Association of cumulative resting heart rate exposure with rapid renal function decline: a prospective cohort study with 27,564 older adults
Xi JIANG, Xian SHAO, Xing LI, Pu-Fei BAI, Hong-Yan LIU, Jia-Mian CHEN,Wei-Xi WU, Zhuang CUI, Fang HOU, Chun-Lan LU, Sai-Jun ZHOU,?, Pei YU,?
1.NHC Key Laboratory of Hormones and Development, Chu Hsien-I Memorial Hospital and Tianjin Institute of Endocrinology, Tianjin Medical University, Tianjin, China; 2.Tianjin Key Laboratory of Metabolic Diseases, Tianjin Medical University, Tianjin, China; 3.Ordos Center Hospital, Ordos, Inner Mongolia, Sudu Street, Kangbashi District, Ordos City, China; 4.Department of Epidemiology and Health Statistics, Tianjin Medical University, Heping District, Tianjin,China; 5.Community Health Service Center, Jiefang Road, Tanggu Street, Binhai New District, Tianjin, China
ABSTRACT OBJECTIVE To evaluate the prospective association between cumulative resting heart rate (cumRHR) and rapid renal function decline (RRFD) in a cohort of individuals aged 60 and older.
Chronic kidney disease (CKD) has emerged as a significant global public health concern, affecting approximately 8%-16%of the population.[1,2]In China, the prevalence of CKD among adults was estimated to be around 8.2%,posing a considerable socioeconomic burden in China.[3]Therefore, the identification of clinically accessible risk factors assumes paramount importance in the early detection and primary prevention of progressive kidney disease.
Resting heart rate (RHR) is a straightforward and non-invasive indicator.However, it is influenced by various factors such as gender, race, age, smoking,alcohol consumption, and exercise,[4]which has led to the underestimation of its clinical significance.RHR serves as an indicator of the sympathetic nervous system (SNS), and an increased RHR is a manifestation of sympathetic activation.[5]The activation of the SNS frequently transpires under ischemic circumstances and induces modifications in the reninangiotensin-aldosterone system (RAAS) and the“heart-renal” axis.[6,7]Several studies indicated that chronic and persistent sympathetic nervous system activation may persist during both the early and terminal stages of CKD, with sympathetic activation potentially increasing as renal function declines.[8]Therefore, it is imperative that extensive populationbased cohort studies be conducted to ascertain the association between the heart rate and kidney.
The conventional range for resting heart rate in adults is typically considered to be between 60 and 100 beats/min, although this consensus among experts has yet to be scientifically substantiated.Several recent studies have demonstrated a significant association between an elevated RHR and the degradation of renal function in patients with hypertension,[9,10]diabetes,[11-13]heart failure,[14]cardiovascular disease[15]and the general population,[16,17]despite the RHR being within the conventional normal range.However, inconsistent observations have also been reported.[18,19]As a result, the association between RHR and the deterioration of renal function in elderly individuals remains uncertain due to the conflicting results of the aforementioned studies.However, most studies focused on the initial measurement of RHR taken at singular time points, without taking into account the cumulative effect over an extended period.Given that the decline in renal function often occurs gradually and that the level of RHR could be influenced by various factors, a single measurement does not accurately reflect the longterm state of sympathetic activation and could not be deemed a reliably predict the outcome.In contrast, cumulative exposure accounts for both the intensity and duration of the exposure, providing a more comprehensive assessment.[20]The Kailuan cohort study has demonstrated that a higher cumulative resting heart rate (cumRHR) was associated with an increased risk of all-cause mortality in the general population.[21]Yu,et al.[22]have reported that an increase in cumRHR was independently associated with a higher risk of stroke.However, the association between cumRHR and rapid renal function decline (RRFD) in the elderly remains inadequately characterized.Our study aims to explore the association between cumRHR and RRFD in a large cohort of elderly population from northern China, using a clinical epidemiological approach.The results of this investigation will broaden research on risk factors for declining renal function in the elderly and offer valuable insights into the prevention of CKD.
METHODS
Study Design and Participants
The Tianjin Chronic Kidney Disease Cohort was the source of data for this study (Supplementary Material Part I).The study participants were recruited from various primary communities in Binhai New Area, Tianjin, China, and were provided with complimentary physical examinations on an annual basis.Trained medical personnel conducted these examinations to obtain information on the demographic characteristics, blood biochemistry,medical history, medication usage and lifestyle habits.The data were uploaded to the Tianjin Community Health Service Information System.
The present study included individuals aged 60 and above who underwent physical examinations annually from 2014 to 2017 and had an estimated glomerular filtration rate (eGFR) of ≥ 60 mL/min per 1.73 m2.The exclusion criteria included: (1) incomplete data on RHR and eGFR; (2) individuals with arrhythmic conditions such as atrial fibrillation/flutter or those who wore a pacemaker; (3) selfreport CKD or other kidney diseases; and (4) individuals who did not undergo the annual examinations from January 1, 2017 to December 31, 2020.Ultimately, the analysis included 27,564 individuals.
Measurement of Cumulative Resting Heart Rate
The cumRHR is expressed in units of beats per minute per year ([(beats/min) * year]).The calculation formula is presented as follows:[21]
The RHR values, denoted as RHR2014, RHR2015,RHR2016and RHR2017, were acquired from physical examinations conducted in the years 2014, 2015,2016 and 2017, respectively.The time intervals between two consecutive examinations, expressed in years, were represented as time2014–2015, time2015–2016,and time2016–2017.
Furthermore, given the relationship between the time interval and the cumRHR, a time-weighted cumRHR was computed.
RHR variability was defined as the fluctuation in RHR measurements across successive health assessments during the follow-up period, and was quantified using the coefficient of variation (CV).
Measurement of Covariates
The study collected data on baseline variables,encompassing demographic characteristics (sex and age), physical examination (height, weight, waist circumference (WC), body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP)), white blood cell count (WBC), hemoglobin (HGB), platelet (PLT), total cholesterol (TC), triglycerides (TG), fasting blood glucose (FBG), aspartate transaminase (AST), alanine transaminase (ALT),total bilirubin (Tbil), and serum creatinine (Cr)).Additionally, the study also recorded the history of diseases (hypertension, cardiovascular and cerebrovascular diseases, and diabetes), medications (anti-hypertensive, antiplatelet, and lipid-lowering drugs),and lifestyle habits (alcohol consumption, exercise,and smoking).The specialist physician completed the questionnaire.
Definition of Outcomes
The primary outcome was RRFD, defined as an annualized decline in eGFR of 5 mL/min per 1.73 m2or greater.[23]The annual rate of decline in eGFR was determined through the least squares method of linear regression.The secondary outcome was progression to CKD, defined as an annualized decline in eGFR of ≥ 5 mL/min per 1.73 m2and < 60 mL/min per 1.73 m2at the final visit.The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI).[24]
Statistical Analysis
The baseline characteristics of the study participants were described based on the cumRHR quartile.The indicators of continuous normal distribution were described by the mean (standard deviation, SD) and compared by ANOVA test.The indicators of continuous non-normal distribution were described as medians (25th-75th), and the Kruskal-Wallis test was used.The Chi-squared test was used for the categorical variables expressed as counts.Additionally, a logistic regression model was used to estimate the association between cum-RHR (with the first quartile serving as the reference group) as a categorical variable or continuous variable (per 1-SD) and the risk of RRFD.Odds ratios(ORs) and 95% confidence intervals (CIs) were calculated.The present study also evaluated the association between RHR variability and RRFD.
To account for potential confounding covariates,four models were employed.Model 1: unadjusted.Model 2: adjusted for age and sex.Model 3: additional adjustments were made for BMI, WC, FBG,SBP, WBC, HGB, PLT, ALT, AST, TC, TG, Tbil,baseline eGFR, history of diseases (hypertension,cardiovascular and cerebrovascular diseases, and diabetes), medications (anti-hypertensive, antiplatelet, and lipid-lowering drugs), and lifestyle habits(alcohol consumption, exercise, and smoking)above model 2.Model 4: to further exclude the effect of baseline RHR, additional adjustments were made for baseline RHR above model 3.
To evaluate the association between cumRHR as a continuous variable and RFFD, a restricted spline regression model was employed, utilizing four knots positioned at the 5th, 35th, 65th, and 95thpercentiles of the cumRHR distribution.Furthermore,the association between cumRHR and RRFD were investigated for the following variables by stratified and interaction analyses: age (< 75vs.≥ 75 years), gender (male and female), BMI (< 24vs.≥24 kg/m2), hypertension (novs.yes), diabetes (novs.yes), smoking status (novs.yes), exercise status (novs.yes), drinking status (novs.yes), and the use of beta-blockers (novs.yes).
The receiver operating characteristic curve (ROC)and area under the curve (AUC) of the model containing cumRHR or baseline RHR resided were calculated to compare the predictive value.The sensitivity analysis excluded participants with a history of cardiovascular or cerebrovascular disease, as well as those taking anti-hypertensive medications or beta-blockers.All statistical analyses were conducted using R software (version 4.0.4).All statistical tests were two-sided, and aPvalue of < 0.05 was considered statistically significant.
RESULTS
Study Participants and Baseline Characteristics
Figure 1 shows a graphic overview of the study.Based on the exclusion criteria, 27,564 individuals were included.The study cohort was comprised of 15,056 (54.62%) males and 12,508 (45.38%) females,with a mean age of 70.62 (5.30) years.The average cumRHR was 207.73 (27.97) [(beats/min) * year].The baseline characteristics of participants stratified by the quartile of cumRHR are given in Table 1.Compared with the lowest quartile of cumRHR (Q1),the highest quartile of cumRHR (Q4) was more likely to be older, have higher FBG, RHR at baseline,TG and SBP, a higher percentage of self-reported hypertension, diabetes, and cerebrovascular disease; and were more likely to be non-smokers and non-drinkers (allP< 0.05, Table 1).
The Association between Cumulative Resting Heart Rate and Renal Outcomes
During a median follow-up of 3.2 years, a total of 4347 (15.77%) participants developed RRFD, and 1432 (5.20%) progressed to CKD.The number of events and incidence rates in each quartile of cum-RHR is presented in Table 2.The incidence rates of RRFD increased in the subjects belonging to the first, second, third, and fourth quartiles of cumRHR,exhibiting rates of 12.85%, 14.21%, 15.97%, and 20.06%, respectively.
Compared with the lowest quartile, individuals with an increased cumRHR had a higher risk of RRFD(Pfor trend < 0.001 for all models).After adjusting for all potential confounders, the OR of the highest quartile was 1.44 (1.28-1.61),Pfor trend < 0.001.After adjusting for all potential variables, the OR(95% CI) of the cumRHR with a 1-SD per change of 27.97 was 1.17 (1.12-1.22),P< 0.001.Comparable findings were observed in relation to progression to CKD.The risk of progression to CKD increased by 24% (1.24 (1.16-1.33),P< 0.001) for each 1-SD (27.97)augmentation in cumRHR after full adjustment for risk factors.Furthermore, in the fully-adjusted model 4, the OR (95% CI) for the highest quartile group was 1.57 (1.31-1.89),Pfor trend < 0.001, compared to the lowest quartile group.
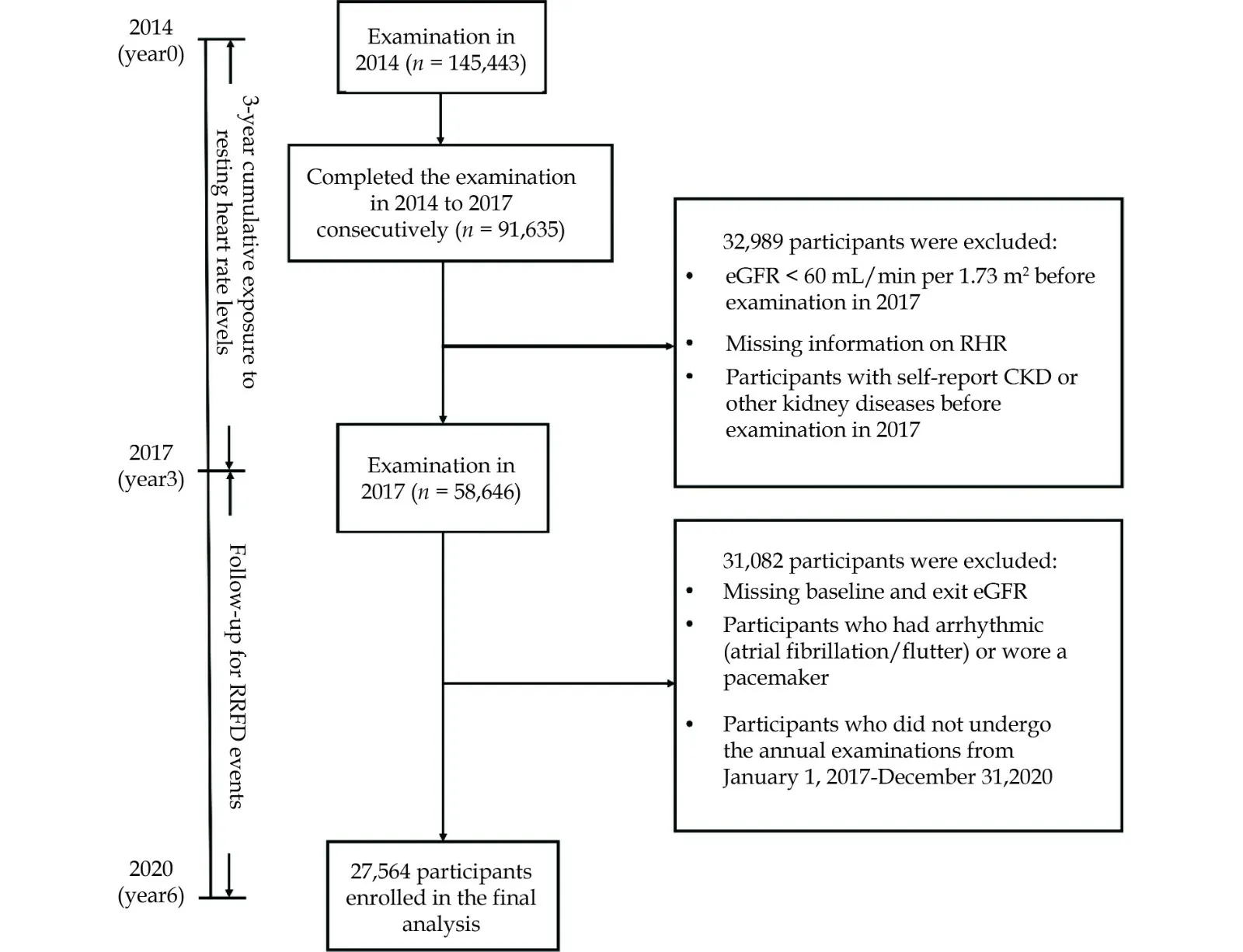
Figure 1 Flow chart of the participants.CKD: chronic kidney disease; RHR: resting heart rate.
Sensitivity Analysis
In the sensitivity analysis, the exclusion of patients who used beta-blockers (Supplemental Table 1), had a history of cardiovascular or cerebrovascular diseases (Supplemental Table 2), or used antihypertensive drugs (Supplemental Table 3) did not yield a significantly impact the association between cumRHR and RRFD, CKD.After full adjusting for confounders, the highest quartile group exhibited a increased risk of 45% to 53% for RRFD and a 64% to 70% increased risk of progression to CKD (allP<0.001).
The Association between Time-weighted cum-RHR, Baseline RHR, Variability of RHR and Renal Outcomes
Similar trends were also observed in the association between time-weighted cumRHR (Supplemental Table 4), baseline RHR (Supplemental Table 5) and renal outcomes.For time-weighted cumRHR,each 1-SD increase was associated with a 14% rise both in the risk of RRFD and progression to CKD(fully-adjusted model 4).For baseline cumRHR,each 1-SD increase was associated with a 9% rise both in the risk of RRFD and progression to CKD(fully-adjusted model 3).In Supplementary Table 6,it was observed that individuals in the highest quartile of RHR variability exhibited a greater risk of RRFD compared with the lowest quartile (OR (95%CI): 1.11 (1.00–1.22),Pfor trend = 0.028).
The Dose-response Association between cum-RHR and the Risk of Rapid Renal Function Decline
After adjusting for all confounders (model 4), The dose-response association between cumRHR and the risk of RRFD was determined using restricted spline regression.As depict in Figure 2, the analysis revealed a positive linear correlation between cum-RHR and the risk of RRFD (Pfor no-linear = 0.803).Notably, the threshold value was identified at 207[(beats/min) * years] as the reference (OR = 1).Furthermore, the risk of RRFD increased rapidly beyond this threshold.
The Supplementary Figure 1 illustrates the nonlinear relationship between cumRHR and the risk of progression to CKD.The graph depicts a significant increase in odds ratio for values exceeding 207(beats/min) * years, resulting in a J-shaped relationship (Pfor non-linear = 0.026).
In the fully adjusted model, ROC analysis demonstrated that the AUCs of cumRHR and baseline RHR were 0.720 and 0.718 for RRFD, and 0.693 and 0.688 for progression to CKD, respectively (Supplementary Figure 2).Significantly higher AUC values were observed for cumRHR compared to baseline RHR for both RRFD (P< 0.001) and progression to CKD (P= 0.002).As previously mentioned, cum-RHR exhibited superior predictive efficacy.
Stratified Analyses
The analysis was stratified based on several factors, including age, sex, BMI, hypertension, diabetes,smoking, exercise, and drinking statuses, as well as the use of beta-blockers.The results, depicted in Figure 3, revealed that individuals who were male, nonhypertensive, and non-exercising were at a greater risk of RRFD with the same level of exposure (Pinteraction values < 0.05).
DISCUSSION
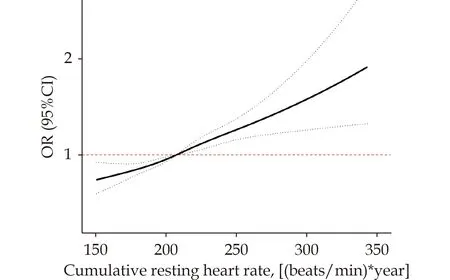
Figure 2 Odds ratio and 95% CI for cumRHR with rapid renal function decline by using restricted cubic spline regression with four knots (P for no-linear = 0.803).Adjusted for BMI, WC,FGB, SBP, WBC, HGB, PLT, ALT, AST, TC, TG, Tbil, eGFR at baseline, smoking, drinking, exercise, history of diabetes, history of hypertension, history of cerebrovascular disease, history of cardiovascular disease, use of beta-blockers, CCB, ACEI, ARB,statins, nitrates and baseline RHR.
In this prospective and longitudinal cohort study of older adults in Northern China, we observed that elevated cumRHR was significantly associated with the increased risk of RRFD and progression to CKD.This association remained significant even after further adjusting for baseline RHR and traditional confounders.Furthermore, the statistical significance of the association between cumRHR and RRFD persisted in sensitivity analyses that excluded patients who were taking β-blockers, had a history of cardiovascular disease, or were using antihypertensive medications.Additional metrics related to RHR,such as time-weighted cumRHR, baseline RHR, and RHR variability, were found to have an influence on renal outcomes, albeit with a lower degree of risk compared to cumRHR.Moreover, the ROC curves also showed that the cumRHR was superior to the RHR baseline and had better predictive performance for CKD.Additionally, we observed a positive linear relationship between cumRHR with RRFD, as well as a “J-shaped” relationship with progression to CKD.Moreover, the proposed dose-response relationship between RHR and renal outcomes suggests that it is optimal to consistently maintain RHR levels below 69 beats/min to promote renal health in Chinese individuals aged 60 and above.Additionally, individuals who were male, nonhypertensive, and non-exercising exhibited a higher susceptibility to RRFD with equivalent levels of exposure.In conclusion, the results indicated that the cumulative effect of persistently higher RHR could significantly impact on renal function among older adults, regardless of RHR measurements at a single time point.These results could provide valuable insights into the prevention of CKD in the elderly population.
This present study were consistent with several previous studies.For instance, the Atherosclerosis Risk in Communities (ARIC) Study[25]followed 13,241 adults aged 45-64 over 16 years and revealed that a significantly association between higher RHR with end-stage renal disease (ESRD) and CKD-related hospitalizations.Additionally, Peng,et al.[10]found that elevated RHR was associated with a high risk of microalbuminuria (MAU) in untreated patients and those with controlled hypertension.Similarly, a prospective study conducted in Japan[16]showed that a higher RHR was a risk factor for CKD in middle-aged or older subjects.B?hm,et al.[15]further substantiated this claim by highlighting RHR as a strong predictor of renal outcomes, including albuminuria, ESRD or doubling of creatinine.Similarly, this present study found a positive linear association between cumRHR and increased risk of RRFD.
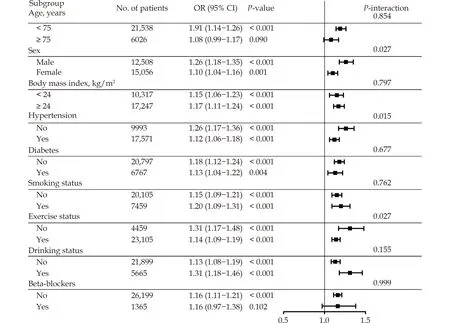
Figure 3 Stratified analyses for the association between change per SD in cumRHR and rapid renal function decline.Adjusted for BMI, WC, FGB, SBP, WBC, HGB, PLT, ALT, AST, TC, TG, Tbil, eGFR at baseline, smoking, drinking, exercise, history of diabetes, history of hypertension, history of cerebrovascular disease, history of cardiovascular disease, use of beta-blockers, CCB, ACEI, ARB, statins, nitrates and baseline RHR (excluding stratification factor); CI: Confidence interval; OR, Odds ratio.
Despite numerous prior investigations, the findings on the association between RHR and renal function have been inconclusive.Pfister,et al.[18]demonstrated no significant association between baseline RHR and MAU in a sample of 5,110 with coexisting diabetes and cardiovascular disease.However,the presence of these comorbidities may have confounded the true relationship between RHR and MAU in this study.Additionally, Miot,et al.[13]have reported that the association between RHR and CKD is contingent upon the presence of a history of cardiovascular disease in diabetic patients.Conversely, Bartáková,et al.[19]did not identify any predictive value of the RHR for the renal outcomes in a sample of 376 diabetic subjects.However, it is important to note that this study had a relatively small sample size, with a majority (77.9%) of the participants exhibiting diabetic kidney disease, 19.2%having a history of cardiovascular disease, and generally poor underlying physical condition.In the stratified analysis, the risk of RRFD was higher in the non-hypertensive group compared to the hypertensive group, despite the same exposure.Consistent with the results of this present study, a cross-sectional study[17]revealed a positive correlation between higher RHR and increased urinary albumin/creatinine ratio (UACR) in the non-hypertension group, while no significant association was observed in hypertensive subjects.Therefore, we posited that the association between RHR and renal function may be obscured by the presence of severe illnesses.At baseline, the present study selected older adults with an eGFR ≥ 60 mL/min per 1.73 m2,while excluding those with a history of cardiovascular or cerebrovascular diseases from sensitivity analyses to mitigate the effect of comorbid states.Despite this precaution, the results of the present study demonstrated that cumulative exposure to higher RHR was independently associated with a higher risk of RRFD.
Moreover, the Prospective Urban and Rural Epidemiology-China study[26]has concluded that an elevated baseline RHR was associated with an increased risk of cardiovascular mortality in the normotensive population, with the association being less pronounced in hypertensive patients.These results indicated that monitoring RHR should be a routine practice for normotensive individuals.
In the majority of studies, the RHR has been assessed solely at baseline.However, the RHR is highly susceptible to various factors resulting the potential for bias.A limited studies have showed a significant association between mean RHR and the risk of MAU in patients with type 2 diabetes.To our knowledge, there is a dearth of research that evaluates the association between cumRHR and the risk of RRFD in older adults.Furthermore, the present study revealed that cumRHR were significantly associated with risk of RRFD independent of baseline RHR.And the ROC analysis has indicated that cumRHR exhibited superior predictive performance, indicating its potential clinical utility in the prevention of CKD.Consequently, we have demonstrated that cumulative exposure to higher RHR was significantly associated with a higher risk of RRFD among older adults.The present study assessed the cumRHR over time, utilizing annual measurements that considered both the intensity and duration of RHR.This approach enhanced the reliability of the measurements and highlighted the potential impact of higher cumRHR on renal function decline.
However, the exact pathogenesis by which a higher RHR predicts renal function decline remains unclear.It is possible that a persistent elevation in RHR may indicate chronic and sustained sympathetic activation, which has been observed in individuals with chronic renal failure in previous studies.[27]Studies conducted on animals have demonstrated that the reduction in heart rate induced by ivabradine has a positive impact on endothelial function, atherosclerosis prevention, and oxidative stress reduction in mice.[28]Specifically, the elevated RHR increased the mechanical stress on the blood vessels, resulting in endothelial cell damage and increased penetration of inflammatory mediators.This, in turn, could mediate the production of MUA and further damage to kidney function.[29]In the geriatric population, the kidneys experience heightened mechanical stress as glomerular perfusion intensifies in response to elevated RHR resulting from diminished myogenic regulation intrinsic.[30]Consequently, augmented glomerular basement membrane permeability, inflammatory effects,[31]pro-atherosclerotic activity,[28]endothelial dysfunction,[29]and increased glomerular mechanical stress[30]have all been hypothesized to be contribute to the decline in renal function associated with high RHR.Notwithstanding, a dearth of studies that are directly pertinent to the elucidation of the specific impacts of cumRHR on renal function persists.The present study, regrettably, did not delve into the underlying mechanisms.Consequently, additional research aimed at elucidating the mechanisms is warranted.
The present study was the first prospective investigation to estimate the correlation between cum-RHR and the risk of RRFD, as evidenced by the literature search results.And annual assessments were performed to evaluate eGFR, and the renal outcome was defined by the trajectory of eGFR,which is less susceptible to short-term eGFR fluctuations.Consequently, our study offers a more precise evaluation of renal function progression over time.Additionally, the repeated measurement of the RHR enabled the calculation of cumulative exposure, which considered both the intensity and duration of exposure.This approach enabled a more accurate estimation of the long-term impact of RHR.Furthermore, a sensitivity analysis was conducted to demonstrate the robustness of the results.
There were some limitations, including the protracted progression of CKD and the potential bias due to the short follow-up period.Additionally, this study was restricted to participants aged 60 and above in Northern China, thus restricting its applicability to other populations.Nonetheless, the large sample size effectively reduced residual confounding caused by socioeconomic and lifestyle disparities.Furthermore, it is important to note that the present study was conducted as an observational analysis, thereby precluding the establishment of a direct causal relationship.Additionally, the final sample size of the study comprised 27,564 individuals out of an initial pool of 145,443 subjects, with the exclusion of those who were deemed ineligible or had significant missing data, which may have introduced selection bias.Despite the adjustment for a range of confounding variables, the potential for residual confounding cannot be entirely ruled out.One potential confounding factor is the impact of dietary habits on renal function.As a result, the details of dietary information during the follow-up will be enhanced.Furthermore, proteinuria and eGFR decline are two distinct yet overlapping manifestations in the progression of CKD, the present study focused on eGFR decline in the current study.Thereby, future studies with larger sample sizes and longer follow-ups are required.
In conclusion, it is recommended that the inclusion of cumRHR into primary prevention strategies for CKD in the elderly population, along with its utilization for risk stratification of CKD events.Furthermore, routine monitoring of RHR in the clinical setting is advised, with a specific focus on managing elevated RHR to delay the decline of renal function and provide substantial benefits in the prevention of CKD.The present study presents a feasible and measurable objective for the elderly demographic,while also promoting interdisciplinary cooperation among cardiologists and nephrologists, with the aim of advancing the strategy to prevent CKD in the elderly.
DISCLOSURE
Funding
This study was funded by Tianjin Science and Technology Major Special Project and Engineering Public Health Science and Technology Major Special Project (No.21ZXGWSY00100).Tianjin Natural Science Foundation Key Projects (22JCZDJC00590,21JCQNJC00460).Tianjin Key Medical Discipline(Specialty) Construct Project (No.TJYXZDXK-032A).The Science and technology talent project of Tianjin Health Commission (No.RC20175).The Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital (No.ZXY-ZDSYSZD-1).China International Medical Exchange Foundation Key Fund Project (No.Z-2017-26-1902).Tianjin Municipal Health Care Commission Scientific Research Fund Project (ZC20128).The funder was not for profit.
Competing Interests
None.
Availability of data and materials
The raw data are not available.However, the data are available from the corresponding author upon reasonable individual request.
Authors' contributions
XJ, XS and XL contributed equally to this study.XJ, XS prepared the figures and wrote the manuscript.XL wrote and revised the manuscript.PY designed this study; SJZ, HYL and JMC revised the manuscript.ZC reviewed and revised the statistical methods.WXW, FEM, PFB, FH, YRZ, CLL, HL acquired the data and revised the manuscript.All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The study was reviewed and approved by the ethics committee of Tianjin Chu Hsien-l Memorial Hospital.Verbal informed consent was obtained from each participant and was recorded by the physician who explained the study procedures.Written informed consent was waived because the data were anonymous and observational.The study was registered in the Chinese Clinical Trial Register with the identification number ChiCTR19000 23701 (2019/06/08).
Consent for Publication
Written informed consent for publication was obtained from all participants.All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgement
The authors thank the staff and participants of the study for their indispensable contributions.
 Journal of Geriatric Cardiology2023年9期
Journal of Geriatric Cardiology2023年9期
- Journal of Geriatric Cardiology的其它文章
- Chinese guideline for lipid management (2023): a new guideline rich in domestic elements for controlling dyslipidemia
- Osimertinib induced adverse cardiac events: a case report
- Atrial fibrillation and dementia: not just a coincidence
- Unfamiliar waveforms spanning from the ST to TP segments only observed in certain limb leads of the standard 12-lead electrocardiogram due to Aslanger’s sign
- Predictive value of bleeding risk scores in elderly patients with atrial fibrillation and oral anticoagulation
- App-based assessment of memory functions in patients after transfemoral aortic valve replacement
