Atrial fibrillation and dementia: not just a coincidence
Udit Choubey, Vasu Bansal?, Priyanshi Shah, FNU Anamika, Vasu Gupta Sweta Sahu,Miran Rezhan, Rohit Jain
1.Shyam Shah Medical College, Rewa, India; 2.Dayanand Medical College and Hospital, Ludhiana, Punjab, India;3.Narendra Modi Medical College, Ahmedabad, India; 4.University College of Medical Sciences, New Delhi, India;5.J.J.M.Medical College, Davangere, Karnataka, India; 6.Pennsylvania State University, State College, Pennsylvania,USA; 7.Penn State Milton S Hershey Medical Center, Hershey, Pennsylvania, USA
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia, affecting 3 to 6 million people in the US, with an increased incidence in women and elderly, with its prevalence estimated to increase from 5.1 million in 2000 to 15.9 million in 2050 in the US population.[1]Age, hypertension, heart failure, myocardial infarction (MI), genetic factors, and associated systemic inflammatory states are well-established risk factors for the development of AF.[2–6]Lifestyle factors including alcohol consumption, smoking, obesity, sedentary lifestyle, and vigorous physical activity have also been implicated to contribute to the development of AF.[2,7–9]Studies suggest that AF drives up medical expenditure.[10]The European Society of Cardiology, based on the duration, responsiveness to treatment, and therapeutic attitude of the patient and physician, classifies AF into 4 types;when AF occurs and terminates by itself or by intervention within 7 days of onset, it is called paroxysmal AF; if sustained for more than a week, with or without intervention, it is called persistent AF.When sustained for more than 12 months on a rhythm control strategy, it is called long standing persistent AF.When accepted by the patient and physician, and all attempts to restore or maintain normal sinus rhythm are abandoned, this is classified as permanent AF, which is a reflection of the therapeutic attitude.Dementia is defined as the progressive decline in cognition in the absence of any disturbances in consciousness, hampering the daily activities of an individual to a significant extent.It can result from various causes, including vascular, age-related, and neurodegenerative changes.[11]The most common primary dementia is Alzheimer’s disease (AD) seen in 60%–80% of patients, followed by vascular dementia (VaD) (15%–25%), Lewy body dementia(5%–10%), and frontotemporal dementia (5%–6%).Often, dementia can present as a combination of two or more of the above types, and as such, it is referred to as "mixed dementia".[12]Overlapping presentations with other disorders, along with insidious onset and progression, make the diagnosis of dementia a challenging task.[13]According to the World Health Organization, global dementia cases reach 55 million, with a financial burden of $1.3 trillion in 2019.The prevalence is expected to almost triple to 150 million by 2050.[14]The risk factors for dementia range from cardiovascular factors such as hypertension, obesity, smoking, and diabetes[15]to increased age and female sex.[3]Both AF and dementia show an increased incidence with aging,and are expected to rise in incidence and prevalence in the coming years.
AF increases the risk of strokes by 4–5 times, and strokes, along with cerebral hypoperfusion, microbleeds, and inflammatory pathways, are proven causes of dementia in the setting of AF.[16,17]While most studies show evidence of the development of cognitive impairment in the setting of AF,[16]the reverse is also true; it has been found that in the setting of AD, the myocardium of such patients also had similar beta-amyloid precursor deposits,[18]and showed diastolic dysfunction.Since diastolic dysfunction is a proven risk factor of AF,[19]these results suggest that the relationship between AF and AD is bidirectional.Taking the aforementioned into account, this article aims to review the AF-AD association and explore dementia development mechanisms in individuals with atrial fibrillation.
AF leads to dementia through cerebral hypoperfusion caused by beat-to-beat variability or reduced cardiac output due to lack of atrioventricular synchrony.[20,21]Anselmino,et al.[20]conducted a study onin-vitromodels of cerebrovascular circulation to assess cerebral blood flow variations which found greater flow variability in AF patients, despite similar mean flow rate compared to sinus rhythm.The Framingham Offspring Study linked decreased cardiac output to incident dementia and AD in 1,039 patients without prior stroke or cognitive decline.However, the mechanisms linking cardiac output and cerebral blood flow to cognitive decline and AD have not been deciphered completely.It was proposed by Bell,et al.[22]in their study that this decline may result from the accumulation of amyloid beta-peptides due to their impaired clearance across the blood-brain barrier.AF's most feared sequelae is cerebral infarction, and microinfarctions are also implicated in AF-related dementia development.[16]Gaita,et al.[23]found that small vessel disease-associated brain pathology serves as a key mechanism linking AF to cognitive decline.Their study revealed that AF patients with a lacunar infarct on magnetic resonance imaging (MRI) experienced a greater annual decline in digit symbol substitution and word fluency over 10 years of follow-up.In contrast, in patients with no evidence of subclinical cerebral ischemia on MRI, incident AF was not associated with cognitive decline.[23]Blood stasis can lead to clot formation and increase the risk of stroke and silent cerebral infarction.The subsequent dementia in stroke patients can be attributed to both necrosis and apoptosis.The infarcted core is necrotic, but there is incomplete activation of caspase-mediated apoptosis in the penumbra due to the failure to implement the pathway fully.[24]Hemodynamic alterations as well as vascular inflammation leaded to an accumulation of amyloid-beta protein and phosphorylation of tau proteins at the cellular level.[25]There are at least five sources of classical complement pathway activation in patients with AD, namely—highly insoluble deposits of abnormal proteins, Aβand tangles, and the exposed cellular by products of degradation, naked DNA, neurofilaments, and myelin fragments.They lead to an increase in the inflammatory markers, and AD has been linked strongly to the upregulation of many of these markers, such as IL-1, IL-6, and tumor necrosis factor alpha.[26]Circulating inflammatory cytokines like CRP and IL-6 have been studied to be associated with incident AF.[27]Therefore, if AF is associated with an inflammatory state, it is probable that patients suffering from AF are more susceptible to blood-brain barrier injury as well as deposition of amyloid, which, in turn, causes earlier cognitive decline and progression of dementia.[28]Ultimately, the actual mechanism by which AF patients suffer from cognitive decline and dementia is an under-researched field, and understanding the pathophysiologic processes involved will allow for better diagnostic and treatment procedures.
AF impacts patients variably, with 10%-40% patients being asymptomatic while others experience symptoms like fatigue, dyspnea, palpitations, chest pain, or dizziness.[29]The diagnosis is made using EKG.Additional tests include blood count, urea,electrolytes, thyroid function test and an echocardiogram can be done to identify valve problems, left atrial size and volume, and ventricular dysfunction.[30]Dementia often presents with memory loss as the primary symptom and also may exhibit language challenges, trouble navigating their surroundings,and alterations in personality and behavior.Medical history obtained from both the patient and a close family member or friend along with cognitive and neurological examinations, are crucial in determining the presence of dementia.The routine work-up also includes tests for vitamin B12, TSH, with MRI being the preferred brain imaging method to identify structural changes.The treatment involves a combination of non-pharmacologic approaches with cognitive, physical, and social activities, and pharmacologic approaches such as an acetylcholinesterase inhibitor for AD.The primary objectives involve mitigating distress stemming from cognitive impairments and associated manifestations like mood and behavioral changes, concurrently decelerating the progression of cognitive deterioration.[31]AF is associated with an increased risk of adverse brain imaging changes over time which show a higher incidence of white matter hyperintensities(WMI), and microbleeds.[16,32,33]The clinical significance of these findings still remains unclear, and there is currently no high-quality evidence to guide recommendations for brain imaging with AF, regardless of cognitive impairment.New onset neurologic issues should prompt urgent neuroimaging,but the interval at which cognitive testing and cerebral imaging should be repeated remains unknown.[16]The current treatment options for AF include drug therapy, catheter ablation, cryoballoon ablation, left atrial appendage closure, and the maze procedure.Drug therapy options include rhythm control, rate control, and anticoagulation for stroke prevention.It is also important to identify and address any reversible causes of AF.Antiarrhythmic drugs are commonly used to maintain sinus rhythm.For rate control therapy, targeting a resting heart rate of less than 110 beats per minute is recommended, with the use of β-blockers, non-dihydropyridine calcium channel blockers (ND-CCBs), digitalis, and amiodarone.[34]The impact of rate control medications on cognitive outcomes in AF is still unknown, but non-pharmacological rhythm management approaches reduce incident dementia and significantly improve Montreal Cognitive Assessment scores in the ablation groups.[16,35]The CHA2DS2-VASc and HAS-BLED scoring systems are utilized to guide anticoagulation therapy.Patients with a CHA2DS2-VASc score of 2 or more in men and 3 or more in women are recommended to receive oral anticoagulants.For those with lower scores, the risks and benefits of therapy should be carefully balanced.[36]Anticoagulant therapy has been proven to reduce the likelihood of dementia, particularly when patients take oral anticoagulants for over 75%of the time.This protective effect is most significant within the first year after diagnosis.[37]Direct-current cardioversion (DCC) is used in patients with heart failure or instability, who are unresponsive to pharmacological therapies.The role of multiple cardioversions on cerebral microemboli and cognitive outcomes is still unknown.A few small studies have demonstrated that successful electric cardioversion can improve cerebral blood flow.[38]However, it is important to note that electric cardioversion may also increase the risk of cerebral microemboli.Studies, such as the NOR-FIB2 study (clinicaltrials.gov; Unique identifier: NCT03816865), are being conducted to evaluate cognitive function and the incidence of new-onset silent cerebral infarcts after programmed direct-current cardioversion.
There is increasing evidence supporting the association between AF and cognitive impairment and dementia, even in the absence of prior stroke.The Intermountain Heart Collaborative Study, which followed a cohort of 37,025 patients, found that AF was independently associated with all types of dementia.In a post hoc analysis of the ONTARGET and TRANSCEND trials, AF was found to be associated with an increased risk of cognitive decline(HR = 1.14; 95% CI: 1.03–1.26), new-onset dementia(HR = 1.30; 95% CI: 1.14–1.49), loss of independence in daily activities (HR = 1.35; 95% CI: 1.19–1.54), and admission to long-term care facilities (HR= 1.53; 95% CI: 1.31–1.79.Chen and colleagues found that AF was linked to a higher cognitive decline and a greater risk of dementia, even after adjusting for cardiovascular risk factors (HR = 1.23;95% CI 1.04–1.45).[39]Meanwhile, Ott and colleagues had previously reported significant positive correlations between atrial fibrillation and both dementia and impaired cognitive function (age- and sex-adjusted odds ratios = 2.3 [95% CI: 1.4–3.7] and 1.7 [95% CI: 1.2–2.5]), respectively, in 1997.A summary of several significant studies can be found in Table 1.[39–42]
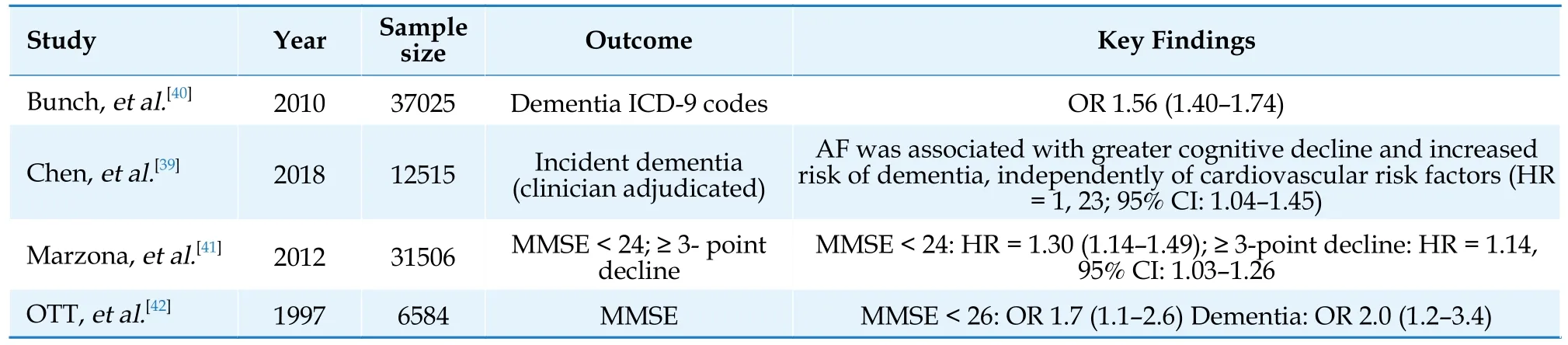
Table 1 Studies highlighting the association between AF and cognitive impairment/dementia.
AF and cognitive impairment are prevalent health concerns projected to escalate significantly in the coming years.Multiple observational studies have identified an association between AF and cognitive dysfunction.The mechanisms are diverse and include such as cerebral hypoperfusion, microinfarctions, and inflammation-related processes.Changes in blood flow and clot formation heighten stroke risk, while vascular inflammation and hemodynamic shifts contribute to the build-up of amyloid-beta and tau proteins.This increased risk of dementia is independent of manifest stroke.AF is also linked to increased adverse brain imaging changes.Treatment options for AF, such as non-pharmacological rhythm management, can reduce dementia incidence and improve cognitive scores.Anticoagulant therapy also lowers dementia risk, especially when taken consistently.Randomized clinical trials are essential to elucidate the connections between AF and cognitive decline, as well as to identify effective interventions for preserving cognitive function and forestalling or postponing dementia onset.
DISCLOSURES
This research received no specific grant from any funding agency in the public, commercial, or notfor-profit sectors.
 Journal of Geriatric Cardiology2023年9期
Journal of Geriatric Cardiology2023年9期
- Journal of Geriatric Cardiology的其它文章
- Chinese guideline for lipid management (2023): a new guideline rich in domestic elements for controlling dyslipidemia
- Osimertinib induced adverse cardiac events: a case report
- Unfamiliar waveforms spanning from the ST to TP segments only observed in certain limb leads of the standard 12-lead electrocardiogram due to Aslanger’s sign
- Predictive value of bleeding risk scores in elderly patients with atrial fibrillation and oral anticoagulation
- Association of cumulative resting heart rate exposure with rapid renal function decline: a prospective cohort study with 27,564 older adults
- App-based assessment of memory functions in patients after transfemoral aortic valve replacement
