Value of enhanced computed tomography in differentiating small mesenchymal tumours of the gastrointestinal from smooth muscle tumours
Wen-Jun Nie, Zhao Jing, Mo Hua
Abstract
Key Words: Smooth muscle tumour; Stomach; Ⅰntestines; Differentiation
INTRODUCTION
Gastric cancer is one of the most common malignancies and is among the top three leading causes of cancer-related deaths worldwide[1-11]. According to the Japanese Classification Criteria for Gastric Cancer, early gastric cancer is defined as a lesion in which tumour infiltration is limited to the mucosa or submucosa without consideration of lymph node metastasis (LNM)[12]. In recent years, with the development of endoscopic techniques, endoscopic submucosal dissection (ESD) has been widely used for the treatment of early gastric cancer without LNM, and the indications for ESD in early gastric cancer published by the Japan Gastric Cancer Association classify gastric cancer into differentiated and undifferentiated types[13]. Gastrointestinal mesenchymal tumours (GIMTs), as a mesenchymal-derived tumour with specific histological features, are mainly located in the gastrointestinal tract and abdominal cavity and have a certain chance of malignant transformation and are therefore often diagnosed and treated differently from gastric smooth muscle tumours in clinical practice[14-20].
Although previous clinical reports have shown that GIMTs are rare[21], recent epidemiological studies have shown that 10%-30% of patients with GIMTs have no obvious clinical symptoms, but 15%-50% of patients may have metastases to the liver and abdominal cavity once detected, missing the best time for treatment. Currently, the clinical diagnosis of GIMTs mainly relies on imaging and pathology; however, imaging methods such as ultrasound endoscopy and computed tomography (CT) are influenced by the operator’s experience and image quality, and cannot accurately determine the nature of the lesion. Besides, the pathological examination requires endoscopy or surgery to obtain the pathological tissue, which is invasive and painful for patients, and pathological examination is not real-time[22-27]. As a convenient and common clinical test, CT has been widely used in the diagnosis, efficacy and prognosis of clinical tumors, and can be used for the identification of GIMTs[28]. Herein, we retrospectively analysed the clinical data of patients (volunteers) who received treatment or health check-ups in our hospital in recent years and investigated the value of CT in the differential diagnosis of patients with gastric mesenchymal tumours and gastric smooth muscle tumours.
MATERIALS AND METHODS
General information
The clinical data of patients with gastric mesenchymal or gastric smooth muscle tumours who were treated in our hospital from May 2018 to April 2023 were retrospectively analyzed. Patients were divided into the gastric mesenchymal tumor group and the gastric smooth muscle tumor group respectively (n= 50 cases per group). Clinical data of 50 healthy volunteers who underwent physical examination in the same hospital during the same period were selected and included in the control group. The gastric mesenchymal tumor group included 24 males and 26 females, aged 38 to 72 years (mean age: 56.73 ± 7.46 years) and with a body mass index (BMI) of 18-24 kg/m2(mean BMI: 21.76 ± 2.21 kg/m2). The gastric smooth muscle tumor group included 22 males and 28 females, aged 36 to 71 years (mean age: 57.11 ± 7.18 years) and with a BMI of 18-24 kg/m2(mean BMI: 21.89 ± 2.14 kg/m2). The control group included 21 males and 29 females, aged 38-70 years (mean age: 55.82 ± 7.39 years) and with a BMI of 18-24 kg/m2(mean BMI: 21.76 ± 2.21 kg/m2). The differences between the three groups were not statistically significant (P> 0. 05) and were comparable. Confidentiality of all patient information was maintained in this study.
Inclusion criteria
Inclusion criteria including: (1) Patients with gastric mesenchymal tumour or gastric smooth muscle tumour, all confirmed by postoperative pathological histology, healthy volunteers with no significant abnormalities by gastric ultrasound; (2) 42-72 years old; and (3) Complete clinical data of patients (or volunteers).
Exclusion criteria
Exclusion criteria including: (1) Organic heart, liver, or kidney dysfunction; (2) Patients with combined cancer of other tissues or a history of radiotherapy; (3) Unable to participate in this study due to psychiatric illness or other reasons; (4) Combined coagulation disorders or autoimmune diseases; and (5) A history of gastrectomy.
Methodology
Approximately 5 mL of fasting venous blood was collected from all patients (or volunteers) during the preoperative examination, centrifuged and stored at -80 °C, and the serum levels of carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), CA-125 and cytokeratin 19 fragment antigen 21-1 (CYFRA21-1) were measured by electrochemiluminescence. The immunoassay was performed using relevant kits on a Beckman Coulter AU5800 fully automated biochemical analyzer (Beckman, United States).
Observation indicators
Serum levels of CEA, AFP, CA19-9, CA-125 and CYFRA21-1 levels were compared among the three groups. The value of CEA and CA19-9 in identifying gastric mesenchymal tumours was analysed using the receiver operating characteristic (ROC) curve. The ROC curves of CEA and CA19-9 in identifying gastric mesenchymal tumours were plotted separately based on the pathological results of the patients. The area of the lower curve for each measure was calculated, and the area of the lower curve > 0.5 indicated that the measure had diagnostic efficacy, and the closer it was to 1, the higher its diagnostic efficacy.
Statistical methods
All data were processed using SPSS 22. 0 statistical software and were expressed as mean ± standard deviation (mean ± SD) or percentages (%). Theχ2test was used to analyze categorical variables. One-way ANOVA was used to compare multiple groups. The ROC curve was used to analyze the value of CEA and CA19-9 in the diagnosis of gastric mesenchymal tumours. The Kappa test was used for consistency.
RESULTS
Gastric mesenchymal and smooth muscle tumours on plain gastroscopy and endoscopic ultrasound
Both intragastric mesenchymal tumours and smooth muscle tumours were found in the fundus and body of the stomach, with no statistically significant difference in the distribution of lesions (P= 0.32). The diameter of mesenchymal tumours was larger than that of smooth muscle tumours, and the difference was statistically significant (P< 0.05). Both mesenchymal and smooth muscle tumours were smooth, erosive, or ulcerated in surface morphology, with no statistically significant difference (P= 0.61). The intrinsic muscular layer and the mucosal muscular layer were the most common sites of origin for both mesenchymal tumours and smooth muscle tumours. The difference was not statistically significant (P= 1.0). At endoscopic ultrasound (EUS), mesenchymal tumours appeared as hypoechoic lesions and smooth muscle tumours appeared as hypoechoic and isoechoic lesions. In terms of echogenicity, the echogenic non-uniformity of mesenchymal tumours was more pronounced than that of smooth muscle tumours (P< 0.05) see Table 1.
Comparison of the levels of each tumour marker among the three groups
CEA levels varied among the three groups in the following order: The gastric mesenchymal tumour group > the control group > the gastric smooth muscle tumour group. CA19-9 levels varied among the three groups in the following order: The gastric mesenchymal group > the gastric smooth muscle group > the control group, the differences were statistically significant (P< 0. 05) see Table 2.
ROC curve analysis of CEA and CA19-9 levels for the identification of gastric mesenchymal tumours
The area under the curve (AUC) for the identification of gastric mesenchymal tumours by CEA and CA19-9 was 0. 879 and 0. 782, respectively (Table 3). The ROC curves for the identification of gastric mesenchymal tumours by CEA and CA19-9 are shown in Figure 1.
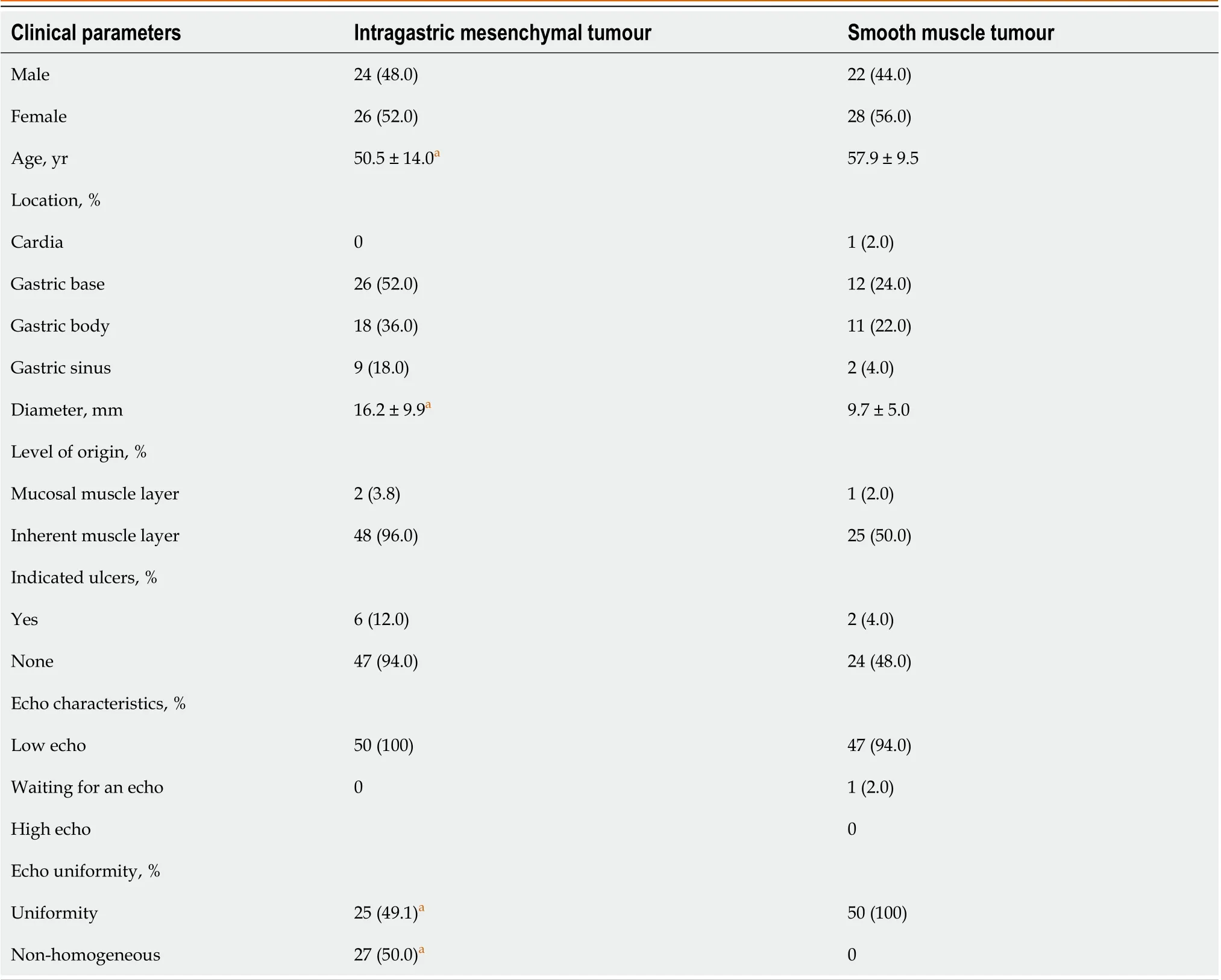
Table 1 Gastric mesenchymal and smooth muscle tumours on plain gastroscopy and endoscopic ultrasound
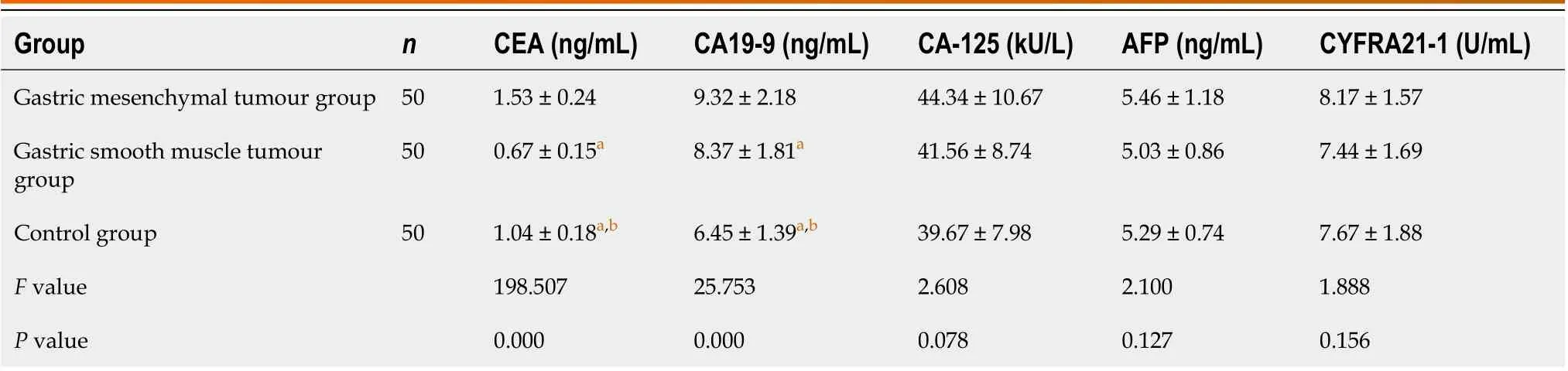
Table 2 Comparison of tumour marker levels among the three groups (mean ± SD)
DISCUSSION
The treatment methods for GIMTs have been rapidly changing with the development of medical treatment technology in recent years, and there are various methods commonly used for differential diagnosis in clinical practice[29-35]. However, the sensitivity of single tumour markers is low, and there is a certain degree of underdiagnosis; thus, combined detection of tumour markers is necessary for the diagnosis of GIMTs[36]. The present study analysed the expression of CEA, AFP, CA19-9, CA-125 and CYFRA21-1 in patients with gastric mesenchymal and smooth muscle tumours to provide a reference for clinical diagnosis. The results showed that CEA levels varied among the three groups in the following order: The gastric mesenchymal tumour group > control group > gastric smooth muscle tumour group, and CA19-9 levels varied in the following order: The gastric mesenchymal tumour group > gastric smooth muscle group > control group, suggesting that CEA and CA19-9 were differentially expressed in patients (or volunteers) with different gastric lesions. Tumour markers are chemical substances that reflect the presence of tumours and are synthesised and released by tumour cells during tumourigenesis and proliferation or are important for the host’s responsiveness to cancer[37]. Their formation or change in expression in the blood can indicate the nature of the tumour and thus help the clinician to understand their role in tumour histogenesis, cell differentiation and cell function. Common tumour markers can be classified into embryonic antigens, glycoproteins, kinins, enzymes and oncogene products according to their composition, with CEA being a protein and CA19-9 and CA-125 being glycoantigens[38].

Table 3 Receiver operating characteristic curve analysis of carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the diagnosis of gastric mesenchymal tumour
Mesenchymal and smooth muscle tumours are the predominant mesenchymal-derived tumours of the gastrointestinal tract and the most common cause of submucosal lesions. Mesenchymal tumours originate from the interstitial cells of Cajal or mesenchymal stem cells in the gastrointestinal tract. It is currently thought that mutations in the C-kit or plateletderived growth factor receptor A gene activation are important causes of mesenchymal tumours. Mesenchymal tumours are characterised by dynamic non-directional differentiation and potential malignancy, and even mesenchymal tumours with very low malignant potential may metastasise[39].
Pathological examination and immunohistochemistry are the gold standard for differentiating mesenchymal tumours from smooth muscle tumours. EUS is a non-invasive method that can assist in the diagnosis of the nature of the lesion and the choice of treatment by observing the level of origin, size and echogenicity of the lesion. The identification of the differences by comparing EUS features of intragastric mesenchymal tumours with those of smooth muscle tumours may spare patients with smooth muscle tumours from undergoing resection, while smaller diameter mesenchymal tumours may be diagnosed early, and intervention may be possible.
The data from this study show that mesenchymal tumours are more common than smooth leiomyosarcomas in the augmentation of the stomach, which is consistent with the finding in national studies[40-43]. Both appear as round or oval submucosal masses on plain endoscopy, with some visible surface erosions or ulcers, making differential diagnosis difficult. On EUS, mesenchymal tumours are usually of intramucosal origin, with a few originating in the mucosal layer, and appear as round or oval masses, which may be homogeneously hypoechoic, heterogeneously echogenic or hyperechoic with hyperechogenicity. A careful analysis of the endoscopic features of the two tumours revealed no statistically significant differences in the distribution of lesions within the stomach or in the level of origin. In terms of tumour size, the diameter of the mesenchymal tumour was larger than that of the smooth muscle tumour, and the difference was statistically significant. Surface ulceration is an important criterion for differentiating benign and malignant GIMTs, and is often used as a criterion for differentiating mesenchymal tumours from smooth muscle tumours[44]. However, our study found no statistically significant difference between mesenchymal and smooth muscle tumours. The difference in internal echogenicity was statistically significant, particularly in the presence of hyperechoic hyperechogenicity, which was only observed in mesenchymal tumours and not in smooth muscle tumours. The greater the difference in the density between the two sides of the interface and the faster the speed of sound, the higher the acoustic impedance and the echogram signal.
Therefore, the number of cells, their tight arrangement, the presence of liquefied necrosis, calcification and the amount of fibrous cell content are the factors that make up the ultrasound interface and the pathological basis of the ultrasound image in submucosal tumours. As previously discussed, mesenchymal tumours are richer in cells, more variable in their morphology and arrangement and more likely to undergo secondary changes than smooth muscle tumours, leading to differences in their echogenic characteristics.
CONCLUSION
In summary, EUS can accurately localise lesion characteristics, and there are significant differences in the echogenic characteristics of intragastric mesenchymal and smooth muscle tumours. Since early metastases can occur in GIMTs of less than 2 cm in diameter, endoscopic resection is recommended for the definitive diagnosis and simultaneous treatment or closer follow-up of intrinsic mesenchymal tumours with a clear echogenic border and less than 2 cm in diameter. The diagnosis of smooth muscle tumour is more likely for lesions with homogeneous echogenicity, well-defined borders and an intrinsic muscle layer of less than 2 cm in diameter, and patients can be advised to follow up. Overall, EUS can provide a strong basis for differentiating mesenchymal and smooth muscle tumours of less than 2 cm in diameter in the stomach and for clinical decision-making.
ARTICLE HIGHLIGHTS

FOOTNOTES
Author contributions:Nie WJ and Hua M contributed equally to this work; Hua M designed the study; Zhao J contributed to the analysis of the manuscript; Nie WJ and Hua M were involved in the data and writing of this article; and all authors have read and approved the final manuscript.
Institutional review board statement:The study was reviewed and approved by the (Changzhou Geriatric Hospital Affiliated to Soochow University, Changzhou No. 7 People’s Hospital Radiology Department) Institutional Review Board.
Informed consent statement:All study participants and their legal guardians provide informed written consent before the study recruitment.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Wen-Jun Nie 0009-0005-7627-2621; Mo Hua 0009-0006-4489-3391.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
