Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
Dong Jiang, Yi Qian, Bi-Bo Tan, Xia-Ling Zhu, Hui Dong, Rong Qian
Abstract
Key Words: Hepatocellular carcinoma; Microvascular invasion; Conventional ultrasound; Shear wave elastography
INTRODUCTION
Hepatocellular carcinoma (HCC), as the third leading cause of cancer-related deaths worldwide and the second leading cause in China, represents a major health concern throughout the world, especially in China[1]. Microvascular invasion (MVI), the invasion of cancer cells into vascular lumen (including microbranches of the portal vein, hepatic artery, and lymphatic vessels), is a very important predictor of poor prognosis, postoperative recurrence, metastasis, and poor survival rate in patients with HCC after surgical resection or liver transplantation[2-5]. Accurate preoperative prediction of MVI in HCC would offer valuable insights to guide therapeutic strategy.
Some studies have demonstrated that preoperative imaging including contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging (MRI) may help in the diagnosis of MVI[6-10]. Related studies have focused on contrast-enhanced ultrasound (CEUS)[11-14]. The study by Qinet al[11] showed that a deep learning model based on CEUS could accurately predict MVI in HCC and help identify high-risk patients. The study by Liet al[12] showed that features such as non-single nodules in the postvascular phase of preoperative Sonazoid CEUS was an independent risk factor for MVI in HCC.
Ultrasound elastography, especially quantitative shear wave elastography (SWE), plays an important role in hepatic imaging[15-17]. Compared with CEUS, contrast-enhanced computed tomography, or contrast-enhanced MRI, SWE has the advantage of the absence of contrast agent allergy and is generally less expensive and less time-consuming than other methods, making it a more practical option for many medical facilities. Zhanget al[7] reported that the stiffness of HCCs based on MR-elastography was an independent risk factor for MVI and may be useful for the preoperative prediction of MVI. However, only a few studies have focused on the value of SWE in the prediction of MVI[18].
In the present study, we explored the value of conventional ultrasound features and SWE in the preoperative prediction of MVI in HCC.
MATERIALS AND METHODS
Study design
This prospective study was approved by the Ethics Committee of Eastern Hepatobiliary Surgery Hospital (Approval No. EHBHKY2021-K-017). Each patient provided written informed consent before the ultrasound examinations.
Patients
Inpatients admitted to the Hepatobiliary Surgery Department at our hospital between November 2021 and July 2022 were included in this study if they met the following criteria: (1) Single liver tumor resected surgically and diagnosed as HCC pathologically; and (2) Ultrasound examinations including SWE successfully performed within 3 d before surgery. The exclusion criteria were: (1) A history of hepatectomy or abdominal malignant tumors; (2) A history of radiotherapy, chemotherapy, and other treatments before surgery; and (3) No definite pathological diagnosis of MVI.
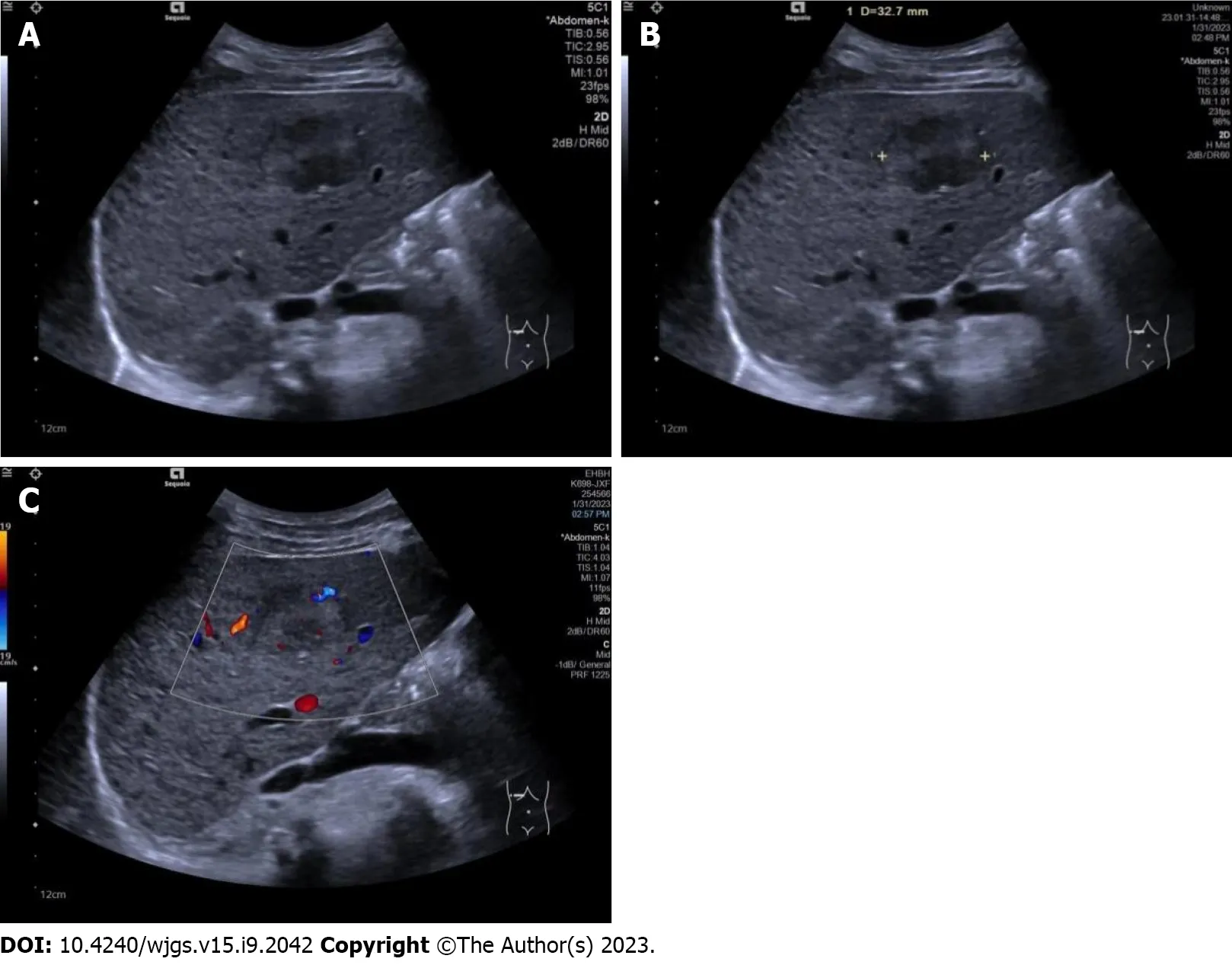
Figure 1 Conventional ultrasound images in patients with a hepatic tumor pathologically diagnosed as hepatocellular carcinoma. A:Conventional ultrasound showed the tumor to be hypoechoic with an unclear boundary and cirrhosis in the surrounding liver tissue; B: Conventional ultrasound measured the maximal diameter of the tumor to be 32.7 mm; C: Color Doppler flow imaging showed one vessel in the tumor, and microvascular invasion was recorded as mild.
Conventional ultrasound examination
All ultrasound examinations were performed within 3 d before surgery, using an Acuson Sequoia diagnostic ultrasound machine and a transabdominal 5C1 probe (Siemens Medical Solutions, Mountain View, CA, United States). The patients were instructed to fast for a minimum of 8 h prior to the examinations.
All conventional ultrasound examinations were performed by a single ultrasound physician with 15 years of experience in liver ultrasound. Maximal tumor diameter, echogenicity (hypo- if the tumor was mainly hypoechoic compared with surrounding liver tissue, or hyper- if the tumor was mainly hyperechoic compared with surrounding liver tissue), boundary (clear or unclear), surrounding hepatic tissue (liver cirrhosis, fatty liver, or normal liver), and tumor vascularity (none if no vessels were seen in the tumor using color Doppler flow imaging, rich if more than three vessels were seen, or mild if one to three vessels were seen) were observed and recorded (Figure 1).
SWE examination
The same ultrasound equipment and probe were used for the SWE examination. Another ultrasound physician with 5 years of experience in both liver ultrasound and ultrasound elastography performed all the SWE examinations. During the examination, the patients were instructed to lie flat and breathe gently. They were instructed to hold their breath for a few seconds if necessary. The targeted tumor was shown on the screen before activation of the SWE mode. The whole tumor (or partial if the tumor was too large) and some surrounding hepatic tissues were included in the region of interest (ROI). Quality mode was used to evaluate the SWE image quality; green in the ROI means image quality is excellent and the results are reliable. In the velocity mode, the speed bar was set as 0.5-4.0 m/s. One circular ROI (diameter of 3 mm) was placed at the stiffest part of the tumor; another ROI of the same size was placed at the periphery of the tumor. The maximum values within the two ROIs were recorded as Emax for both the tumor and the periphery of the tumor, respectively. These values were then used for further analysis (Figure 2).
Pathological MVI examination
One pathologist, who had 20 years of experience in HCC pathology and was blind to all clinical data, reviewed the specimens. The extent of MVI was graded as MVI-negative (no MVI detected), mild MVI (MVI ≤ 5, occurring in the proximal non-neoplastic adjacent hepatic tissues), and severe MVI (MVI > 5, in non-neoplastic adjacent hepatic tissues, Figure 3)[2].

Figure 2 Shear wave elastography images in a pathologically confirmed hepatocellular carcinoma. A: Quality mode showed hepatocellular carcinoma (HCC) as green, indicating that the 2D-shear wave elastography image was of good quality; B: Velocity mode showed that the HCC was stiffer than the surrounding liver tissue. The speed barb was set as 0.5-4.0 m/s; C: Two circular regions of interest (with a diameter of 3 mm) were placed at the stiffest part of the HCC and at the periphery of the HCC; D: Maximal values of the two regions of interest were recorded as maximal elasticity.
Statistical analysis
For statistical analysis, SPSS version 24.0 software (IBM Corporation, Armonk, NY, United States) was used.P< 0.05 was considered statistically significant. Measurement data with normal distribution were reported as mean ± standard deviation and compared using the independent samplet-test; otherwise, data were reported as median (25th-75thpercentile) and compared using the Mann-Whitney test. The cutoff point of Emax was calculated by a receiver operating characteristic curve.
Enumerative data were described as numbers and percentage and compared using the Pearsonc2test. Bivariate logistic regression analysis was performed to determine independent predictors of MVI from the ultrasound characteristics that showed statistical significance using univariate analysis.
RESULTS
Patients and MVI results
One hundred and eighty-eight patients with single HCCs (156 males and 32 females; aged 24-76 years, mean age 56.25 years ± 9.93 years) were enrolled in this study, including 86 who were MVI-negative and 102 who were MVI-positive (54 had mild MVI and 48 had severe MVI). Sex and age were not significantly different between the MVI-negative patients (70 males and 16 females; mean age 56.94 years ± 10.12 years) and the MVI-positive patients (86 males and 16 females; mean age 55.67 years ± 9.77 years).
Conventional ultrasound results
Comparisons of conventional ultrasound results between MVI-negative and MVI-positive HCCs are shown in Table 1. The maximal diameters of MVI-positive HCCs were significantly greater than those of MVI-negative HCCs. The cutoff point for maximal tumor diameters was 61.95 mm with an area under the curve (AUC) of 0.663. MVI-positive HCCs were more likely to have a background of liver cirrhosis and a rich blood flow and less likely to have a fatty liver background.
Comparisons of conventional ultrasound results between mild MVI and severe MVI HCCs are shown in Table 2. There were no statistically significant differences in either maximal tumor diameter or other ultrasound characteristics between the two groups.
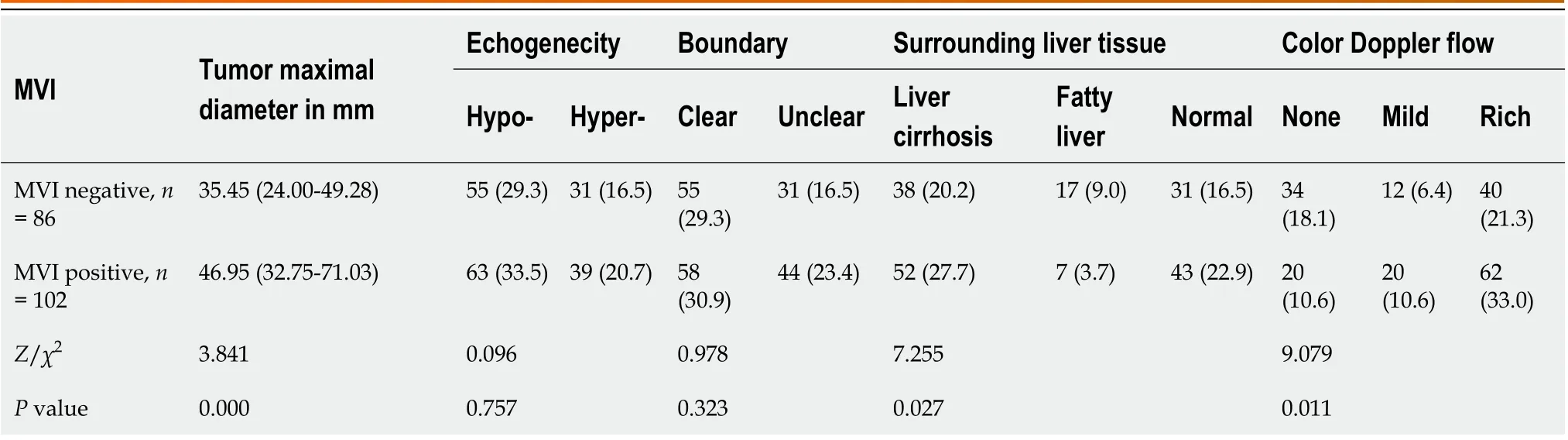
Table 1 Comparisons of conventional ultrasound results between microvascular invasion-negative and microvascular invasion-positive hepatocellular carcinomas, n (%)

Table 2 Comparisons of conventional ultrasound results between mild microvascular invasion and severe microvascular invasion hepatocellular carcinomas
SWE results
The differences in SWE results between MVI-negative and MVI-positive HCCs are shown in Table 3. The Emax of MVIpositive HCCs was significantly higher than that of MVI-negative HCCs. The Emax of the periphery of MVI-positive HCCs was significantly higher than that of MVI-negative HCCs. The cutoff point for the Emax of HCCs was 2.340 with an AUC of 0.598; the cutoff point for the Emax of the periphery of HCCs was 1.305 with an AUC of 0.622 (Figure 4).
The differences in SWE results between mild MVI and severe MVI HCCs are shown in Table 4. There were no significant differences between the Emax of mild MVI and severe MVI HCCs. However, the Emax of the periphery of severe MVI HCCs was significantly higher than that of mild MVI HCCs.
Bivariate logistic regression results
The results of bivariate logistic regression of the features suggestive of positive MVI are shown in Table 5. Higher Emax of the periphery of HCCs and larger maximal diameters were independent risk factors for MVI, with odds ratios of 2.820 and 1.021, respectively.
DISCUSSION
In this study, we investigated the efficacy of conventional ultrasound features and SWE in the preoperative prediction of MVI in HCC. Our findings revealed that a higher Emax in the periphery of HCCs coupled with larger maximal diameters were independent risk factors for MVI.
There were no significant differences in the distributions of sex and age between patients with MVI-positive HCCs and those with MVI-negative HCCs. These results were similar to those in a previous study[19]. Our results also showed that the maximal diameters of MVI-positive HCCs were significantly larger than those of MVI-negative HCC, and a larger maximal diameter was an independent risk factor for MVI-positive HCCs with an odds ratio of 1.021. Tumor size is an established independent prognostic factor for HCC[20,21]. Our research has revealed that HCC size was also an independent prognostic factor for positive MVI. Consequently, it is of utmost importance to have precise preoperative measurements of HCC size for accurate prediction of MVI status and prognosis.

Table 3 Comparisons of shear wave elastography results between microvascular invasion-negative and microvascular invasionpositive hepatocellular carcinomas

Table 4 Comparisons of shear wave elastography results between mild microvascular invasion and severe microvascular invasion hepatocellular carcinomas

Table 5 Results of bivariate logistic regression of the features suggestive of positive microvascular invasion
Conventional ultrasound is the first imaging choice for hepatology and plays an important role in both focal hepatic lesions and diffuse liver diseases. Our study showed that ultrasound features such as tumor boundary or tumor echogenicity were not significantly different between MVI-positive and MVI-negative HCCs, similar to the results of Zhouet al[22], which showed that ultrasound features including echogenicity, margin, shape, and halo sign were not significantly different between MVI-positive and MVI-negative HCCs. According to our findings, MVI-positive HCCs were more likely to have a rich blood flow. As MVI reflects the invasion of cancer cells into microvessels, it may cause a change in the blood supply in tumors. Some CEUS studies have also confirmed that MVI-positive HCCs have increased blood flow perfusion, compared with MVI-negative HCCs[13,14]. However, the study by Zhouet al[22] showed different results as the distribution of blood flow was not significantly different between MVI-positive and MVI-negative HCCs. The reason for this may be due to the different ultrasound machines used. The sensitivity of color Doppler can vary greatly between ultrasound machines. Also, the correct setting of machine parameters is very important[23]. The application of new Doppler techniques, such as superb microvascular imaging, would be useful[24]. Our study revealed that MVI-positive HCCs were more likely to have a background of liver cirrhosis and less likely to have a background of fatty liver, indicating that HCCs with a background of liver cirrhosis are likely MVI-positive. The surrounding liver background of HCCs should be taken into consideration for preoperative evaluation.
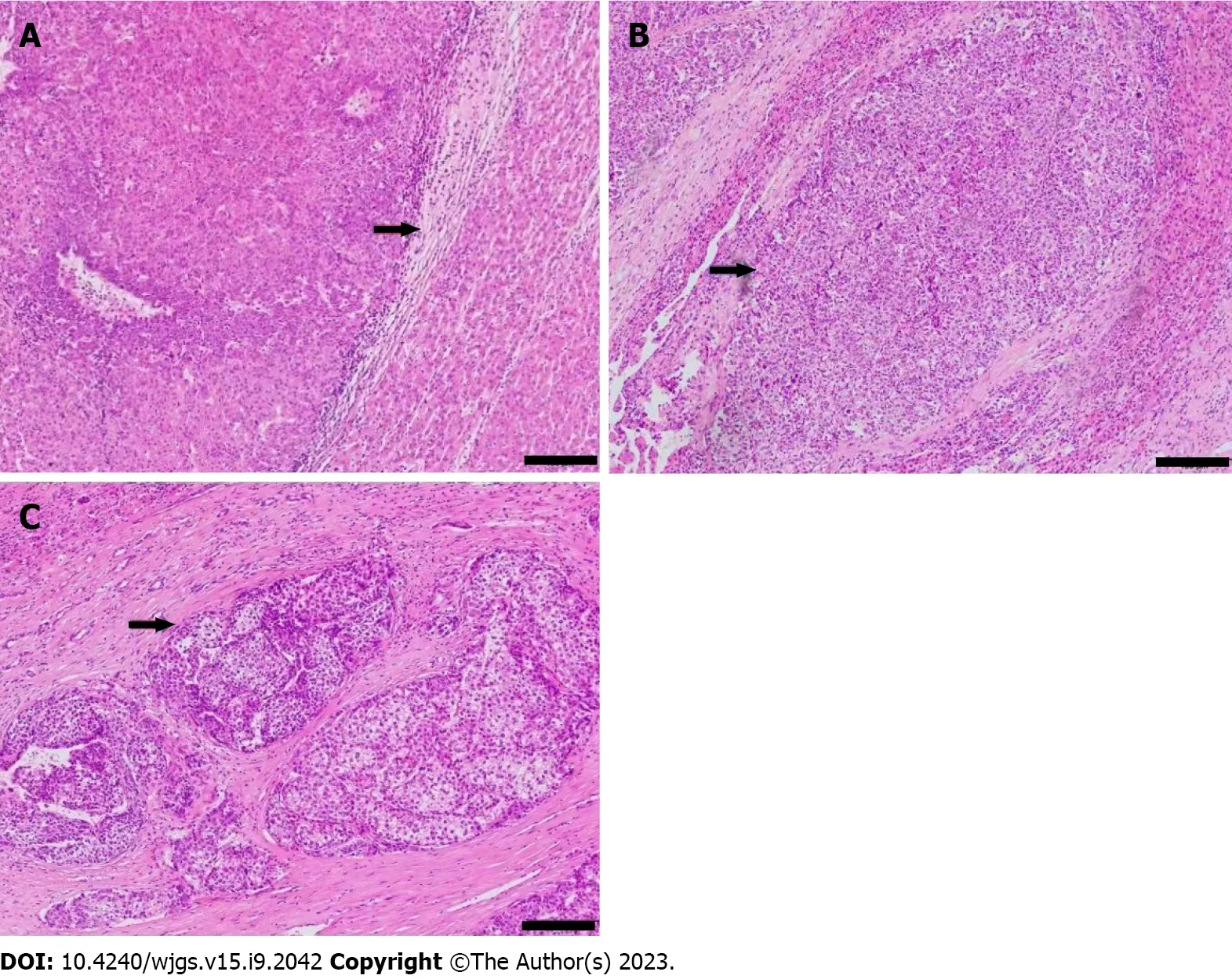
Figure 3 Pathological images showed microvascular invasion in hepatocellular carcinoma. Hematoxylin and eosin stain, magnification: × 100. A:No microvascular invasion (MVI) detected, recorded as MVI-negative; B: One MVI detected, recorded as mild MVI; C: More than 5 MVIs detected, recorded as severe MVI. Scale bar: 100 μm.

Figure 4 Receiver operating characteristic curve for maximal elasticity of the periphery of the hepatocellular carcinoma and maximal elasticity of the hepatocellular carcinoma. Emax: Maximal elasticity; HCC: Hepatocellular carcinoma.
Our previous studies have shown that the value of SWE with Emax in the differential diagnosis between benign and malignant focal liver lesions or among different pathological types of malignant focal liver lesions[15]. In this study, we found that the Emax of HCCs and the Emax of the periphery of HCCs were significantly different between MVI-positive HCCs and MVI-negative HCCs. The Emax of the periphery of HCCs was an independent risk factor for MVI-positive HCC with an odds ratio of 2.820. Our results indicated that MVI-positive HCCs were stiffer than MVI-negative HCCs, and this was similar to the results of other studies based on MR-elastography[7,25]. One probable reason is that positive MVI may change the blood supply in the tumor and then modify its stiffness. As MVI usually invades the capsule of HCCs first, the periphery of the HCC is usually involved early[2]. This early change in blood flow and tissue stiffness could potentially have significant implications. Zhanget al[26] reported rim enhancement in the arterial phase and peritumoral hypointensity in the hepatobiliary phase in gadobenate-enhanced MRI as independent risk factors for MVI. In addition, our findings suggest that the stiffness of the periphery of HCCs may serve as an important independent predictor of MVI risk. Specifically, we observed that higher stiffness in this region of HCCs was significantly associated with an increased risk of developing MVI. Furthermore, differences in Emax values at the periphery of HCCs were found to distinguish between HCCs with mild and severe MVI, highlighting the potential diagnostic value of this parameter in MVI detection.
There were some limitations to our study that should be acknowledged. First, laboratory data, including total bilirubin and alpha fetoprotein, were not taken into account. The inclusion of these laboratory indices in conjunction with the ultrasound indices would be valuable in developing a predictive model. This will be a focus of our future research.
CONCLUSION
In summary, HCC size and stiffness of the periphery of HCCs are useful ultrasound criteria for predicting positive MVI. Thus, preoperative ultrasound and SWE could provide useful information for the prediction of MVI in HCCs.
ARTICLE HIGHLIGHTS
FOOTNOTES
Author contributions:Jiang D, Dong H, and Qian R designed the study; Qian Y, Tan BB, and Zhu XL performed the research and collected ultrasound data; Dong H reviewed and analyzed pathological specimens; Jiang D and Qian R performed statistical analysis; Jiang D and Qian R wrote the manuscript; All authors read and approved the final manuscript.
Supported bythe Key Program of Science and Technology Commission Foundation of Changning, No. CNKW2022Y61.
Institutional review board statement:This prospective study was approved by the Ethics Committee of Eastern Hepatobiliary Surgery Hospital (Approval No. EHBHKY2021-K-017).
Clinical trial registration statement:This study is registered at clinical hospital center “Eastern Hepatobiliary Surgery Hospital, Naval Medical University” trial registry. The registration identification number is ChiCTR2100049831.
Informed consent statement:Each patient provided written informed consent before the ultrasound examinations.
Conflict-of-interest statement:All authors confirm having no conflicts of interest.
Data sharing statement:No additional data are available.
CONSORT 2010 statement:The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Rong Qian 0009-0007-2567-8722.
S-Editor:Chen YL
L-Editor:Filipodia
P-Editor:Chen YL
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
- Surgical decompression for the management of abdominal compartment syndrome with severe acute pancreatitis: A narrative review
