Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
Ji Hyeong Song, Tae Jeong Oh, Sungwhan An, Kyung Ha Lee, Ji Yeon Kim, Jin Soo Kim
Abstract
Key Words: Biomarkers; DNA methylation; Syndecan-2; Colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and second leading cause of cancer-related deaths worldwide[1]. According to the 2019 Korean statistics, the prevalence of CRC was the third highest, following thyroid and lung cancer. From 2015 to 2019, the 5-year survival rate of CRC patients in Korea was 74.3%[2]. Patients with metastatic CRC have a 5-year survival rate of < 10%, whereas those with early detection of CRC have a 5-year survival rate of > 90%[3]. Therefore, the early detection of CRC is essential to reduce cancer-related morbidity and mortality.
Most patients with early-stage CRC are mostly asymptomatic. There are several screening tools for detecting asymptomatic CRC. The fecal occult blood test and fecal immunochemical test are non-invasive, inexpensive, and convenient methods. However, these tests have low sensitivity and specificity[4]. Computed tomography colonography can be used as an alternative examination for colonoscopy, which non-invasively examines the entire colon beyond the obstructive. However, there are concerns about radiation hazards and the disadvantage of detecting small polyps < 5mm in size. Colonoscopy is the most accurate method with the advantage of being able to perform procedures such as biopsy and polypectomy. In the NordICC trial, a prospective, multinational, and randomized controlled trial investigating the effectiveness of colonoscopy on CRC incidence and mortality, colonoscopy as a screening tool showed good compliance, a high rate of adenoma yield, and adequate performance[5]. However, colonoscopy is an invasive procedure that requires mechanical bowel preparation and carries the risk of complications, such as perforation or electrolyte imbalance. Therefore, there is a need to develop a non-invasive and highly accurate CRC screening tool, especially for those reluctant to undergo colonoscopy.
CRC carcinogenesis is associated with the accumulation of genetic and epigenetic alterations[6]. Aberrant DNA methylation is one of the most common molecular alterations involved in CRC[7]. Because exfoliated cells from tumors are present in stool samples from CRC patients, detection of aberrant methylation is emerging as a non-invasive diagnostic tool for CRC[8]. Several stool DNA (sDNA)-based methylation markers are available for the early detection of CRC[9,10]. Among these, syndecan-2 (SDC2) has been reported as a potential biomarker for sDNA testing[11-13]. Aberrant DNA methylation is an early epigenetic event during tumorigenesis that can occur in the normal colonic mucosa during aging[14], which may compromise the sDNA test results. If preoperatively methylatedSDC2(meSDC2) normalizes after the surgical resection of CRC, it may have diagnostic value and may be used for postoperative surveillance[15]. Therefore, we conducted a prospective observational study to compareSDC2methylation in the sDNA of patients with CRC before and after surgery.
MATERIALS AND METHODS
Patients
In this prospective study, we enrolled 151 patients diagnosed with CRC who underwent surgical resection with curative intent between September 2016 and May 2020 at the Department of Surgery, Chungnam National University Hospital (South Korea). This observational study was approved by the Institutional Review Board of our institute (No. 2016-05-018). The exclusion criteria were as follows: (1) Patients under 18 or over 80 years of age; (2) Difficulty in obtaining informed consent due to mental health issues; (3) Tumor complications such as bleeding, perforation, or obstruction; and (4) Palliative operation. Among the 151 enrolled patients, four dropped out. Two patients were transferred to other hospitals, and two were unresectable. Clinical data were collected for 147 patients. Stool samples were collected from 123 patients preoperatively and 122 patients postoperatively, excluding those due to poor sample quality and inadequate sample amount. A total of 104 samples were collected from both preoperative and postoperative patients. Figure 1 shows the flowchart of the enrolled patients.
Stool collection, DNA isolation, and bisulfite treatment
For each subject, at least 2 g of voided stool sample was collected in 20 mL of preservative buffer (Genomictree, Inc., Daejeon, South Korea) using a disposable spatula from four to five different locations. The samples were collected from the patients before and after definitive surgery. Inadequate stool specimens were not included in the methylation analysis (e.g., diarrhea or loose stools).
sDNA was isolated using the GT Nucleic Acid PREP Kit II (Genomictree, Inc., Daejeon, South Korea) according to the manufacturer’s instructions. The Qubit dsDNA BR Assay Kit (Thermo Fisher Scientific, MA, United States) was used to determine the DNA concentration. Briefly, all stool samples were weighed and homogenized in a preservative buffer using a multiple vortex mixer (MIULAB, Hangzhou, China). After homogenization, 1–2 g of each stool sample was used for DNA isolation.
Each two μg of stool-derived genomic DNA was chemically modified with sodium bisulfite using the EZ DNA Methylation-Gold Kit (ZYMO Research, CA, United States) according to the manufacturer’s instructions. Bisulfiteconverted DNA was purified using a Zymo-Spin IC column (ZYMO Research) and eluted with 10 μL of distilled water. Bisulfite-converted DNA was either used immediately for methylation analysis or stored at -20 oC until use.
Analysis of SDC2 methylation in sDNA using meSDC2 linear target enrichment-quantitative methylation-specific realtime polymerase chain reaction test and data
The meSDC2linear target enrichment (LTE)-quantitative methylation-specific real-time polymerase chain reaction (qMSP) assay was performed in duplicate reactions for each sample as described by Hanet al[13]. A highly sensitive twostep meSDC2LTE-qMSP assay was used to measureSDC2methylation in sDNA. First, LTE was used to enrich meSDC2target DNA and controlCOL2A1DNA from the bisulfite-modified DNA. The region of theCOL2A1gene lacking CpG dinucleotides was used as a control to estimate the amount of amplifiable template and the adequacy of bisulfite conversion. The LTE reaction mixture (20 μL) containing 2.0 μg of bisulfite-converted sDNA, 50 nmol/L each ofSDC2methylation-specific antisense (5′-AAAGATTCGGCGACCACCGAACGACTCAAACTCGAAAACTCG-3’) andCOL2A1gene-specific antisense primers (5′-AAAGATTCGGCGACCACCGACTAICCCAAAAAAACCCAATCCTA-3′) attached to a 5’ universal sequence (5’-AAAGATTCGGCGACCACCGA-3’), and 4 μL of 5× AptaTaq polymerase chain reaction (PCR) master mix (Roche Diagnostics, Basel, Switzerland) was prepared. The thermal cycling conditions were as follows: 95 °C for 5 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 60 s. Next, the reaction mixture volume was scaled up to 40 μL, containing 8 μL of 5× AptaTaq PCR master mix, 250 nmol/LSDC2methylation-specific sense primer (5′-GTAGAAATTAATAAGTGAGAGGGC-3′), 125 nmol/LSDC2probe (5′-FAM-TTCGGGGCGTAGTTGCGGGCGG-3′), 125 nmol/LCOL2A1sense primer (5′GTAATGTTAGGAGTATTTTGTGGITA-3′), 62.5 nmol/LCOL2A1probe (5′-Cy5-AGAAGAAGGGAGGGGTGTTAGGAGAGG-3′), and 250 nmol/L universal sequence primer. Thermal cycling conditions were as follows: 95 °C for 5 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Heating and cooling rates were 20 °C/s and 15 °C/s, respectively. For each run, bisulfite-converted methylated (HCT116) and unmethylated genomic DNA (whole-genome amplified human lymphocyte DNA) were used as methylation controls. Non-template and nontemplate bisulfite-converted controls were also included in the study.
PCR forSDC2andCOL2A1was performed in a single tube. meSDC2LTE-qMSP was performed using Rotor-Gene Q real-time PCR instrument (Qiagen, Hilden, Germany). The cycle threshold (CT) value was calculated using the Rotor-Gene Q software. Lower CTvalues indicated higher levels ofSDC2methylation. For PCR analysis,SDC2methylation was detected if the CTwas less than 40 cycles. It was not detected if CTwas not measurable. Samples were categorized as positive if at least one of the two reactions showed detectableSDC2, and they were considered negative ifSDC2methylation was not measurable in both reactions. The test results were acceptable only when the CTvalue ofCOL2A1was < 31. IfCOL2A1was not detected, or the CTvalue was > 31, the test was repeated. Neither the personnel involved in laboratory work nor data analysis of theSDC2methylation results was informed of colonoscopic findings or pathology outcomes as reference standards. Oh TJ and An S are employees and shareholders of Genomictree, Inc.
Statistical analysis
Statistical analysis of the data was performed using IBM SPSS Statistics 26 (SPSS Inc., Chicago, IL, United States). Chisquare and/or Fisher’s exact tests were used for categorical variables. Detection rates were evaluated dichotomously as ‘0’ for methylation-negative and ‘1’ for methylation-positive.
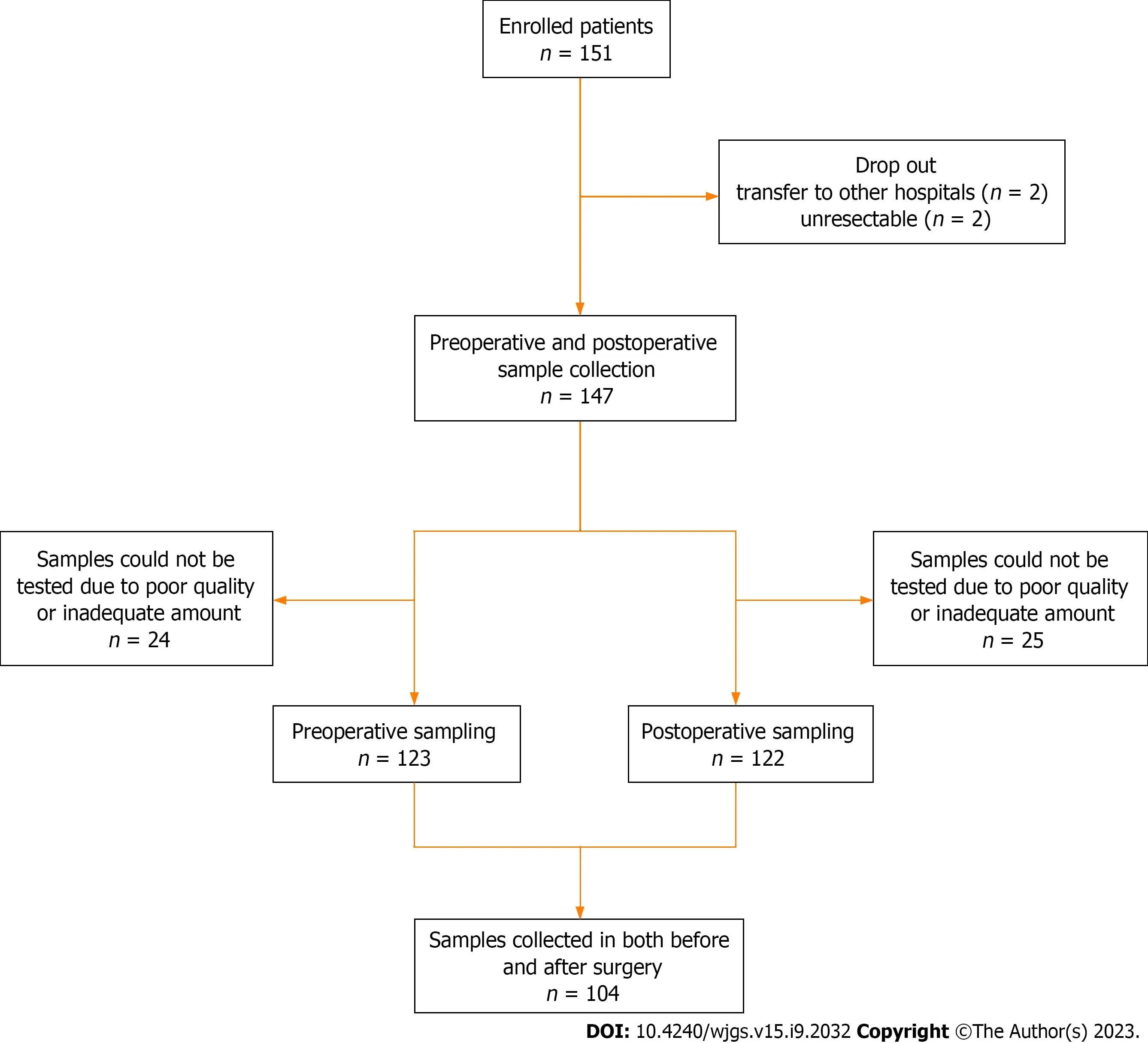
Figure 1 Flowchart of preoperative and postoperative sample collection in patients with colorectal cancer.
The CTvalues before and after surgery according to tumor node metastasis (TNM) stage were compared using analysis of variance (ANOVA). A pairedt-test was performed to compare CTvalues before and after surgery for each patient. APvalue < 0.05 was considered statistically significant.
In addition, we retrieved literatures by Reference Citation Analysis to supplement the latest cutting-edge research results.
RESULTS
Patient characteristics are described in Table 1. In total, 97 male (66%) and 50 female (34%) patients were included in the preoperative or postoperative sampling. The mean age was 62.3. Total colonoscopy was performed before surgery in 133 patients (90.5%), and remnant advanced adenoma (RAA) was present in 18 patients (12.2%). Among the 18 patients with RAA, five remainedSDC2methylation-positive after surgery. The recurrence rate was 6.8% in all patients.
Detection rates of the SDC2 methylation test
Figure 2 shows the detection rates ofSDC2methylation according to TNM stage. The positivity rate of theSDC2methylation test before surgery was 88.6% (109/123), and the false negative rate was 11.4% (14/123). The positivity rates for stages 0, I, II, III, and IV were 75% (3/4), 83.9% (26/31), 91.1% (41/45), 89.2% (33/37), and 100% (6/6), respectively (Figure 2A). The detection rate ofSDC2methylation after surgery was 19.7% (24/122). The detection rates for stages 0, I, II, III, and IV were 25% (1/4), 31.2% (10/32), 17.5% (7/40), 14.0 (6/43), and 0% (0/3), respectively (Figure 2B). The demographic analysis of patients according to the preoperative and postoperativeSDC2methylation results is presented in Table 2. Large tumor size (3 cm,P= 0.019) and advanced T stage (T3–T4,P= 0.033) were associated with positivity ofSDC2methylation before surgery. Additionally, female patients showed more false positives after surgery (P= 0.030).
CT values of SDC2 methylation
The distribution of CTvalues in preoperative and postoperativeSDC2methylation according to TNM stage is shown in Figure 3. There were no significant differences in the CTvalues ofSDC2methylation preoperatively (P= 0.901) or postoperatively (P= 0.332) according to TNM stage.

Table 1 Characteristics of patients
Figure 4 shows the CTvalues of meSDC2for each patient before and after surgery. Among the 104 patients from whom stool samples were obtained before and after surgery, 92 patients showed positive results for preoperativeSDC2methylation. The postoperative negative conversion rate for preoperatively meSDC2was 79.3% (73/92). The CTvalues ofSDC2methylation significantly decreased postoperatively compared to the preoperative values (P< 0.001).
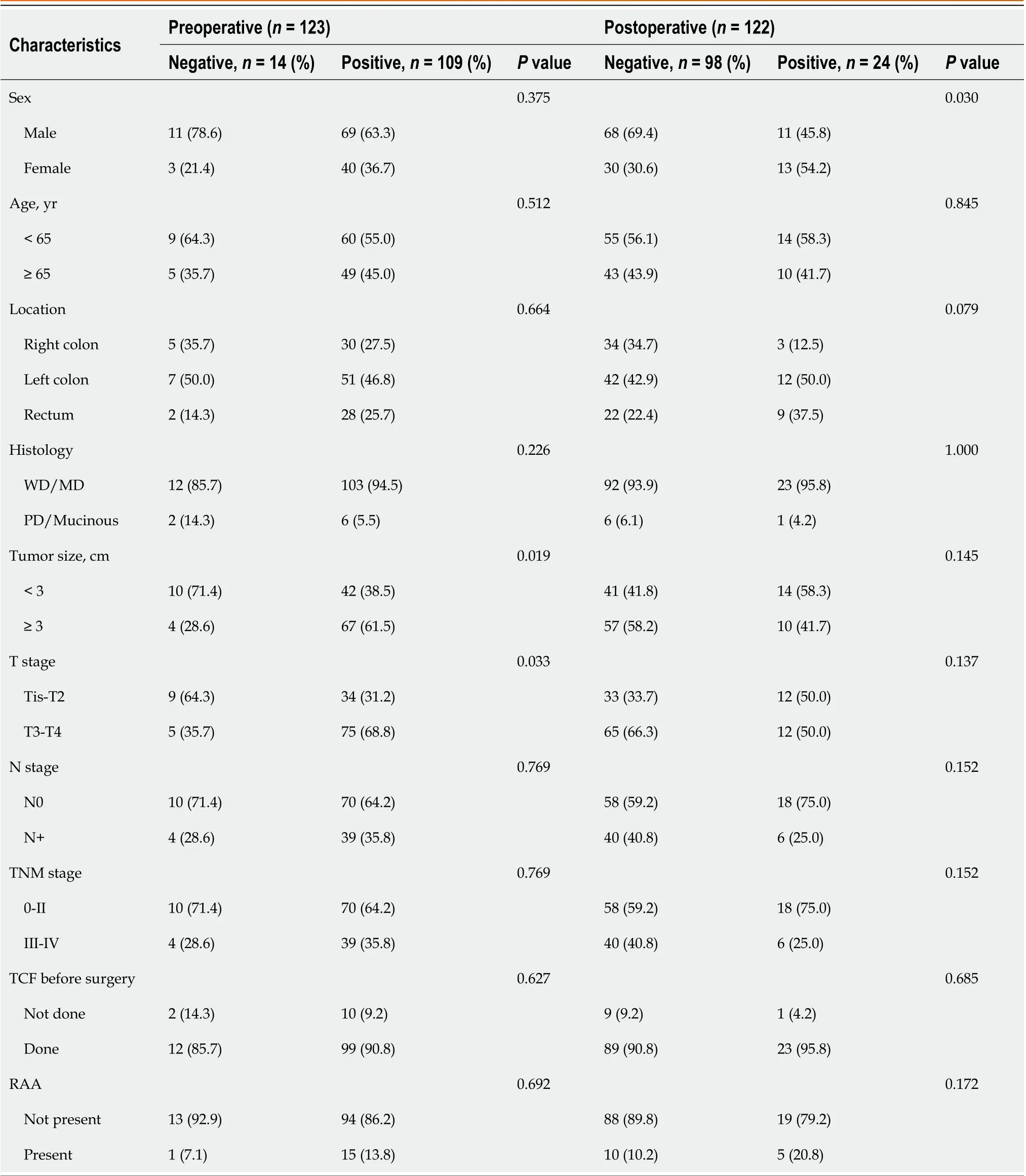
Table 2 Demographic analysis of patients according to preoperative and postoperative syndecan-2 methylation test
DISCUSSION
Various screening tools are used for the early detection of CRC to reduce cancer-related morbidity and mortality. Among the screening tools, colonoscopy is the most accurate method and is considered the gold standard with high sensitivity and specificity. However, colonoscopy is an invasive procedure that requires mechanical bowel preparation and carries the risk of complications, such as perforation or electrolyte imbalance. Hence, it is not yet useful as a mass-screening test[16]. Recently, non-invasive and highly accurate tests using sDNA methylation have been reported[6,7,9,10]. Among the several known sDNA biomarkers,SDC2was found to be the most accurate single gene[17]. Therefore,SDC2methylation in sDNA has been proposed as a non-invasive mass-screening tool for the early detection of CRC.
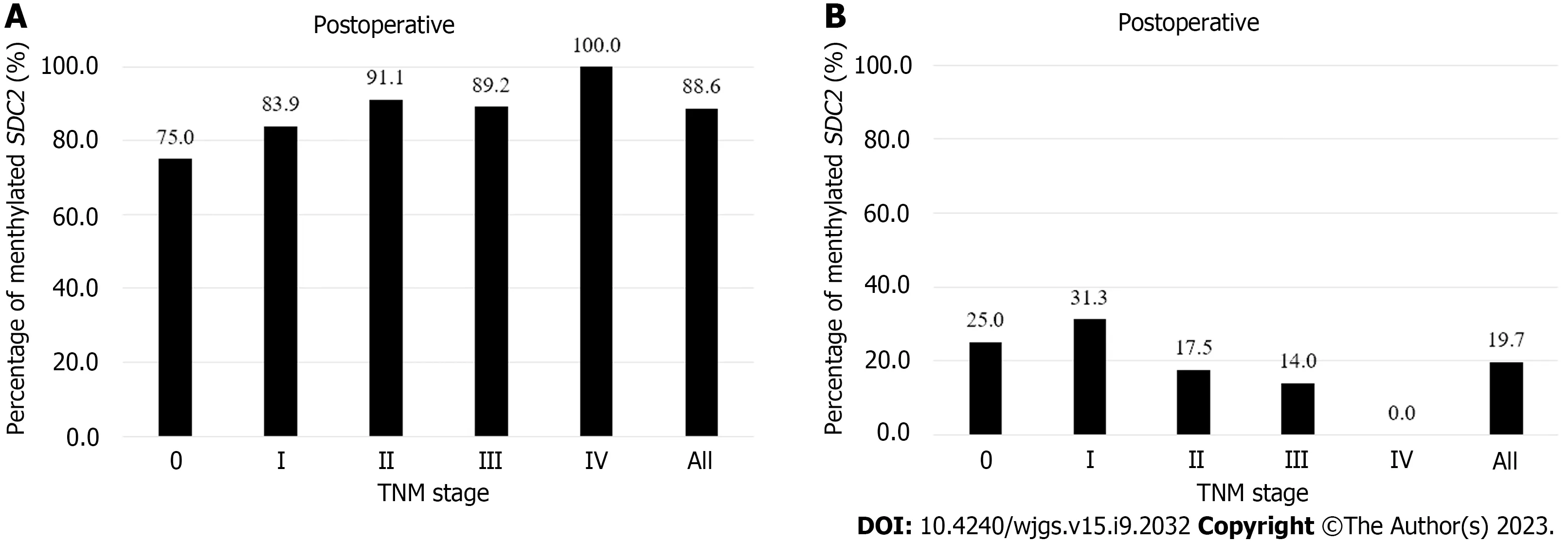
Figure 2 Percentages of syndecan-2 methylation test results according to tumor node metastasis stage. Using the 1/2 algorithm, the percentage of samples with detectable methylated syndecan-2 is presented by bars. A: Preoperative stool samples were collected from 123 patients. Overall sensitivity was 88.6%; B: Postoperative stool samples were collected from 122 patients. Overall specificity was 80.3%. SDC2: Syndecan-2; TNM: Tumor node metastasis.

Figure 3 Distribution of syndecan-2 methylation according to tumor node metastasis stage. The cycle threshold (CT) values for each sample were calculated as 40-CT. A higher 40-CT indicates a higher methylation level of syndecan-2 (SDC2). If SDC2 methylation was not detected, it was expressed as 0. A:Preoperative (n = 123); B: Postoperative (n = 122). SDC2: Syndecan-2; CT: Cycle threshold; TNM: Tumor node metastasis.
Recently, Wanget al[18] reported a meta-analysis that evaluated the diagnostic performance of theSDC2methylation test for detecting CRC. Twelve studies were included in the meta-analysis, and all articles were retrospective studies. Among them, seven studies measured meSDC2in sDNA, and five studies measured it in blood. The overall sensitivity was 80% and the specificity was 95%; and the sensitivity and specificity of sDNA test were 83% and 94%, respectively. In the present study, the detection rates ofSDC2methylation in preoperative and postoperative were 88.6% and 19.7%, respectively. These values represent a sensitivity of 88.6% (109/123, 95%CI: 82%–96%) and specificity of 80.3% (98/122, 95%CI: 72%–87%), and the results were comparable to those of several previous studies.
Zhaoet al[19] reported the detection rate of meSDC2in stool samples from 94 patients with CRC and 124 normal healthy individuals. There were no significant differences in the detection rates of meSDC2based on the age, sex, or stage. As the tumor size increased, the positive detection rate of meSDC2also increased (P< 0.05). Similarly, we found that a large preoperative tumor size and advanced T stage were associated with high detection rates ofSDC2methylation. Females showed more false positive results after surgery. This result was thought to be due to the small sample size. The combination ofSDC2with other biomarkers may improve CRC detection rates[9,19].
Several studies have reported thatSDC2methylation is not related to the clinical stage[11,13,18]. The present study also showed similar results. There was no association between the detection rate ofSDC2methylation and clinical stage. Although there was no statistically significant difference, the detection rate ofSDC2methylation according to stage showed a gradual increase. Moreover, Ohet al[12] found that the sensitivity ofSDC2methylation test tended to gradually increase with an increase in the stage. Further studies are needed to validate whether clinical stage affects the detection rate of meSDC2.
Few studies have compared sDNA test results before and after surgery. Kisielet al[15] evaluated sDNA markers (NDRG4andBMP3) in 22 patients with CRC before and after surgery. They demonstrated that methylated sDNA markers present in patients normalized following surgical resection. Nishiokaet al[20] compared the preoperative and postoperative sDNA levels of 54 patients with CRC who underwent surgical resection. Aberrant methylation of sDNA markers (CDH4andGATA5) was detected in 23 (42.3%) preoperative stool samples from patients with CRC. Methylated alleles of these genes were not found in the postoperative sDNA. To the best of our knowledge, no studies have compared meSDC2levels before and after surgery. We compared the preoperative and postoperative CTvalues of meSDC2in 92 patients who tested positive forSDC2methylation before surgery. The CTvalues ofSDC2methylation in sDNA were significantly decreased postoperatively compared to the preoperative values. These results indicate thatSDC2methylation test has diagnostic value and may be used for surveillance.

Figure 4 Paired cycle threshold values of syndecan-2 methylation before and after surgery for each patient (n = 104). The cycle threshold (CT)values were calculated as 40-CT. A higher 40-CT indicated a higher methylation level of syndecan-2 (SDC2). If SDC2 methylation was not detected, it was expressed as 0. P value was calculated using a paired t-test. SDC2: Syndecan-2; CT: Cycle threshold.
In our study, twenty-four (19.7%) patients with CRC remained positive for meSDC2in the sDNA after surgical resection. Among these patients, there was no recurrence after surgery during the median follow-up period of 46 (1–67) months. Since molecular-level methylation precedes phenotypic tumorigenesis, these patients require follow-up with concern for possible recurrence. However, six patients who experienced recurrence after surgery showed negative results forSDC2methylation. The correlation between postoperativeSDC2methylation and recurrence was not statistically significant (P= 0.597). Therefore, based on these results, the present study seemed unlikely to show the usefulness of meSDC2in the sDNA as surveillance after CRC surgery. In addition, the liver and peritoneum are the most common sites for CRC metastasis[21], with rare occurrences in other parts of the body[22]. These recurrent cases of metastasis are difficult to detect with a sDNA test, making it difficult to use stool tests as a surveillance tool.
Similar to stool DNA testing, dysbiosis in the microbiota can be used as a test for early detection of CRC by obtaining fecal samples[23]. Several microbial species, such asF. nucleatum,B. fragilis, andF. Prausnitzii, are known to act as a driving force in the occurrence of CRC. Hence, detecting these microbial species in stool samples can aid in the early identification of CRC[24]. Non-invasive fecal biomarkers, such as aberrant methylation of sDNA tests or dysbiosis in the microbiota, can be expected to make important contributions to the early diagnosis and therapeutic implications of CRC in the future.
The present study had several limitations. First, selection bias may have existed because this study was conducted at a single institution with a small sample size. Second, it is insufficient to determine its value as a surveillance tool becauseSDC2methylation was only compared before and after surgery, without long-term follow-up. Therefore, multicenter prospective studies with long-term follow-up are necessary to assess the feasibility of surveillance.
CONCLUSION
We demonstrated that theSDC2methylation test in sDNA has acceptable sensitivity and specificity. However, small-size and early T stage tumors are associated with a low detection rate ofSDC2methylation. As the CTvalues significantly decrease after surgery,SDC2methylation of the sDNA test exhibits diagnostic value for CRC.
ARTICLE HIGHLIGHTS
FOOTNOTES
Author contributions:Song JH collected and analyzed the clinical data, drafted the manuscript, and prepared the figures; Oh TJ and An S drafted the manuscript; Lee KH and Kim JY collected the stool samples; Kim JS participated in study design, collected the stool samples, collected and analyzed the clinical data, drafted the manuscript, and prepared the figures; All authors read and approved the final manuscript.
Supported bythe Research Fund of Chungnam National University, No. 2018-0626-01.
Institutional review board statement:This observational study was approved by the Institutional Review Board of our institute (IRB No. 2016-05-018).
Informed consent statement:Written informed consent was obtained from all subjects.
Conflict-of-interest statement:The authors declare that they have no competing interests.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:South Korea
ORCID number:Ji Hyeong Song 0000-0002-7501-7259; Tae Jeong Oh 0000-0001-6212-1949; Sungwhan An 0000-0001-8781-4186; Kyung Ha Lee 0000-0002-0035-9000; Ji Yeon Kim 0000-0001-7964-5060; Jin Soo Kim 0000-0002-3362-574X.
S-Editor:Zhang H
L-Editor:A
P-Editor:Cai YX
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
- Surgical decompression for the management of abdominal compartment syndrome with severe acute pancreatitis: A narrative review
