Osimertinib induced adverse cardiac events: a case report
Shang-Xin LU, Yun-Li XING,?, Ye MIAO, Xiao-Jie ZHANG, Hong-Wei LI?
1.Department of Geriatrics, Beijing Friendship Hospital, Capital Medical University, Beijing, China; 2.Radiology Department, Beijing Friendship Hospital, Capital Medical University, Beijing, China; 3.Department of Cardiology, Beijing Friendship Hospital, Capital Medical University, Yongan Road, Beijing, China
Osimertinib is a third-generation epidermal growth factor receptor (EGFR)—tyrosine kinase inhibitors (TKI) selective for both EGFR-TKI sensitizing and T790M resistance mutations.It is also recommended as firstline therapy for patients with non-small-cell lung cancer (NSCLC) who have targetable EGFR mutations.[1]Recently, some emerging evidence revealed the significant cardiac toxicity induced by osimertinib.However, osimertinib-induced dilated cardiomyopathy and congestive heart failure has never been reported in China.Here, we report a rare case of dilated cardiomyopathy with heart failure exacerbation which developed during osimertinib treatment.
A 74-year old male was diagnosed with stage IVb adenocarcinoma of the lung harboring an EGFR deletion mutation in exon 19.He started on first-generation EGFR-TKI gefitinib 250 mg once daily orally.Six months later, he experienced the T790M EGFR mutation, and began osimertinib 80mg afterwards.Before starting gefitinib, he had a normal electrocardiograph (ECG) (Figure 1A), and transthoracic ultrasound cardiography (UCG) with left ventricular ejection fractions (LVEF) of 65%.
Five months after osimertinib administration, the patient was admitted to our hospital with dyspnea and bilateral lower limb edema.The N-terminal probrain natriuretic peptide (NT-proBNP) was significantly elevated to 35000 pg/mL, while the creatine kinase myocardial band isozymes were normal.The ECG indicated sinus tachycardia with normal QTc(Figure 1B).Furthermore, transthoracic UCG showed cardiomegaly and severe hypokinesis (LVEDD 65mm, LVEF 27%).The cardiac magnetic resonance imaging subsequently confirmed severe left ventricular systolic dysfunction with no late gadolinium enhancement to suggest focal fibrosis and no evidence of myocarditis (Figure 2).Based on these findings, the patient was diagnosed with dilated cardiomyopathy and congestive heart failure.Osimertinib-associated cardiotoxicity was suspected and discontinued its use.
Two days after admission, the patient complained of palpitation, and an supraventricular tachycardia of 165 beats/min was evident on ECG (Figure 1C).We administered intravenous amiodarone.The ECG revealed a markedly prolonged QT interval of 496 msec (QTc 606 ms) (Figure 1D), and tremendous AST/ALT elevation was found.The amiodarone was discontinued.The patient was prescribed Sacubitril/valsartan, metoprolol, spironolactone,furosemide, and ivabradine.At 1 month, 2 months,and 4 months after osimertinib discontinuation, his LVEF was improved to 35%, 41%, and 40%, respectively, LVEDD was reduced to 61 mm, 59 mm, and 59 mm, accordingly.The NT-proBNP decreased to 195 pg/mL eventually.Moreover, the AST/ALT level returned to normal over a couple of days.He did not take any other treatment for lung cancer and died of cancer progression and pulmonary infection 6 months later.
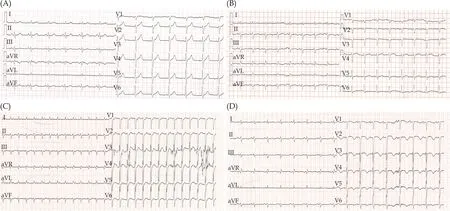
Figure 1 The ECGs of the patient during hospitalization.(A): Normal ECG before osimertinib treatment; (B): ECG on admission showed sinus tachycardia; (C): ECG before amiodarone administration showed supraventricular tachycardia with a heart rate of 165 beats/min; (D): ECG after amiodarone administration showed sinus rhythm with QT prolongation.ECG: electrocardiogram.
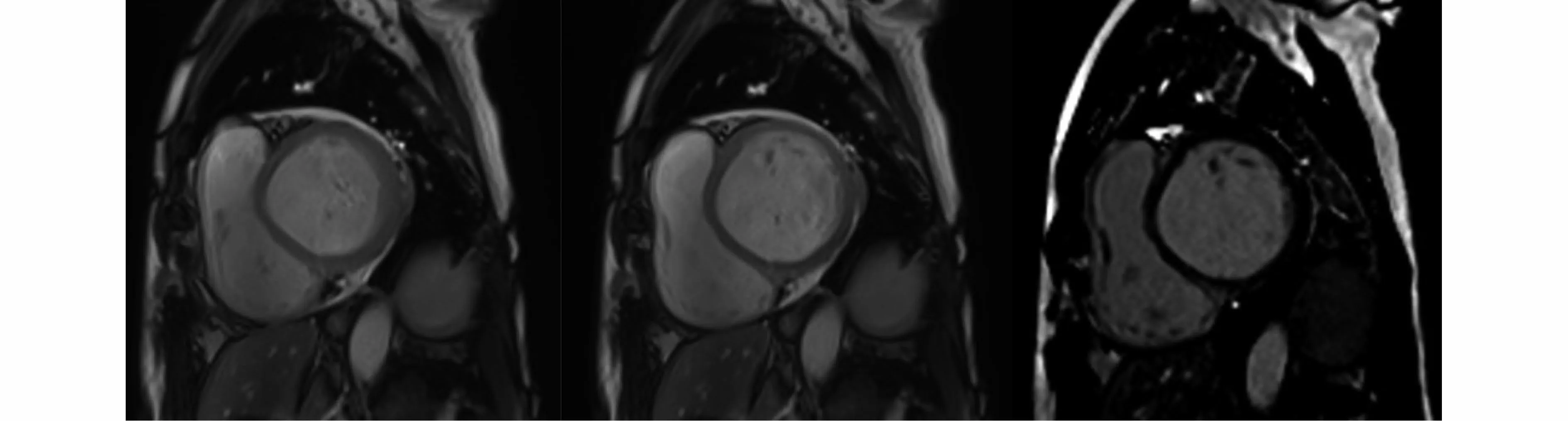
Figure 2 Cardiac magnetic resonance imaging revealed severe left ventricular systolic dysfunction with no late gadolinium enhancement.
Osimertinib is standard-of-care therapy for patients with previously untreated metastatic non–small cell lung cancer with epidermal growth factor receptor mutations.Recently, some emerging evidence revealed the significant cardiac toxicity induced by osimertinib.In a Japanese retrospective cohort enrolled 123 patients treated with osimertinib, the incidence of cardiac adverse events was 4.9%.The cardiac adverse events included one case of acute myocardial infarction, three cases of heart failure, and two cases of valvular heart disease.[2]An observational study based on the U.S.Food and Drug Administration Adverse Events Reporting System described osimertinib as responsible for nearly 6.1% of cardiac adverse events, including cardiac failure (2.3%),atrial fibrillation (1.2%), QT prolongation (1.3%),myocardial infarction (0.7%) and pericardial effusion (0.6%).[3]The study also revealed that osimertinib was associated with a higher risk of heart failure [odds ratio (OR) = 2.2, 95% confidence interval (CI): 1.5–3.2], atrial fibrillation (OR = 2.1, 95%CI:1.3–3.5) and QT prolongation (OR = 6.6, 95%CI:3.4–12.8) compared to the other EGFR TKIs.[3]Our patient presented with shortness of breath after osimertinib initiation.Thus, we suspected that Osimertinib, rather than that gefitinib more likely caused the cardiac toxicity of this patient.Several cases of osimertinib induced cardiotoxicity have been reported since 2017 (Table 1).[2,4–14]These patients experienced cardiac enlargement, LVEF reduction, QT prolongation, or sudden TdP.Among them, a patient experienced heart failure only 12 days after osimertinib administration.Almost all reported cases demonstrated the development of cardiac adverse events at a daily dose of 80 mg of osimertinib.In our case, the patient appeared cardiomegaly, LVEF reduction, atrial tachycardia, and QT prolongation (combined with amiodarone) five months after initiating osimertinib at a daily dose of 80 mg.
The underlying etiology of cardiotoxicity in human EGFR-targeted therapies is currently unclear.Although osimertinib is highly specific for EGFR, it has been observed that osimertinib inhibited the human epidermal growth factor receptor 2 (HER2)more strongly than erlotinib or afatinib in the mouse model.HER2 is expressed in multiple organs, including cardiomyocyte membranes, and is critical to normal cardiac physiology.[15]It has been confirmed that HER2- knockout mice have a 50% reduction in cardiac contractility with severe dilated cardiomyopathy.[16]Thus, we suspected that osimertinib inhibits HER2, potentially causing heart dysfunction and cardiomyopathy such as trastuzumab.The cardiac toxicities might be reversible when cessation of osimertinib if the mechanistic is similar to that observed with other HER2 inhibitor targeted therapy.[17]Among the reported patients, 15 of 24 patients had improved LVEF after discontinuing osimertinib.In our case, the patients’ cardiac structure and function were improved but not completely reversed after osimertinib discontinuation.We suspect there may be some potential mechanisms for the cardiotoxicity of osimertinib that result in irreversible myocardial damage.
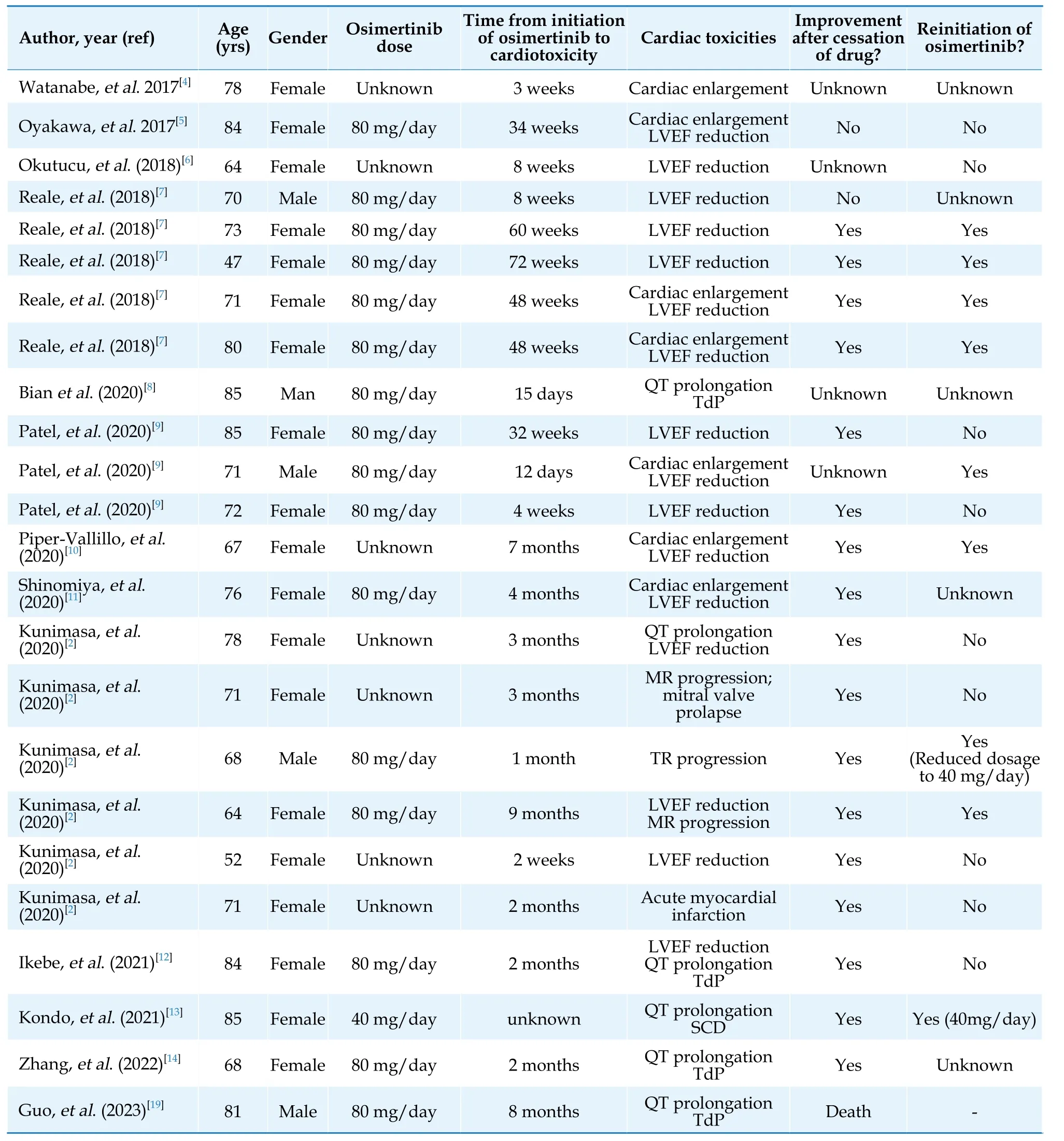
Table 1 Clinical characteristic of reported patients with cardiotoxicity due to osimertinib.
Notably, few patients underwent serial ECG or UCG before and after osimertinib administration,especially those without underlying heart diseases.Our patient didn’t perform an ECG or UCG after receiving EGFR-TKI treatment until he was suspected of heart failure.In the Japanese cohort, among the 123 NSCLC patients treated with osimertinib, there were only 72 patients who underwent serial ECG and 36 who underwent UCG both before and after osimertinib treatment.[2]This indicated the low awareness regarding cardiotoxicity induced by osimertinib.The cardiotoxicity of anticancer treatments for lung cancer seemed underestimated because of the poor prognosis of advanced lung cancer.According to the American Cancer Society, the 5-year survival rates for non-small cell lung cancer is reached 25%,[18]and the rate will be higher since the application of the novel anticancer therapy.Thus,we believe much more cardiac adverse events will be observed in the future.To improve the quality of life and increase the survival of patients with cancer,the comprehensive management of a multidisciplinary team is exceptionally urgent.
In conclusion, osimertinib-induced dilated cardiomyopathy and congestive heart failure has never been reported in China, especially for the elderly adenocarcinoma of the lung.Cardiac adverse events due to osimertinib is obviously lack of awareness,which have high rate of morbidity and mortality.For its HER2 inhibitor and weak inhibitor of the cardiac potassium ion channel, Osimertinib could induce cardiac dysfunction and QT prolongation.Physicians should be more cautious when administering medication potentially cardiotoxic to patients undertaking osimertinib.Routine surveillance of ECG and UCG for cardiac adverse events is also necessary during osimertinib treatment.The guideline or expert consensus for the diagnosis and treatment of osimertinib related cardiotoxicity is quite urgent.
DISCLOSURE
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The patients provided their written informed consent to participate in this study.Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Funding
This work was supported by National Science and Technology Innovation 2030 of China (2021ZD0111000).
 Journal of Geriatric Cardiology2023年9期
Journal of Geriatric Cardiology2023年9期
- Journal of Geriatric Cardiology的其它文章
- Chinese guideline for lipid management (2023): a new guideline rich in domestic elements for controlling dyslipidemia
- Atrial fibrillation and dementia: not just a coincidence
- Unfamiliar waveforms spanning from the ST to TP segments only observed in certain limb leads of the standard 12-lead electrocardiogram due to Aslanger’s sign
- Predictive value of bleeding risk scores in elderly patients with atrial fibrillation and oral anticoagulation
- Association of cumulative resting heart rate exposure with rapid renal function decline: a prospective cohort study with 27,564 older adults
- App-based assessment of memory functions in patients after transfemoral aortic valve replacement
