Etiology and classification of acute pancreatitis in children admitted to ICU using the Pediatric Sequential Organ Failure Assessment (pSOFA)score
Vratislav Smolka, Marie Rohanova, Miroslav Seda, Eva Karaskova, Oksana Tkachyk,Martin Zapalka, Jana Volejnikova
Department of Pediatrics, Faculty of Medicine and Dentistry, Palacky University and University Hospital Olomouc, I. P. Pavlova 185/6, Olomouc 779 00,Czech Republic
ARTICLE INFO Article history:Received 8 September 2021 Accepted 29 June 2022 Available online 4 July 2022
Keywords:Acute pancreatitis Children Organ dysfunction Prognosis pSOFA score
ABSTRACT Background: Pediatric acute pancreatitis (AP) is rare but increasing. Severe AP is associated with higher morbidity and mortality. However, there are no universally accepted prognostic criteria for AP.Methods: This retrospective study included children with AP admitted to an intensive care unit (ICU) of our tertiary pediatric center between January 2009 and December 2018. The severity of organ dysfunction in AP was assessed according to the modified Atlanta criteria using the Pediatric Sequential Organ Failure Assessment (pSOFA) and Computed Tomography Severity Index (CTSI).Results: Seventy acute episodes of AP were evaluated in 55 children with primary pancreatitis. In addition, secondary AP was diagnosed in 15 patients originally admitted to ICU for different indications. Mild AP [no organ dysfunction, normal computed tomography (CT) finding] was the most prevalent (64/85 episodes in 49 children), followed by moderate AP (15 children; pSOFA 2-9 points, CTSI 3-4 points on admission). Severe AP (pSOFA 4-17 points, CTSI 6-10 points) was diagnosed in 6 children with traumatic or secondary AP. The most frequent etiologies of primary AP episodes were idiopathic (39%) and biliary(31%). Children with idiopathic AP had frequent relapses and comorbidities. Hereditary AP was typically mild, but presented with high pancreatic enzyme levels and recurrence rates. Admission at ICU and an interval without enteral nutrition (EN) were relatively short in drug-induced AP and relatively long in secondary and traumatic AP. Endoscopic retrograde cholangiopancreatography (ERCP) was performed in 13 patients with biliary AP and in 4 patients with traumatic AP. No AP-related death was observed.Conclusion: pSOFA score accurately reflects the severity and prognosis of AP in children.
Introduction
Acute pancreatitis (AP) is rare in childhood, but its incidence has been increasing. Contrary to pancreatitis in adults with predominant alcoholic and obstructive etiology, causes of pediatric AP are much more diverse. The causes of pediatric AP include biliary tract diseases and structural anomalies, abdominal injury, systemic conditions, medications, infections and metabolic disorders, but also frequently remain unknown (idiopathic AP).The incidence of AP in children was estimated at 3.6-13.2 cases per 100 000 patients/year [ 1 , 2 ]. AP can be classified as mild, moderate or severe [3] . The mild form is prevailing and associated with excellent prognosis, whereas severe AP is uncommon in children.The mortality of pediatric AP does not exceed 11% and is higher in patients with comorbidities [ 2 , 4 ].
Categorization of AP severity is currently based on a definition of systemic inflammatory response syndrome (SIRS) and organ dysfunction in children by the International Pediatric Sepsis Consensus Conference from 2005 [5] . AP classification according to the Atlanta criteria modified for pediatric population includes cardiovascular, respiratory and renal dysfunctions [3] . However, meeting these criteria would already indicate a severe organ dysfunction.The utility of scoring systems for adults in a pediatric setting is limited and unambiguous, and criteria predicting the development of severe AP in children are lacking. Pediatric score of AP severity according to DeBanto et al. [6] has a relatively low specificity and requires a 48 h monitoring for evaluation. Even other published prognostic models based on clinical, biochemical or radiological results did not reach sufficient specificity [7-11] .
Here we report on patients admitted to pediatric intensive care unit (ICU) due to AP or presence of pancreatitis-associated clinical,laboratory or radiologic signs. To assess AP severity, we employed Atlanta criteria modified for pediatric population using the Pediatric Sequential Organ Failure Assessment (pSOFA) score reflecting organ dysfunction ( Table 1 ) [12] . Furthermore, the ICU length of stay and time to restarting enteral nutrition (EN) were evaluated.

Table 1pSOFA: the pediatric sequential organ failure assessment.
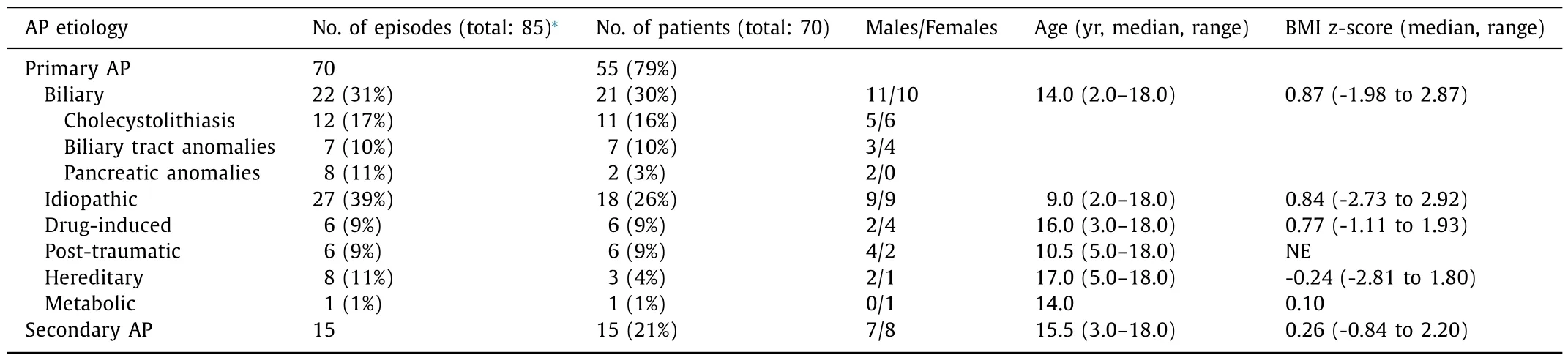
Table 2 Distribution and characteristics of children admitted to ICU due to AP.
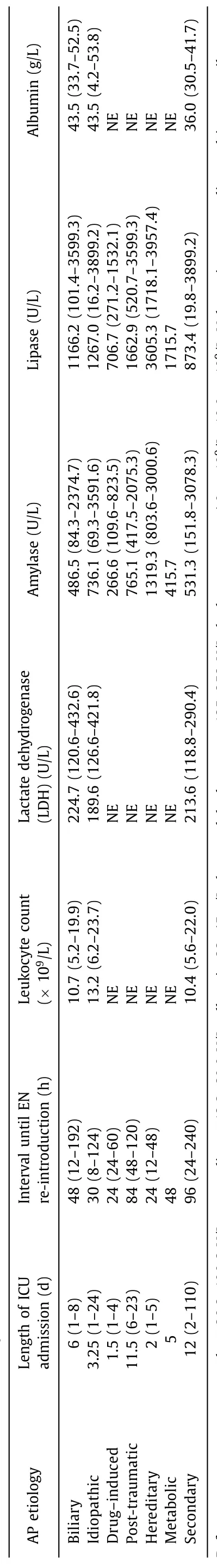
Table 3 Admission course and laboratory results of children admitted to ICU due to AP.
Patients and methods
This retrospective study recruited patients under 19 years of age hospitalized at ICU of a tertiary pediatric care center between January 2009 and December 2018. This included children with AP being the original reason of admission (primary AP) and children admitted with a different diagnosis but subsequently rediagnosed with AP during their ICU stay (secondary AP).
All patients underwent clinical, biochemical and immunological examination, nutritional status assessment (body mass index standard deviation, BMI z-score) and an abdominal ultrasonography (USG) within 24 h after admission. Computed tomography (CT)and/or magnetic resonance imaging (MRI) were indicated according to clinical condition, biochemical and USG results [7] . Endoscopic retrograde cholangiopancreatography (ERCP) was performed in cases with suspected biliary/pancreatic duct obstruction, either for therapeutic intervention or to clarify magnetic resonance cholangiopancreatography (MRCP) finding. In patients with recurrent AP of unknown origin, mutations in the following possibly causative genes were examined:PRSS1(serine protease 1, cationic trypsinogen),SPINK1(serine peptidase inhibitor Kazal type 1) andCFTR(cystic fibrosis transmembrane conductance regulator).
Diagnosis of AP was established based on the INSPIRE (International Study Group of Pediatric Pancreatitis: In Search for a Cure)definition [13] . Presence of at least two from the following criteria was required: (i) abdominal pain; (ii) serum amylase and/or lipase levels greater than 3-fold the upper reference limit and (iii) characteristic radiographic findings of AP. Recurrent AP was defined as a minimum of two AP episodes without diagnosed chronicity, where an asymptomatic interval and/or normalization of pancreatic enzyme levels lasted for more than 1 month between the episodes.
The severity of AP was evaluated according to the modified Atlanta criteria using the Pediatric Sequential Organ Failure Assessment (pSOFA), which excludes hepatic, hematologic parameters and consciousness ( Table 1 ) [12] . The pSOFA score was assessed within 6 h after admission and re-evaluated at 48 h after admission; here we reported the pSOFA values at 6 h after admission.Organ dysfunction was present in pSOFA ≥2 points. Local complications were scored according to the CT Severity Index (CTSI) [ 7 , 8 ].AP was classified as mild (no organ dysfunction or local complications), moderate (transient organ dysfunction<48 h, CTSI ≥3 points) or severe (organ dysfunction ≥48 h).
Results
Within the evaluated period, seventy episodes of confirmed primary AP occurred in 55 patients. Secondary AP was observed in 15 patients, who were originally admitted to ICU for other reasons.Our cohort included 35 boys and 35 girls with a median age of 12.4 (range 2.0-18.0) years. The prevailing reason for admission was abdominal pain with elevated serum lipase and/or amylase levels ( Tables 2 and 3 ). CT was indicated in 21 cases: of these, 9 children had mild AP (CTSI 1-2), 8 children moderate AP (CTSI 3-4) and 4 children severe AP (CTSI 6-10) ( Table 4 ). MRI of the pancreas and biliary tract was necessary in 8 cases (5 obstructive AP,3 idiopathic AP). All patients in our cohort survived.

Table 4 Clinical and laboratory characteristics of mild, moderate and severe AP.
Biliary AP
Twenty-one children (11 boys and 10 girls) were admitted with biliary (obstructive) pancreatitis and had 22 acute episodes of primary AP. Cholecystolithiasis, choledocholithiasis or biliary sludge were diagnosed in 11 patients. Congenital malformations of the biliary tract were present in 7 patients, including choledochal cyst in 5 patients and distal stenosis of the choledochus in 2 patients.Two patients had pancreas divisum. In the remaining one patient, biliary obstruction was caused by a pancreatic pseudocyst of unknown etiology. AP was diagnosed at a significantly younger age (2-5 years) in choledochal cysts than in cholelithiasis (10-18 years). Two children had cholecystolithiasis underlied by hereditary hemolytic anemia. CT was performed in 7 patients, four of whom had anatomical anomalies of biliary tract and/or pancreas.
Diagnostic MRCP was performed in 5 cases, ERCP in 13 cases and papillosphincterotomy in 9 cases. Biliary stent was inserted in 10 children. Cholecystectomy and other biliary tract surgeries were required in 14 patients within 6 weeks after admission. None of the patients had severe AP, however, 5 cases were evaluated as moderate AP (pSOFA on admission 2, 2, 3, 3 and 4 points, respectively). The duration of ICU admission and the interval until reintroduction of EN in biliary AP were relatively longer than those in drug-induced and hereditary AP ( Table 3 ), but relatively shorter than those in post-traumatic AP ( Table 3 ).
Idiopathic AP
The etiology of AP remained unresolved (idiopathic AP) in 9 girls and 9 boys with total 27 episodes of primary AP. Four of these children had obesity with metabolic syndrome, contrariwise,three patients were malnourished. Two patients were followed with genetic disorders (1p36 microdeletion syndrome, Williams-Beuren syndrome), one with hyperinsulinism and sleep apnea and one with cerebral palsy with spastic quadriplegia and severe psychomotor retardation. History of antecedent respiratory or gastrointestinal infection was recorded in 10 children. The idiopathic AP was mostly mild with only 4 episodes classified as moderate(pSOFA 2, 3, 3 and 4 points, respectively; CTSI 4 points). Median age, BMI z-score ( Table 2 ), levels of amylase, lipase, lactate dehydrogenase (LDH) and albumin, leukocyte count ( Table 3 ) and length of ICU admission (5.1 days) ( Tables 3 ) were comparable with biliary AP. However, EN was re-introduced earlier, and the lipase level was higher in idiopathic AP.
Drug-induced AP
Drug-induced AP occurred in 6 patients. Five of them were treated for inflammatory bowel disease (IBD) with azathioprine,cyclosporine A and mesalazine, the remaining one patient was receiving valproic acid due to epilepsy. All these children had mild AP, which resolved rapidly after drug cessation. The median duration of ICU admission was 1.5 days and EN was re-introduced 24 h after diagnosis.
Post-traumatic AP
Another 6 children suffered from post-traumatic pancreatitis following blunt abdominal injury. All these patients underwent CT examination and 4 of them also had emergent ( ≤48 h after admission) ERCP due to a suspected pancreatic duct rupture. Five children underwent surgery. In 3 cases, the AP was severe (pSOFA 4,4 and 8 points, respectively; CTSI 6-8 points) and in 3 cases moderate (pSOFA 4, 4 and 6 points, respectively; CTSI 4-6 points). The median duration of ICU stay was 11.8 days and EN was started 84 h after diagnosis.
Hereditary AP
Hereditary AP was confirmed in 3 patients, two of whom subsequently fulfilled the criteria for chronic pancreatitis. Heterozygous mutations inPRSS1gene were identified in 2 cases andSPINKmutation in one case. The total number of AP attacks was 8, ICU admission lasted 2 days and EN was administered after 24 h. In one patient, the first flare-up was of a biliary origin. All 3 patients had mild AP, but their amylase and lipase levels were significantly higher than those in other AP subgroups.
Metabolic AP
Only one patient presented with decompensated diabetes mellitus with severe ketoacidosis and obtained EN 48 h after admission.
Secondary AP
Secondary AP was diagnosed in 15 patients. Five of them had undergone abdominal surgery due to IBD, 4 had suffered polytrauma and 3 sustained septic shock. In 3 cases, AP developed as a complication after ERCP was performed for various indications.
In 3 patients, secondary AP was severe (pSOFA 7, 14 and 17 points, respectively). One of these patients manifested septic shock and multiorgan failure with pSOFA 12 points and CTSI 10 points following ERCP. The second patient was admitted after drug intoxication with respiratory failure, hypotension and hypothermia(pSOFA 9 points). The third patient had undergone surgery for significant intestinal stenosis and abscesses in Crohn’s disease. Three patients had moderate AP (pSOFA 5, 7 and 9 points, respectively)and 9 patients had mild secondary AP.
CT of the pancreas was carried out in only 1 of secondary AP patients. In the majority (10/15) of children, AP was diagnosed 5 to 12 days since ICU admission. Secondary AP presented with lower albumin levels and with lower lipase and amylase levels ( Table 3 ).However, the duration of ICU stay (12 days) and interval until EN re-introduction (96 h) were by far the longest of the whole cohort.
Overall, of the observed 85 AP episodes, sixty-four (75%) were mild, fifteen (18%) moderate and 6 (7%) severe. Only cases of posttraumatic and secondary AP progressed into a severe course. Contrarily, all cases of drug-induced and hereditary AP were mild. The severity of AP correlated both with the duration of ICU admission and with an interval until EN re-introduction ( Table 4 ). Median albumin levels were relatively lower in severe than in moderate/mild AP, but the other evaluated parameters were similar.
Discussion
Severe AP is characterized by cardiovascular, renal and pulmonary dysfunction and is associated with significant morbidity and mortality. Pediatric AP is a heterogeneous entity with diverse pathogenesis, which complicates prediction of the disease course.Different from Coffey et al. [9] , we did not confirm absolute serum lipase level to be a reliable predictive marker in this regard. In agreement with the currently used prognostic model [10] , we observed a correlation of albumin levels with AP severity, however,LDH and leukocyte count did not appear to be significantly relevant. CT imaging including the CTSI score [11] is not a suitable early prognostic marker in pediatric AP due to a radiation burden. The consensus for CT indication in children with AP is lacking.AP in childhood is predominantly mild with pancreatic edema detected by USG and does not require further radiographic imaging.In our cohort, CT was indicated in complicated AP with persistent pain, vomiting, organ dysfunction and predominantly in pancreatic injuries. In accordance with the literature [14] , CTSI was the lowest in mild AP with a subsequent increase in moderate and severe AP.ICU admission in children, who required CT assessment based on clinical criteria, is currently endorsed in CTSI ≥3 [15] , which approximately corresponds to our patients with moderate and severe AP.
In this study, we evaluated organ condition using an adapted and validated pSOFA score for pediatric age, which was originally designed for the purposes of sepsis assessment. The following aspects of pSOFA have enabled scoring also in cases with less severe organ dysfunction: (i) an easier monitoring of the peripheral oxygen saturation/administered oxygen fraction (SpO 2 /FiO 2 ) ratio;(ii) a facilitated evaluation of absolute blood pressure values or the need for inotropic support and (iii) a relation of serum creatinine level to the patient’s age. The median pSOFA in severe AP was 8, which represents an optimal cutoff for mortality prediction in critically ill patients, both children and adults [ 5 , 16 ].A higher pSOFA could identify children with major multiorgan dysfunction [17] and, more specifically, predict a severe course of AP. SIRS criteria fulfillment can be used for AP severity assessment on admission, including ICU indication [18-20] , because it was proven to be an independent predictor of admission duration and interval until EN re-introduction [ 19 , 21 ]. However, the presence of SIRS on admission failed to predict severe AP development in 65.5% of pediatric patients [21] . Whether AP may deteriorate and progress into severe AP even in an absence of SIRS, is unclear.
The majority of children in our study presented with mild AP,which did not necessarily require an ICU hospitalization [ 9 , 22 ]. Yet,due to an unknown etiology of AP in some patients at the time of admission and due to a risk of an imminent disease progression,all children with AP were initially admitted to ICU. Furthermore,because of a different organization of pediatric beds at our hospital, there is no specialized pediatric emergency department and the initial emergency care is provided directly at the ICU. Children with a mild disease course are subsequently transferred to a standard pediatric gastroenterology department.
We observed significantly less cases of severe AP (7%) than the reported 15%-34% [ 1 , 7 , 9 , 12 ], exclusively in abdominal injuries and secondary AP. The severity of pancreatic and particularly pancreatic duct injuries directly correlates with morbidity and mortality [23] . Pancreatic trauma in our cohort also represented a frequent reason for ERCP indication (the second most prevalent after biliary pancreatitis). We reported an inverse ratio of primary and secondary AP than other studies [ 24 , 25 ], which may be explained by the abovementioned cautious approach to ICU admissions in our center. Secondary AP showed relatively long duration of ICU admission and interval until EN re-introduction, and high pSOFA and CTSI. Severe AP cases following ERCP were associated with infections leading to a septic shock or sepsis-induced organ dysfunction.
All cases of drug-induced and hereditary AP in our cohort were mild with relatively short ICU admission and interval until EN reintroduction. Interestingly, the levels of pancreatic enzymes were relatively low in drug-induced AP but high in hereditary AP. We observed more frequent recurrence in hereditary AP, thus confirming that mutations in the known hereditary pancreatitis-related genes represent a risk factor for the development of recurrent AP and chronic pancreatitis [26] . The number of hereditary AP episodes was in accordance with the literature [27] with estimated recurrence rates of 15%-35% [28] .
Drug-induced AP accounts for 1.4%-25.6% AP cases [29-32] and correspondingly, it represented 11% of cases in our study. According to our previous experience, it can manifest as a severe necrotizing pancreatitis during valproic acid administration in primary epilepsy [33] . The majority of drug-induced AP occurred during an IBD treatment, and further frequent comorbidities may include other convulsive diseases on antiepileptic therapy and acute lymphoblastic leukemia [29] . However, our cohort did not comprise patients with acute leukemia receiving asparaginase, because these children were admitted to a specialized hemato-oncology unit.
The most frequent AP etiology was biliary (by the number of patients) or idiopathic (by the number of episodes, due to a frequent recurrence). Congenital or acquired biliary tract disorders are amongst the most frequent triggers of AP in childhood. Obesity is an unambiguous risk factor for gallstone- and sludge-induced pancreatitis [34] ; such AP usually occurs during adolescence, contrary to congenital bile duct anomalies which are diagnosed at an early age. The length of ICU admission in biliary AP was dependent on whether ERCP was performed ( Table 3 ). However, the interval until EN re-introduction was relatively long in biliary AP. The ICU admission in idiopathic AP could be prolonged by frequent presence of comorbidities [21] . In both biliary and idiopathic AP, only moderate (5 and 4 patients, respectively) and mild courses were seen.The median age of children with idiopathic AP was relatively low.
Our study has some limitations. First, it is a retrospective study,which recruited patients during a considerable time span. Next, the cohort size is limited as it includes only children from a single tertiary care center. We assume that the retrospective nature of our study did not affect the diagnosis of primary AP according to the current definition, but might have been more problematic for secondary AP diagnosis (with an increase in pSOFA due to the primary condition).
In conclusion, pSOFA represents a promising tool to evaluate AP severity and prognosis. Further prospective multicentric studies and their comparison with the currently suggested prognostic markers are necessary to implement pSOFA into pediatric clinical practice.
Acknowledgments
None.
CRediTauthorshipcontributionstatement
VratislavSmolka:Conceptualization, Data curation, Formal analysis, Investigation, Writing - original draft, Writing - review &editing.MarieRohanova:Investigation.MiroslavSeda:Investigation.EvaKaraskova:Investigation.OksanaTkachyk:Investigation.MartinZapalka:Data curation, Formal analysis.JanaVolejnikova:Conceptualization, Funding acquisition, Writing - original draft, Writing - review & editing.
Funding
This work was supported by grants from the European Regional Development Fund - Project ENOCH ( CZ.02.1.01/0.0/0.0/16_019/0 0 0 0868 ) and the Ministry of Health , Czech Republic - conceptual development of research organization (MH DRO; grant FNOL,0098892 ).
Ethicalapproval
This study was approved by the Ethics Committee of the Palacky University and University Hospital Olomouc.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2023年3期
Hepatobiliary & Pancreatic Diseases International2023年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Narrative medicine principles and organ donation communications
- Hepatobiliary&Pancreatic Diseases International
- Laparoscopic extended right hepatectomy for posterior and completely caudate massive liver tumor (with videos)
- Endoscopic ultrasound-guided drainage of peripancreatic fluid collections: What impacts treatment duration?
- Percutaneous ultrasound and endoscopic ultrasound-guided biopsy of solid pancreatic lesions: An analysis of 1074 lesions
- Increased incidence of indeterminate pancreatic cysts and changes of management pattern: Evidence from nationwide data
