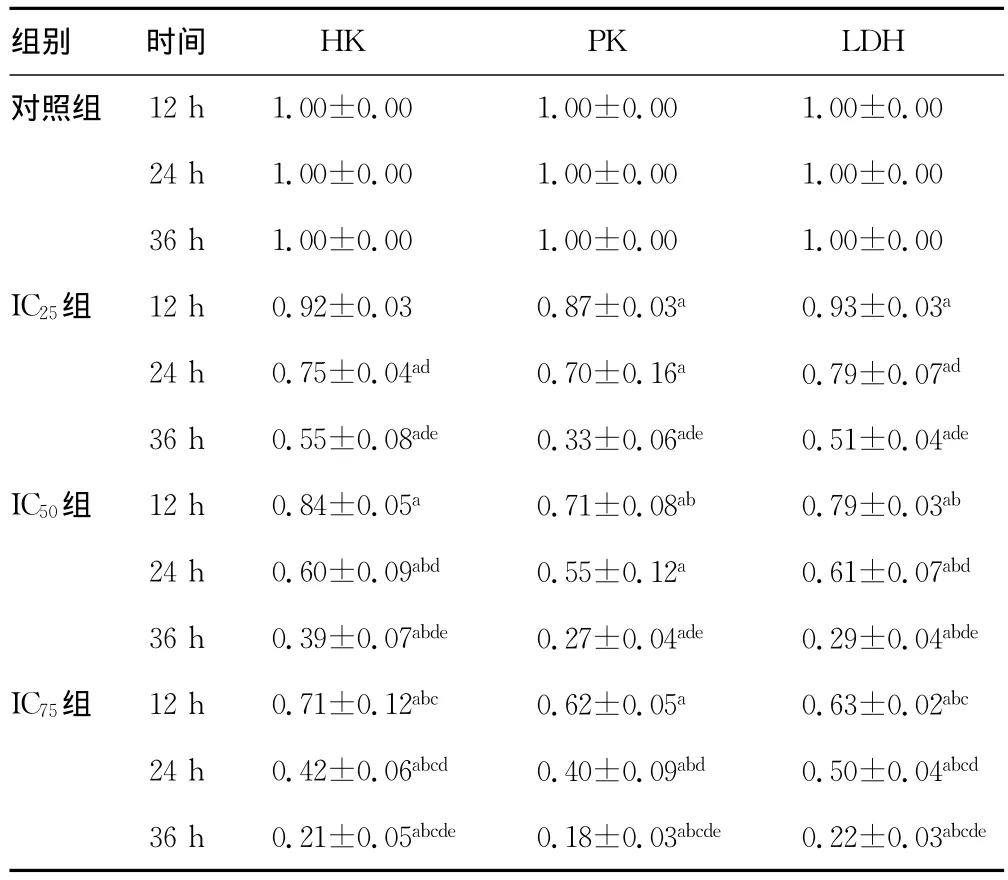
表2 各組HK?PK?LDH相對(duì)活性檢測(cè)結(jié)果(±s)
組別 時(shí)間HK PK LDH對(duì)照組12h 1.00±0.00 1.00±0.00 1.00±0.00 24h 1.00±0.00 1.00±0.00 1.00±0.00 36h 1.00±0.00 1.00±0.00 1.00±0.00 IC25組 12h 0.92±0.03 0.87±0.03a 0.93±0.03a 24h 0.75±0.04ad 0.70±0.16a 0.79±0.07ad 36h 0.55±0.08ade 0.33±0.06ade 0.51±0.04ade IC50組 12h 0.84±0.05a 0.71±0.08ab 0.79±0.03ab 24h 0.60±0.09abd 0.55±0.12a 0.61±0.07abd 36h 0.39±0.07abde 0.27±0.04ade 0.29±0.04abde IC75組 12h 0.71±0.12abc 0.62±0.05a 0.63±0.02abc 24h 0.42±0.06abcd 0.40±0.09abd 0.50±0.04abcd 36h 0.21±0.05abcde 0.18±0.03abcde 0.22±0.03abcde
a:P0.05,與同時(shí)間對(duì)照組比較;b:P0.05,與同時(shí)間IC25組比較;c:P0.05,與同時(shí)間IC50組比較;d:P0.05,與同組內(nèi)12h時(shí)比較;e:P0.05,與同組內(nèi)24h時(shí)比較?
表3 各組細(xì)胞不同時(shí)間Akt?Myc mRNA表達(dá)結(jié)果(±s)
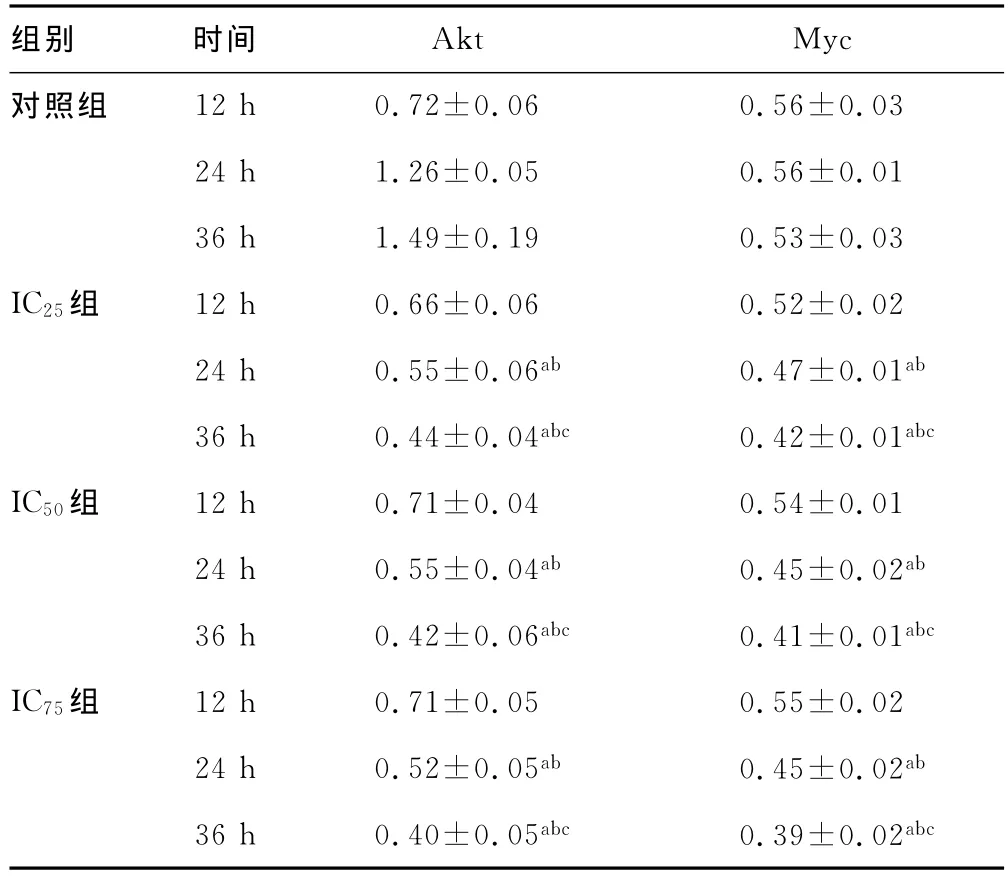
表3 各組細(xì)胞不同時(shí)間Akt?Myc mRNA表達(dá)結(jié)果(±s)
組別 時(shí)間 Akt Myc對(duì)照組12h 0.72±0.06 0.56±0.03 24h 1.26±0.05 0.56±0.01 36h 1.49±0.19 0.53±0.03 IC25組 12h 0.66±0.06 0.52±0.02 24h 0.55±0.06ab 0.47±0.01ab 36h 0.44±0.04abc 0.42±0.01abc IC50組 12h 0.71±0.04 0.54±0.01 24h 0.55±0.04ab 0.45±0.02ab 36h 0.42±0.06abc 0.41±0.01abc IC75組 12h 0.71±0.05 0.55±0.02 24h 0.52±0.05ab 0.45±0.02ab 36h 0.40±0.05abc 0.39±0.02abc
2.3 Akt和Myc mRNA表達(dá)結(jié)果 作用12h,ABZ不能明顯下調(diào)Akt?Myc mRNA表達(dá),作用時(shí)間延長(zhǎng),兩種基因表達(dá)水平均明顯下降,即ABZ下調(diào)作用有時(shí)間依賴性,見表3,圖1?2?

圖1 各組細(xì)胞不同時(shí)間Akt mRNA的PCR凝膠電泳圖
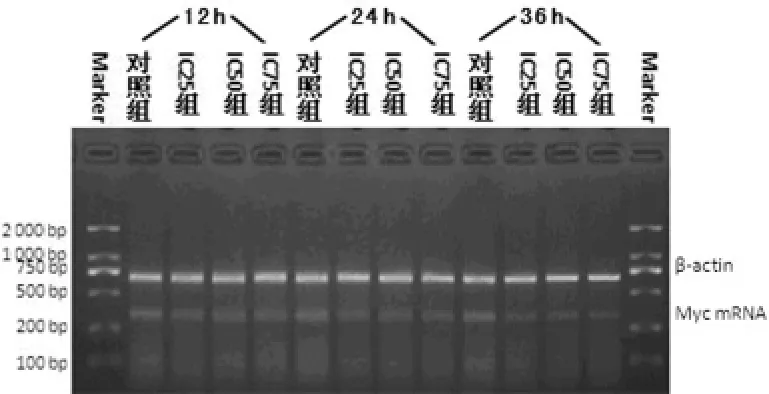
圖2 各組細(xì)胞不同時(shí)間Myc mRNA的PCR凝膠電泳圖
2.4 各組細(xì)胞不同時(shí)間細(xì)胞周期和細(xì)胞凋亡率比較 各組細(xì)胞不同時(shí)間細(xì)胞周期分布見表4,細(xì)胞凋亡率比較見表5?
表4 各組細(xì)胞不同時(shí)間細(xì)胞周期分布(±s,%)

表4 各組細(xì)胞不同時(shí)間細(xì)胞周期分布(±s,%)
a:P0.05,與同時(shí)間對(duì)照組比較;b:P0.05,與同組內(nèi)12h時(shí)比較;c:P0.05,與同組內(nèi)24h時(shí)比較?
組別 時(shí)間 G1期 G2期 S期對(duì)照組12h 93.68±1.39 4.81±1.04 1.50±0.46 24h 89.62±1.47 2.36±0.65 8.02±1.08 36h 62.48±1.08 12.48±0.60 25.38±0.74 IC25組 12h 65.01±1.58a 12.46±1.19a 22.53±0.44a 24h 52.98±1.80ab 14.27±1.00a 32.75±1.25ab 36h 12.03±1.18abc 53.09±2.92abc 34.87±2.92abc IC50組 12h 64.97±2.21a 13.50±0.93a 21.53±1.60a 24h 54.65±1.17ab 14.78±0.79a 31.41±1.08ab 36h 11.37±0.92abc 54.51±1.38abc 34.12±2.00a IC75組 12h 65.67±1.42a 13.46±1.23a 20.87±2.62a 24h 54.00±1.06ab 14.06±0.66a 31.94±1.68a 36h 10.38±1.06abc 54.52±2.15abc 35.10±3.18ab
表5 各組細(xì)胞不同時(shí)間細(xì)胞凋亡率比較(±s,%)

表5 各組細(xì)胞不同時(shí)間細(xì)胞凋亡率比較(±s,%)
分組 時(shí)間 晚期凋亡細(xì)胞比例 正常細(xì)胞比例 早期凋亡細(xì)胞比例對(duì)照組12h 1.28±0.18 94.39±0.69 1.95±0.10 24h 0.58±0.11 94.87±0.80 2.61±0.25 36h 1.53±0.33 93.23±1.23 2.54±0.37 IC25組 12h 3.52±0.44 85.39±1.64a 5.71±0.86a 24h 5.46±0.45ad 78.19±1.84ad 9.74±1.25ad 36h 10.27±1.37ade 72.30±1.71ade 9.80±1.10ad IC50組 12h 8.71±0.92ab 77.78±1.31ab 7.78±0.38ab
續(xù)表5 各組細(xì)胞不同時(shí)間細(xì)胞凋亡率比較(±s,%)
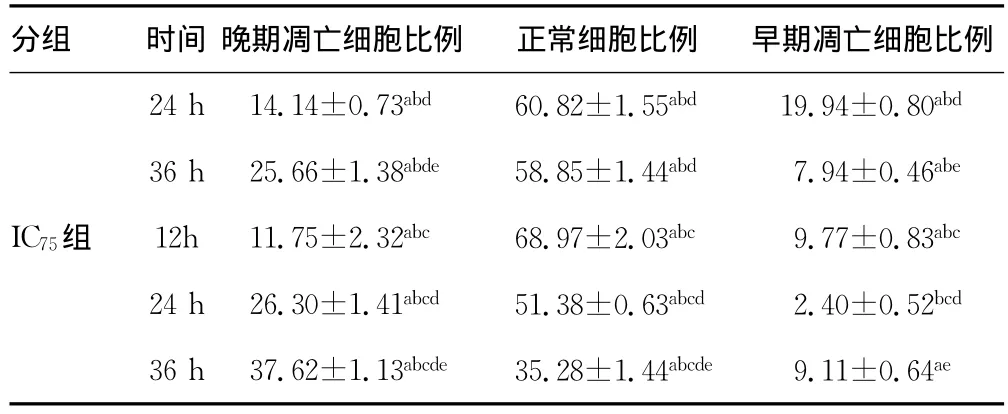
續(xù)表5 各組細(xì)胞不同時(shí)間細(xì)胞凋亡率比較(±s,%)
a:P0.05,與同時(shí)間對(duì)照組比較;b:P0.05,與同時(shí)間IC25組比較;c:P0.05,與同時(shí)間IC50組比較;d:P0.05,與同組內(nèi)12h時(shí)比較;e:P0.05,與同組內(nèi)24h時(shí)比較?
分組 時(shí)間 晚期凋亡細(xì)胞比例 正常細(xì)胞比例 早期凋亡細(xì)胞比例24h14.14±0.73abd 60.82±1.55abd 19.94±0.80abd 36h25.66±1.38abde 58.85±1.44abd 7.94±0.46abe IC75組 12h11.75±2.32abc 68.97±2.03abc 9.77±0.83abc 24h26.30±1.41abcd 51.38±0.63abcd 2.40±0.52bcd 36h37.62±1.13abcde 35.28±1.44abcde 9.11±0.64ae
3 討 論
大部分學(xué)者認(rèn)為腫瘤細(xì)胞活躍的糖酵解代謝與腫瘤細(xì)胞線粒體功能障礙[8],酶譜改變,尤其是糖酵解關(guān)鍵酶活性增加和同工酶譜改變有關(guān)?HK是腫瘤細(xì)胞糖酵解的限速酶,它在腫瘤細(xì)胞中活性明顯增加,從而保證腫瘤細(xì)胞生長(zhǎng)和增殖所需要的能量及物質(zhì)[9]?PK是糖酵解的第2個(gè)關(guān)鍵酶,它的活性不依賴于氧含量,它與ATP凈生成增加有關(guān),從而保證了腫瘤細(xì)胞在低氧環(huán)境中也能生存[10]?LDH催化糖酵解產(chǎn)物變?yōu)槿樗?酸化微環(huán)境,有利于腫瘤生存與生長(zhǎng)[11]?另外,在糖酵解過程中,Akt基因和 Myc基因也發(fā)揮了重要作用?Akt基因刺激腫瘤細(xì)胞選擇有氧糖酵解,且使腫瘤細(xì)胞依賴糖酵解生存和生長(zhǎng)[12]?Myc基因能夠直接上調(diào)GLUT-1和LDH的表達(dá),促進(jìn)葡萄糖攝取和加強(qiáng)葡萄糖轉(zhuǎn)化為乳酸[13]?
肺癌的發(fā)病率和病死率極高[14],耐藥是臨床面臨的難題?糖酵解與腫瘤惡性程度及耐藥相關(guān)[15-16]?抑制糖酵解,阻斷能量來源,可殺死腫瘤細(xì)胞[17-20]?
本實(shí)驗(yàn)中,A549/DDP細(xì)胞PK和LDH活性非常高,糖酵解相當(dāng)活躍,這可能與其高度惡性及耐藥有關(guān)?ABZ作用后,HK?PK?LDH活性明顯降低,Akt?Myc基因的表達(dá)明顯下調(diào),細(xì)胞周期阻滯在G2和S期,凋亡明顯?
ABZ作為安全有效且價(jià)格低廉的臨床用藥,抗腫瘤的作用機(jī)制廣泛?微管蛋白是ABZ的主要靶分子,但通過抑制微管蛋白聚合誘導(dǎo)腫瘤細(xì)胞凋亡,非ABZ抗腫瘤的惟一機(jī)制[21]?通過本實(shí)驗(yàn),可以看出,ABZ對(duì)耐藥腫瘤細(xì)胞仍具有較強(qiáng)的抗腫瘤活性,可能與它抑制糖酵解,切斷腫瘤的能量來源有關(guān)?作為一種經(jīng)濟(jì)?有效?低毒的臨床用藥,在腫瘤基礎(chǔ)研究中,發(fā)現(xiàn)了ABZ抑制腫瘤細(xì)胞糖酵解,誘導(dǎo)腫瘤細(xì)胞凋亡的新用途,希望經(jīng)過深入研究后,能使ABZ運(yùn)用到臨床腫瘤治療中,給肺癌患者帶來福音?
[1] Hsu PP,Sabatini DM.Cancer cell metabolism:warburg and beyond[J].Cell,2008,134(5):703-707.
[2] Luo W,Semenza GL.Pyruvate kinase M2regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1in cancer cells[J].Oncotarget,2011,2(7):551-556.
[3] Robey RB,Hay N.Is Akt the“warburg kinase”?——Akt-energy metabolism interactions and oncogenesis[J].Semin Cancer Biol,2009,19(1):25-31.
[4] Dang CV.Rethinking the warburg effect with Myc micromanaging glutamine metabolism[J].Cancer Res,2010,70(3):859-862.
[5] Chu SW,Badar S,Morris DL,et al.Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22human ovarian cancer cells by albendazole[J].Anticancer Res,2009,29(10):3791-3796.
[6] Pourgholami MH,Cai ZY,Wang L,et al.Inhibition of cell proliferation,vascular endothelial growth factor and tumor growth by albendazole[J].Cancer Invest,2009,27(2):171-177.
[7] Pourgholami MH,Cai ZY,Badar S,et al.Potent inhibition of tumoral hypoxia-inducible factor 1alpaha by albendazole[J].BMC Cancer,2010,10(7):143-148.
[8] Wu M,Neilson A,Swift AL,et al.Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells[J].Am J Physiol Cell Physiol,2007,292(1):C125-136.
[9] Marín-Hernández A,Rodríguez-Enríquez S,Vital-González PA,et al.Determining and understanding the control of glycolysis in fast-growth tumorcells.Flux control by an over-expressed but strongly product-inhibited hexokinase[J].FEBS J,2006,273(9):1975-1988.
[10]Mazurek S,Boschek CB,Hugo F,et al.Pyruvate kinase type M2and its role in tumor growth and spreading[J].Semin Cancer Biol,2005,15(4):300-308.
[11]Fantin VR,St-Pierre J,Leder P.Attenuation of LDH-A expression uncovers a link between glycolysis,mitochondrial physiology,and tumor maintenance[J].Cancer Cell,2006,9(6):425-434.
[12]Elstrom RL,Bauer DE,Buzzai M,et al.Akt Stimulates Aerobic Glycolysis in Cancer Cells[J].Cancer Res,2004,64(11):3892-3899.
[13]Osthus RC,Shim H,Kim S,et al.Deregulation of glucose transporter 1and glycolytic gene expression by c-My[J].J Biol Chem,2000,275(29):21797-21800.
[14]Siegel R,Ward E,Brawley O,et al.Cancer statistics,2011:the impact of eliminating socioeconomic and racial disparities on premature cancer deaths[J].CA Cancer J Clin,2011,61(4):212-236.
[15]Sattler UG,Mueller-Klieser W.The anti-oxidant capacity of tumour glycolysis[J].Int J Radiat Biol,2009,85(11):963-971.
[16]Milane L,Duan Z,Amiji M.Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multidrug resistant tumor model in nude mice using hypoxic pre-conditioning[J].Cancer Cell Int,2011,11(4):3-12.
[17]Gatenby RA,Gillies RJ.Glycolysis in cancer:apotential target for therapy[J].Int J Biochem Cell Biol,2007,39(7/8):1358-1366.
[18]Kim W,Yoon JH,Jeong JM,et al.Apoptosis-inducing antitumor efficacy of hexokinaseⅡinhibitor in hepatocellular carcinoma[J].Mol Cancer Ther,2007,6(9):2554-2562.
[19]Le A,Cooper CR,Gouw AM,et al.Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression[J].Proc Natl Acad Sci USA,2010,107(5):2037-2042.
[20]Priebe A,Tan L,Wahl H,et al.Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells[J].Gynecol Oncol,2011,122(2):389-395.
[21]Sebastian M.Review of catumaxomab in the treatment of malignant ascites[J].Cancer Manag Res,2010,2:283-286.
Effects of albendazole on glycolysis,cell cycle and cell apoptosis of cisplatin-resistant lung cancer cells
ObjectiveTo investigate effect of albendazole(ABZ)on glycolysis,cell cycle and cell apoptosis of human cisplatin-resistant lung cancer cells(A549/DDP cells).MethodsEffect of ABZ on proliferation of A549/DDP cells was detected by methyl thiazolyl tetrazolium(MTT)colorimetry,then according to inhibition concentrations(IC)of 0%,25%,50%,75%to devide cells into 4 groups-control group,IC25group,IC50group,IC75group,moreover,based on ABZ action time to invide every group into 12,24and 36hsub-groups.At the setting points of action time,activitie of hexokinase(HK),pyruvate kinase(PK),dehydrogenase(LDH)were detected by colorimetry and ELIASA,expression of Akt and Myc mRNA were detected by RT-PCR,cell cycle and cell apoptosis were detected by flow cytometry(FCM).ResultsABZ inhibited proliferation of A549/DDP cells in a dose-dependent manner,IC0,IC25,IC50and IC75respectively were(0.00±0.00)μmol/L,(0.99±0.11)μmol/L,(5.73±0.65)μmol/L,(33.15±3.94)μmol/L.The activity of LDH,HK and PK of test groups decreased,expression of Akt mRNA and Myc mRNA was downregulated.Cell cycle was blocked,apoptosis was obvious.ConclusionABZ can inhibit activity of glycolytic enzymes and expression of glycolysis-related genes,block cell cycle and induce apoptosis in vitro.
albendazole;glycolysis;cell cycle;apoptosis;tumor cells,cultured
10.3969/j.issn.1671-8348.2012.18.009
A
1671-8348(2012)18-1811-04
△通訊作者,E-mail:nescafe_1985@126.com?
2011-12-07
2012-02-22)
?論 著?

