Laparoscopic extended right hepatectomy for posterior and completely caudate massive liver tumor (with videos)
Liang Chen , Lu-Zheng Liu , Jia-Cheng Chen, Da-Feng Xu, Cheng Chen, Shi-Xun Lin,Xiang-Xiang Luo, Jin-Cai Wu
Division of Hepatobiliary Pancreatic Surgery, Hainan Affiliated Hospital of Hainan Medical University (Hainan General Hospital), Haikou 570311, China
TotheEditor:
Liver tumor may occur in any hepatic segment or lobe, and thus the liver resection is individualized as per the location and size of the tumor. In addition, the resection of the posterior and caudate lobes of the liver is especially difficult amongst all types of hepatectomy. Kawaguchi et al. believed that the laparoscopic resection of right posterior liver lobe was a difficult surgical procedure [1] .Besides, since the hepatic caudate lobe is deep in the anatomical position and wrapped by the three porta hepatis, with limited exposure space, it has always been one of the most challenging complex operations in hepatic surgery [2] . If the patient has poor liver function due to liver cirrhosis, this will undoubtedly increase surgical difficulty. In case of a massive tumor that involves the hepatic posterior and caudate lobes, the requirement for the surgical evaluation and techniques is even higher, and it might be a challenge to conduct laparoscopic hepatectomy. On November 11, 2021, we completed one case of laparoscopic anatomically extended right posterior and complete caudate lobe resection.
A 41-year-old male patient was admitted to the hospital because of “repeated pain in the back and loin for one week” and had more than 10 years of alcohol abuse. The examinations were completed after the patient was admitted to the hospital. The preoperative blood biochemistry examination showed: total protein (TP)62.9 g/L, albumin 27.2 g/L, total bilirubin (TBil) 22.10μmol/L, direct bilirubin (DBil) 13.04μmol/L, alanine aminotransferase (ALT) 51.2 U/L, and C-reactive protein (CRP) 94.15 mg/L. The blood routine examination showed: red blood cell (RBC) 3.57 × 1012/L, hemoglobin(Hb) 128 g/L, and blood platelet count 171 × 109/L. The coagulation function test showed: prothrombin time (PT) 10.0 s, international normalized ratio (INR) 0.86, and activated partial thromboplastin time (APTT) 22.6 s. HBsAg was negative, hepatitis C virus (HCV)antibody test was positive ( + ), and the PCR quantitative test result was normal. The multi-tumor marker protein chip detection showed: alpha-fetoprotein (AFP) 3.7 ng/mL, carbohydrate antigen 19-9 (CA19-9) 108.26 U/mL. The epigastric computed tomography(CT) showed: a mass (19.9 × 10.4 cm) and nodular shadows in the hepatic right and caudate lobes, partially exceeding the contour of the liver ( Fig. 1 A), and splenomegaly without ascites. The preoperative diagnosis indicated massive space-occupying lesion in the liver, probably focal nodular hyperplasia (FNH).
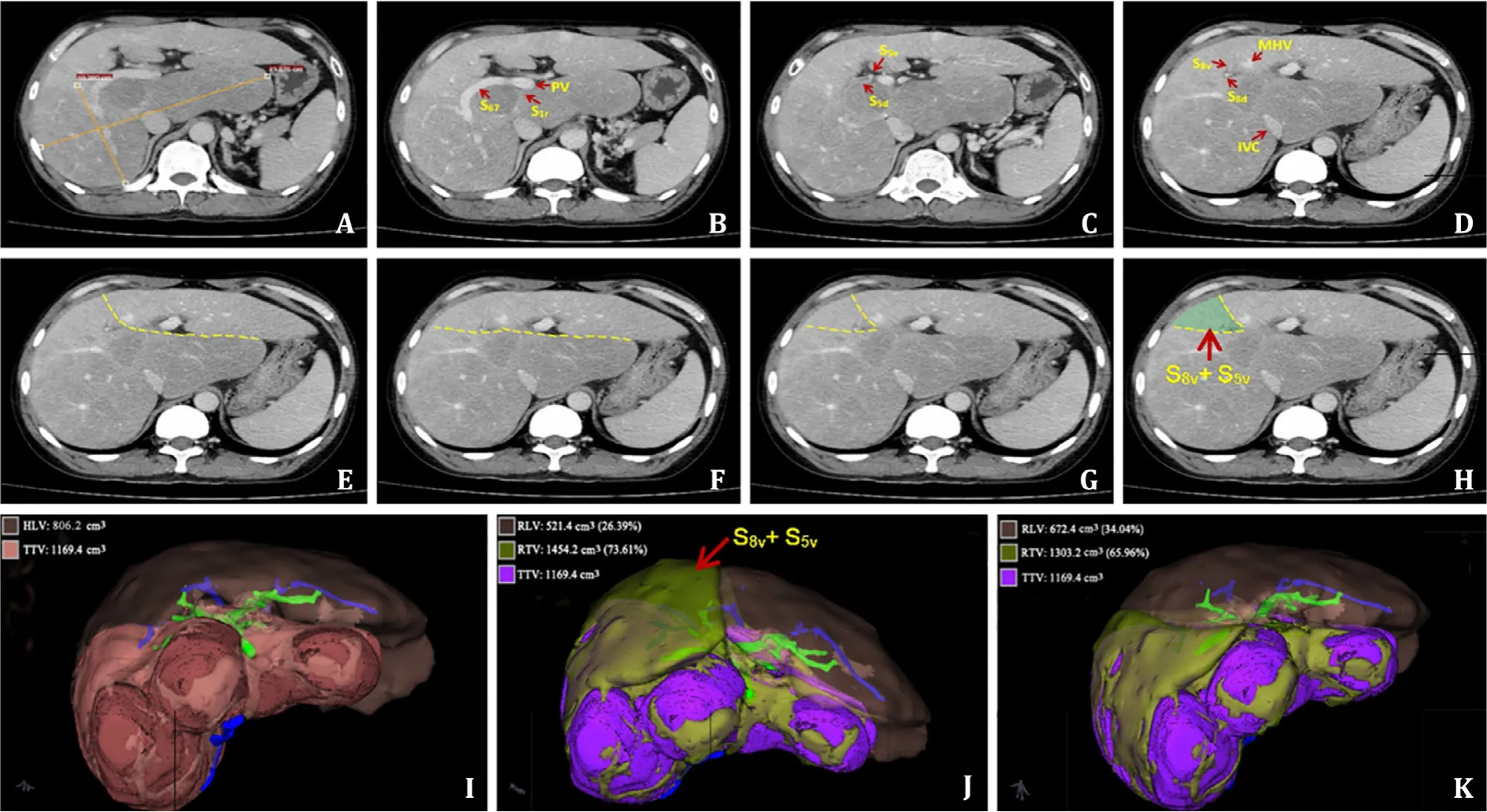
Fig. 1. Preoperative CT examination and surgical design. The massive focal nodular hyperplasia (FNH) occupied the right posterior and the whole caudate lobe of the liver,with a dimension of approximately 19.9 × 10.4 cm ( A ). The tumor involved the right anterior lobe (S5 + 8) ( B ), of which the dorsal segments of S5 + 8 (S5d + 8d) were occupied( C ), while the ventral segments of S5 + 8 (S5v + 8v) were retainable ( D - H ). According to three-dimensional imaging, the healthy liver volume (HLV) was 806.2 cm 3 , while the tumor total volume (TTV) was 1169.4 cm 3 ( I ). If the right hepatic lobe and the complete caudate lobe (S1 + S5 + 8) are resected, the resected total volumn (RTV) is 1454.2 cm 3 (including 284.8 cm 3 of HLV) and the remaining liver volumn (RLV) is 521.4 cm 3 , accounting for 26.39% of the total liver volume (TLV) ( J ). If the right hepatic segments S6 + 7, the dorsal segments of S5 + 8, and the segment S1 (S1 + S6 + 7 + S5d + 8d) are resected, RTV is 1303.2 cm 3 (including 133.8 cm 3 of HLV), while RLV is 672.4 cm 3 ,accounting for 34.04% of the TLV ( K ). The HLV of the retained wedged part of S5v + 8v (151.0 cm 3 ) could obtain blood outflow from the middle hepatic vein (MHV). CT:computed tomography; PV: portal vein; IVC: inferior vena cava.
The CT examination showed that, according to the Couinaud segmentation system, the tumor was in the right liver lobe (S5 + 8 segments) and the complete caudate lobe (S1) (Video S1). The indocyanine green excretion test for liver function (ICG15) was 5.2%.The Child-Pugh score was 7 (albumin 27.2 g/L, Child-Pugh Grade B) when the patient was admitted to the hospital, and was adjusted to 5 after infusion of albumin (albumin 39.7 g/L, Child-Pugh Grade A). According to liver volume calculation based on three-dimensional imaging (Myrian software, Intrasense, Montpellier, France) [ 3 , 4 ], the healthy liver volume (HLV) was 806.2 cm3,while the tumor total volume (TTV) was 1169.4 cm3( Fig. 1 I). It was relatively easy if the laparoscopic right hemihepatectomy and complete caudate lobe resection was conducted, but the resected total volume (RTV) was 1454.2 cm3(including 284.8 cm3of the HLV),and the remnant liver volume (RLV) was 521.4 cm3, accounting for 26.39% of the total liver volume (TLV) ( Fig. 1 J), which might result in post-hepatectomy liver failure (PHLF). Upon elaborate preoperative evaluation of the CT images, we found that the tumor involved the right anterior lobe (S5 + 8) and the dorsal segment of S5 + 8(S5d + 8d) was occupied, but the ventral segment (S5v + 8v) could be preserved ( Fig. 1 B-H). The resection of the right hepatic S6 + 7,S5 + 8 dorsal and S1 segments (S6 + 7 + S5d + 8d + S1) was planned.In that case, the RTV was 1303.2 cm3(including 133.8 cm3of the HLV) with 672.4 cm3of RLV which accounted for 34.04% of the TLV ( Fig. 1 K, Video S2). The preserved wedged S5v + 8v segments of the HLV (151.0 cm3) ( Fig. 1 H-K) could obtain blood outflow from the middle hepatic vein (MHV), and might be greatly helpful for the recovery of postoperative liver function and improving surgical safety, despite highly-increased surgical difficulty. Therefore, after the comprehensive evaluation, we decided to adopt the latter option.
The patient was placed supine with his right side increased by 15 ° and was treated with the 8-hole method ( Fig. 2 A). It was detected that the liver showed mild cirrhosis, enlarged right hepatic lobe, exophytic mass growth at the right caudate with a size of approximately 7 × 8 × 8 cm, and enlarged left caudate lobe. After the gallbladder was resected, the Pringle method was adopted to occlude the porta hepatis, and hepatic pedicles of the right posterior lobe, right caudate and left caudate were dissected separately and then respectively transected after ligation. Consequently an ischemic curve appeared on the liver surface, the dissection underwent along the curve on the right posterior lobe,with the cephalic-directed dissecting towards the MHV and the foot-directed dissecting towards the dorsal and ventral pedicles branches of segment S5 + 8. After the dorsal and ventral branches of the segment S5 + 8 were identified in the liver parenchyma, the dorsal branch pedicles of the segment S5 + 8 (S5d + 8d) were ligated and transected. Dissection was conducted from the cephalic side along the trunk of MHV to display the right venous wall, and then turned to the dorsal wall of the MHV to join the dorsal surface of the Arantius duct. At that moment, the resected specimen was mostly separated from the preserved hepatic surface. Afterwards, the short hepatic veins (SHVs) were ligated and transected along inferior vena cava (IVC), the specimen was removed from IVC and finally the right hepatic vein (RHV) was transected. The specimen was completely resected. The surgical field was thoroughly inspected, and no active bleeding or bile leakage was found. An abdominal tube was placed at the Winslow foramen and under the left hepatic lobe. The operation underwent smoothly and took about 280 min with 300 mL of bleeding. We infused 20 0 0 mL of crystalloid fluid and 500 mL of colloid fluid to the patient. The porta hepatis was occluded 12 times in total (15 min per time with an interval of 5 min). The intraoperative situations were shown in Fig. 2 B-L and Video S3.
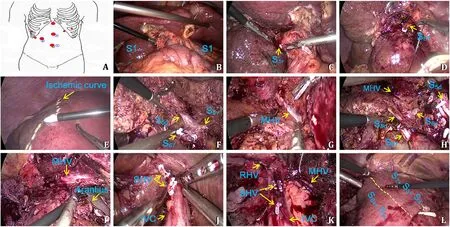
Fig. 2. Surgical procedure. The patient was placed supine with his right side raised by 15 °, and treated with the 8-hole method. Among them, the holes 1 ○2 ○3 ○can be used for observation or operation as necessary ( A ). The liver showed mild cirrhosis, enlarged right hepatic lobe, exophytic mass growth at the right caudate, with a size of approximately 7 × 8 × 8 cm and a complete capsule, and enlarged left caudate lobe ( B ). After the gallbladder was resected, the Pringle method was adopted to separate pedicle of the right caudate pedicle S1r ( C ), right posterior lobe S6 + 7, and the left caudate ( D ) by dissecting outside sheath at the first porta hepatis and then respectively transected after ligation. When an ischemic curve ( E ) appeared on the liver surface of the right posterior lobe, the liver was dissected along the ischemic curve, with the cephalic resection towards the middle hepatic vein (MHV) and the foot-side dissection towards the dorsal and ventral branches of the S5 + 8 pedicle. Then, the dorsal(S5d + 8d) and ventral branches (S5v + 8v) of the segment S5 + 8 were identified and the S5d + 8d branch was ligated and transected ( F ). Dissection was conducted from the cephalic side along the trunk of MHV to reveal the right venous wall, and then turned to the dorsal wall ( G and H ) of the MHV to join the dorsal plane with Arantius duct.At that moment, the specimen was mostly separated from the preserved hepatic surface ( I ). Afterwards, the short hepatic veins (SHVs) were ligated and transected along with inferior vena cava (IVC) ( J ), the resected specimen was completely removed from IVC ( K ), and finally the right hepatic vein (RHV) was transected, with the wedged part of S5v + 8v retained ( L ). IVC: inferior vena cava; SHV: short hepatic vein; RHV: right hepatic vein.
The postoperative gross specimen was shown in Fig. 3 A.The histology confirmed FNH ( Fig. 3 B and C). The postoperative transaminase level was slightly increased without jaundice. The patient was able to get out of bed 3 days after surgery and began to eat. The CT re-examination 5 days after surgery was shown in Fig. 3 D-F and Video S4. The patient was discharged from hospital 9 days after surgery. The liver function of the patient completely recovered in 2 months after surgery.
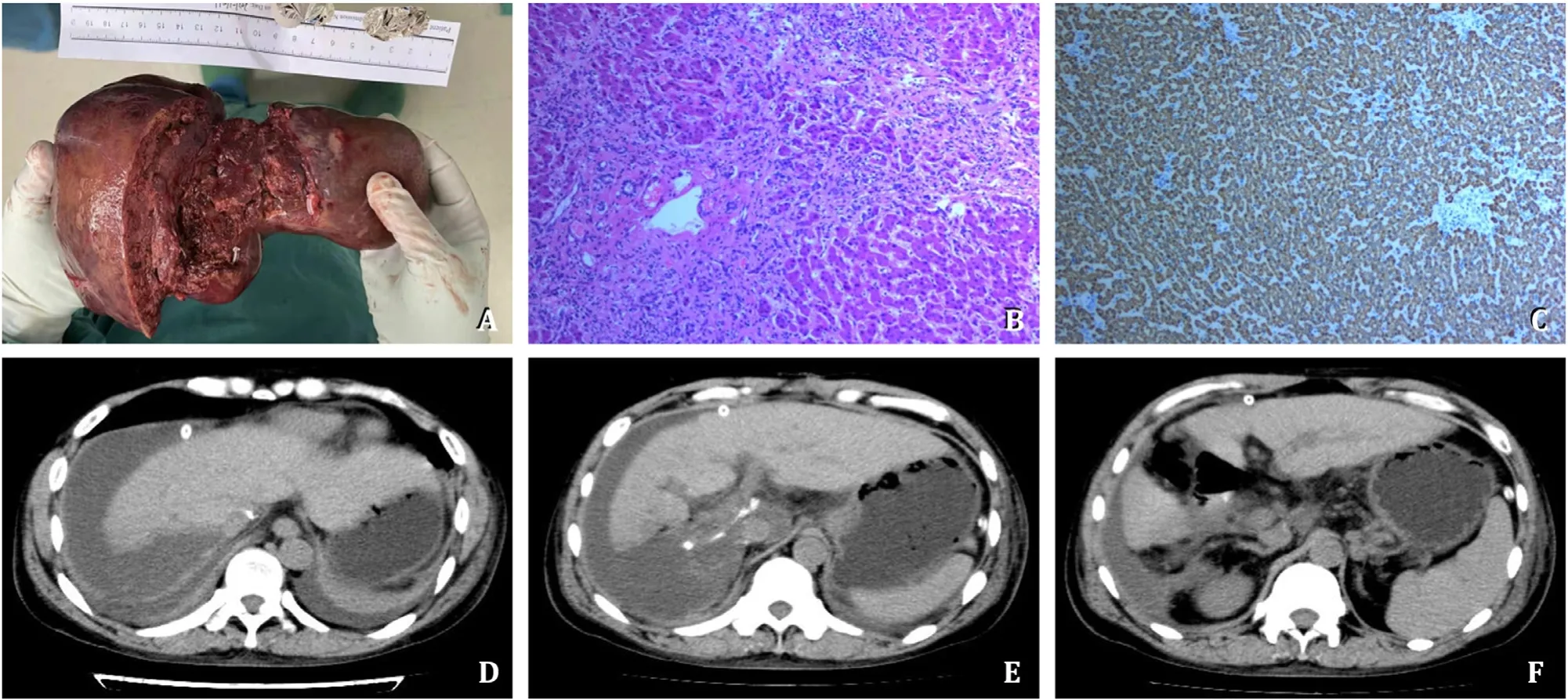
Fig. 3. Postoperative pathological examination and CT re-examination. The postoperative gross specimen was shown ( A ). The pathological examination reported focal nodular hyperplasia (FNH) in HE staining and Hep-1 immumohistochemical staining ( B and C ), original magnification × 200. D-F : The postoperative CT re-examination on the 5th day after surgery. CT: computed tomography; HE: hematoxylin-eosin.
FNH is a rare benign hepatic tumor-like lesion, with an incidence of 0.9%-3.0% [1] , accounting for 8% of primary hepatic tumors [ 5 , 6 ]. Surgical resection is considered when the clinical diagnosis is unclear or possibly a malignant hepatic tumor or hepatic adenoma, and the diameter of the tumor is above 5 cm [7-9] . The case reported herein was a patient with a massive hepatic space-occupying lesion whose preoperative CT scan showed no obvious enhancement in arterial phase, with CA19-9 increased to 108.26 U/mL. The patient had a long history of drinking and infection of HCV. The tumor was massive and the possibility of a malignant lesion cannot be ruled out, and thus was resected.
The tumor was mostly located in the right posterior lobe and entire caudate lobe of the liver. For preoperative surgical planning,the right hemihepatectony together with the resection of caudate lobe could acquire relatively large exposure space, which decreased the surgical difficulty, but may result in PHLF since the patient had history of alcohol abuse and HCV infection, with liver cirrhosis,splenomegaly and the small volume of the left hepatic lobe. Therefore, we decided to preserve the ventral part of the segments S5 and S8 and conduct extended right posterior and completely caudate lobe resection in order to additionally preserve a wedged liver with the MHV for blood outflow ( Fig. 1 H and K) and increase the volume of the RLV from 26.39% to 34.04%, improving the surgical safety and decreasing the possibility of PHLF [10] . Nonetheless, it also narrowed down the space for surgery and increased the surgical difficulty.
Exophytic growth occurred to the right caudate process of the patient, causing the part of the tumor located in the complete caudate lobe to be hypertrophic and wrap IVC at the same time. The choice of surgical method had to be made between laparotomy and laparoscopy. With the development of the laparoscopic technology, Dorovinis et al. and Jin et al. considered laparoscopy superior to laparotomy regarding caudate lobe resection and safety as well [ 11 , 12 ]. Furthermore, we believed that laparoscopy had its unique advantages regarding surgical field and operating space.Meanwhile, with the laparoscopic design we included the increase of the right side of the patient by 15 ° and the distribution of an observing hole in the left, middle and right areas, respectively, based on the actual situation since the left and right areas of the mass were both large and the right hepatic mass was next to the dorsal part ( Fig. 2 ). The 2 ○and 3 ○holes in Fig. 2 A could be used for observation or operation. The three operating areas combined with the inclination degree of the operation table made it possible to conduct the surgery from multiple routes and angles.
The difficulties and risks throughout the whole surgical procedure were as follows: 1) the tumor was massive and had exceeded the posterior lobe area and compressed the middle hepatic area.The ventral part of the tumor was next to the dorsal part of the MHV. If the tumor had to be completely resected, the MHV needed to be lifted so that the tumor of the caudate lobe could be separated from the dorsal part of the MHV. However, in case that the MHV was intraoperatively torn, it would be difficult to carry out intraoperative repair and suture since the operating space was narrowed by the ventral part of the preserved segments S5 and S8,probably resulting in massive hemorrhage and anemia; 2) since the tumor in the caudate lobe area was hypertrophic and wrapped IVC annularly, and the operating space for exposing and transecting the SHVs was extremely narrow, it would be even harder to deal with bleeding.
When dissecting liver, we used the small-mouth clamp or the attached energy of high intensity focused ultrasound, in order to expose the intrahepatic ducts better without causing damage.When those ducts were clearly exposed and identified, it was easier to decide which ones to be transected or retained. The vasa with a diameter below 2 mm were directly transected, and bipolar coagulation was adopted for the oozing bleeding at the wound;those with a diameter above 2 mm were transected with biological clip. We chose to first transect the hepatic blood inflow (including the hepatic pedicles of the right posterior lobe and the left and right caudate lobes) from the diseased hepatic side, which could decrease the blood stasis on the diseased hepatic side. In case of a malignant lesion, this could also decrease the possibility of tumor metastasis via portal vein (PV) during the operation. It was easier to identify the ducts and shorten the surgery duration by dissecting hepatic pedicles outside the sheath [13] . The bleeding on the cutting surface of the wound was obviously decreased during the hepatectomy by the three techniques we adopted, i.e. priority transecting the hepatic pedicles outside the sheath, Pringle maneuver,and low central venous pressure.
Regarding the control over the cutting plane of hepatectomy,a wedged ventral part of the segments S5 and S8 needed to be preserved after the extended right posterior lobe resection. Since the case in this report was considered a benign lesion, we could access the plane first via the ischemic line of the right posterior lobe and then approach to the tumor capsule, or dissect the liver with an angle with intraoperative ultrasound towards the MHV. After preserving the hepatic blood inflow in the ventral part of the segments S5 and S8 at the first porta hepatis, we carried out dissection to expose the root of the MHV at the second porta hepatis,and then conducted separation along the right wall of the MHV from the cephalic to the foot direction (dissection of the MHV from the foot to the cephalic required more care, as it can easily tear the MHV branches retrogradely). The separation plane was changed to the dorsal wall of the MHV when the separation was close to the first porta hepatis so that it joined the plane between the Arantius duct and the left caudate lobe. The operation underwent along the MHV during the whole procedure, with two cutting planes established between the three lines, i.e. the right posterior lobe ischemic line, the MHV and the Arantius duct. With the MHV as the axis, the cutting surface was changed from the right side to their dorsal side, which was the key to the joint of the whole cutting surface. Previous experience considered it dangerous to expose the veins. However, transecting the liver along the MHV all through the procedure of anatomical hepatectomy, on the one hand, could use the MHV as the anatomic landmark to guide the completion of the cutting plane and the lesion resection. One the other hand,it helped identify the branches of the MHV and the blood flow branches of the caudate lobe clearly and dissect the branch veins one by one safely from the root to decrease the risk of bleeding.In case of missing of cutting plane, the MHV wrapped in the liver parenchyma was more likely to be damaged compared to the completely exposed vein during liver resection. It surely needed carefulness for the separation of the hepatic vein, especially for a patient with liver cirrhosis because the hepatic tissue was seriously fibrotic and clearly adhered to the hepatic vein, probably resulting in hepatic vein tearing and then massive hemorrhage or gas embolism. The 5-0 Prolene sutures could be used to repair a venous crevasse during operation in case of any, and the anatomical hepatectomy with the purpose of exposing the hepatic vein must be aborted or the surgical method must be converted to laparotomy in time when necessary.
Since the patient had a massive tumor that needed to be completely removed, we adopted the strategy of first separating the diseased hepatic part from the preserved hepatic part and then removing the diseased hepatic part around IVC. When the diseased part was separated from the healthy part completely, the blood outflow of the diseased hepatic part was normal, but its blood inflow had already been transected. Meanwhile, the blood supply to the collateral circulation in liver parenchyma had been practically transected. Therefore, the diseased hepatic part was dry without blood supply. Regarding the transection of the SHVs, it was even feasible only to properly ligate the SHVs on IVC-side without applying ligation to the hepatic side. In the past, the SHVs had to be first treated prior to caudate lobe resection, which caused congestion at the caudate lobe. Therefore, uncontrollable massive hemorrhage might probably occur due to the detachment of biological clips on the diseased hepatic side during the dissection of the SHVs.
Based on the whole procedures above, we found that the laparoscopic hepatectomy had two key points: 1) bleeding control: it was safe and effective to control intraoperative bleeding following the principle of transecting incoming hepatic ducts first and outgoing ducts afterwards; 2) on the liver dissection and cutting plane:it was better to control the cutting surface of the liver by establishing a virtual plane using the important ducts, including hepatic ischemic lines, hepatic veins, Arantius duct, IVC, etc., as anatomic landmarks. As long as the two key points above were followed, we believe that it is safe and feasible to conduct laparoscopic extended right posterior and caudate lobe resection for massive right and caudate lobe tumors.
Acknowledgments
None.
CRediTauthorshipcontributionstatement
LiangChen:Writing - original draft, Writing - review & editing.Lu-ZhengLiu:Writing - original draft, Writing - review &editing.Jia-ChengChen:Funding acquisition.Da-FengXu:Visualization.ChengChen:Software.Shi-XunLin:Resources.Xiang-XiangLuo:Software.Jin-CaiWu:Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81660489), Hainan Province Science and Technology Special Fund (ZDYF2020134), the Specific Research Fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202005) and the Innovative Research Project for Postgraduates of Hainan Province (Hys2020-355).
Ethicalapproval
The consent was obtained from the patient for publication of this report.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementarymaterials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.hbpd.2022.10.008 .
 Hepatobiliary & Pancreatic Diseases International2023年3期
Hepatobiliary & Pancreatic Diseases International2023年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Narrative medicine principles and organ donation communications
- Hepatobiliary&Pancreatic Diseases International
- Etiology and classification of acute pancreatitis in children admitted to ICU using the Pediatric Sequential Organ Failure Assessment (pSOFA)score
- Endoscopic ultrasound-guided drainage of peripancreatic fluid collections: What impacts treatment duration?
- Percutaneous ultrasound and endoscopic ultrasound-guided biopsy of solid pancreatic lesions: An analysis of 1074 lesions
- Increased incidence of indeterminate pancreatic cysts and changes of management pattern: Evidence from nationwide data
