Leaf stoichiometry of Leontopodium lentopodioides at high altitudes on the northeastern Qinghai-Tibetan Plateau, China
WANG Hairu, SU Haohai, Asim BISWAS, CAO Jianjun,3,4*
1 College of Geography and Environmental Science, Northwest Normal University, Lanzhou 730070, China;
2 School of Environmental Sciences, University of Guelph, 50 Stone Road East, Guelph, Ontario N1G 2W1, Canada;
3 Key Laboratory of Eco-Functional Polymer Materials of the Ministry of Education, Northwest Normal University, Lanzhou 730070, China;
4 Key Laboratory of Resource Environment and Sustainable Development of Oasis, College of Geography and Environmental Science, Northwest Normal University, Lanzhou 730070, China
Abstract: Altitude affects leaf stoichiometry by regulating temperature and precipitation, and influencing soil properties in mountain ecosystems. Leaf carbon concentration (C), leaf nitrogen concentration (N),leaf phosphorous concentration (P), and their stoichiometric ratios of Leontopodium lentopodioides (Willd.)Beauv., a widespread species in degraded grasslands, were investigated to explore its response and adaptation strategy to environmental changes along four altitude gradients (2500, 3000, 3500, and 3800 m a.s.l.) on the northeastern Qinghai-Tibetan Plateau (QTP), China. The leaf C significantly varied but without any clear trend with increasing altitude. Leaf N showed an increasing trend, and leaf P showed a little change with increasing altitude, with a lower value of leaf P at 3500 m than those at other altitudes.Similarity, leaf C:P and N:P exhibited a little change with increasing altitude, which both had greater values at 3500 m than those at other altitudes. However, leaf C:N exhibited a decreasing trend with increasing altitude. Soil NH+ 4-N, soil pH, soil total phosphorus (STP), mean annual temperature (MAT), and mean annual precipitation (MAP) were identified as the main factors driving the variations in leaf stoichiometry of L. lentopodioides across all altitudes, with NH+ 4-N alone accounting for 50.8% of its total variation.Specifically, leaf C and N were mainly controlled by MAT, soil pH, and NH+ 4-N, while leaf P by MAP and STP. In the study area, it seems that the growth of L. lentopodioides may be mainly limited by STP. The results could help to strengthen our understanding of the plasticity of plant growth to environmental changes and provide new information on global grassland management and restoration.
Keywords: alpine area; environmental changes; leaf elements; nutrient limitation; Qilian Mountains
1 Introduction
A variety of elements closely control the growth of biological organisms and maintain the ecosystem balance (Moe et al., 2005; ?gren and Weih, 2012). These elements include carbon (C)nitrogen (N), and phosphorus (P), in which C constitutes about 50% of a plant's dry mass (?gren,2008); N, a crucial component of proteins and enzymes, plays a vital role in plant photosynthesis,respiration, and litter decomposition (Thomson et al., 2012; Yang et al., 2018); and P, an essential element in ribosome production, affects the production of N-rich proteins (?gren, 2008; Reich et al., 2009). Therefore, C, N, and P are all interactive, and their composition in organisms determines the main processes of the ecosystem (Vitousek et al., 2010). In the context of global biodiversity loss and global change, ecological stoichiometry focusing on the balances (relative proportions) of these elements, and their interactions in ecosystem has emerged (Elser et al., 1996,2000; Sterner and Elser, 2002; Moe et al., 2005). It not only provides a new method to integrate evolutionary biology and ecosystem science, but also plays a critical role in plant functional traits(He et al., 2009), plant productivity (Tang et al., 2018), ecosystem carbon storage (Reich et al.,2005; Hu et al., 2021), and ecosystem function (Tian et al., 2019; Lin et al., 2022).
Leaf, the main organ of plant photosynthesis, is the primary place for material and energy exchange between plants and environment (Sun et al., 2017), and its stoichiometry is essential for understanding plant nutrient limitation, nutrient utilization efficiency, and adaptation strategies to environmental change (Schreeg et al.,2014), and formulating the rational management policies(Xia et al., 2014; Su et al., 2022). Leaf stoichiometry has been studied widely, both at the plant community level (Bai et al., 2012; Xu et al., 2014; Sardans et al., 2016; Gong et al., 2020) and at the individual species level (Ai et al., 2017; Wang et al., 2019; Cao et al., 2020; Liu et al., 2020;Su et al., 2021; Tao et al., 2021) under various disturbances and environmental changes such as altitude change.
Generally, altitude can control plant growth metabolic rate and nutrient uptake efficiency by influencing various environmental gradients such as climate and soil properties (Qin et al., 2016;Feng et al., 2021). At the regional scale, altitude is a vital and fundamental factor for environmental change (Jiang et al., 2019). The high altitude, low CO2concentration, strong radiation, and low temperature in the Qinghai-Tibetan Plateau (QTP), China make its plants extremely sensitive to environmental changes, and develop unique adaptation mechanisms through long-term natural selection of environmental stress (Xu et al., 2005). In this region, leaf stoichiometry of some plants was studied previously. For instance, Wang et al. (2019) found that leaf N and P concentration ofJuniperus przewalskiiKomarovin the northeastern QTP significantly varied with temperature. Cao et al. (2020) reported that leaf C, N, and P ofOxytropis ochrocephalaBunge in the Qilian Mountains (northeastern QTP) were significantly higher at high altitudes than at low altitudes. However, Guo et al. (2021) observed that leaf C and N ofStellera chamaejasmeL. on the QTP maintained relative stability, in accordance with homeostasis theory that suggests the stability of leaf nutrient composition when plants face the environmental changes (Sterner and Elser, 2002). These results indicate that the response of leaf stoichiometry to environmental changes is species-specific, which is beneficial to the metabolic and phenological processes of plant, and nutrient combination for increasing plant growth rate by self-regulation(Kang et al., 2011; Li et al., 2015).
Leontopodium lentopodioides(Willd.) Beauv., a common perennial herbaceous plant in the degraded grasslands on the QTP. Zhang et al. (2020) observed that the coverage and abundanceofL. lentopodioidespopulation gradually decreased from 2900 to 3800 m, and the spatial distribution of the population shifted from aggregated to random. Sun et al. (2016) found that the structural morphology of its leaves changed with increasing altitude, such as increasing the density of stomata in the upper and lower epidermis of the leaves to capture more CO2, and strengthening the palisade and spongy tissue to resist strong radiation and low temperature.However, leaf stoichiometry ofL. lentopodioidesalong high altitudes in this region is not documented.
In the present study, we aimed to: (1) explore changes in leaf stoichiometry ofL.lentopodioidesfrom 2500 to 3800 m, and identify the dominant factor influencing the change; (2)identify direct and indirect factors affecting leaf nutrients ofL.lentopodioidesalong altitude; and(3)identify the main factor of limitingL.lentopodioidesgrowth on the northeastern QTP. Based on current theories and existing studies, we hypothesize that altitude may affect leaf stoichiometry ofL. lentopodioidesthrough exerting effects of climate and soil properties.
2 Materials and methods
2.1 Study area
Qilian Mountains located in the northeastern part of the QTP, Northwest China (36°30′-39°42′N,93°30′-103°01′E). These mountains have a high western and low eastern topography, and altitudes between 2000 and 5500 m a.s.l. The mountains above 4600 m are covered with snow all year round, and glaciers are widely distributed, making them the birthplace of many rivers. These mountains with a plateau and continental climate have a mean annual temperature (MAT) of 6.21°C, and a mean annual precipitation (MAP) of 233.95 mm (Liu et al., 2020). Grassland is the most important vegetation type, accounting for about 53% of the total area (Yang et al., 2022).The vegetation mainly includesPotentilla chinensisSer.,Elymus dahuricusturcz.,Agropyroncristatum(L.) Gaertn.,Carex tristachyaThunb.,Stipa capillataLinn., etc. And the soil types are mainly mountain gray cinnamon soil, chestnut soil, and alpine meadow soil (Zhu et al.,2016).
2.2 Sampling and analyses
In August 2018, based on the distribution altitude (2200-3800 m) ofL.lentopodioidesin the Qilian Mountains, we conducted a field survey. In order to control the selected hillsides with sunny slope aspects and similar slopes, we selected four altitudes of 2500, 3000, 3500, and 3800 m (Fig. 1). The basic characteristics of each site are presented in Table 1. At each altitude gradient, three 10 m×10 m sample plots with 10-m intervals were randomly placed, and a total of 12 plots (4 altitudes×3 plots) were selected. From each plot, 12-15 healthy, approximately the same size ofL.lentopodioideswere selected, and 4-5 leaves from the middle of the plant were collected and stored in an envelope. Additionally, three 1 m×1 m sample quadrats were identified within each plot to eliminate the effect of small-scale spatial heterogeneity on soil properties, and a total of 36 quadrats (12 plots×3 quadrats) were identified. In each quadrat, after manually removing soil surface litter, soil samples at 0-10, 10-20, and 20-40 cm depths were collected from the center and corners of each quadrat with a 35-mm diameter soil drill. Within each quadrat,soil samples from the same soil depth were mixed in self-sealing bags, marked carefully, and taken back to the laboratory.
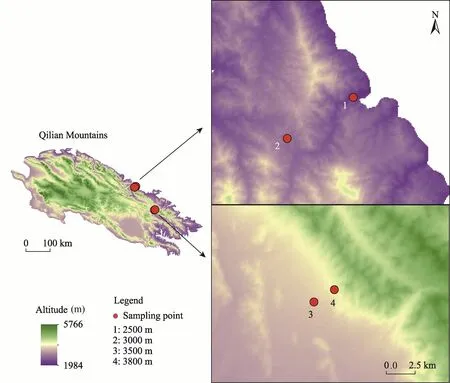
Fig. 1 Four sampled altitudes (2500, 3000, 3500, and 3800 m) in the study area
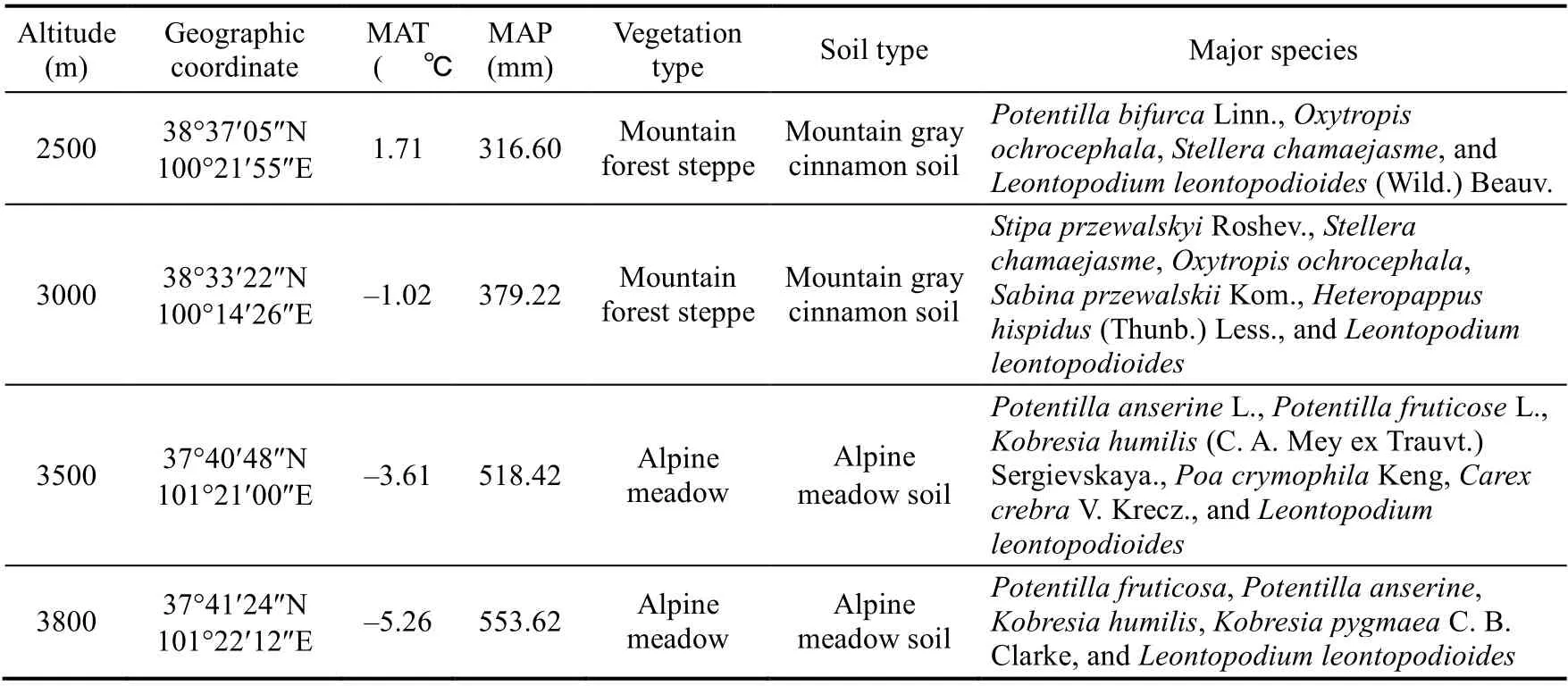
Table 1 Basic characteristics of sample site in the northeastern Qinghai-Tibetan Plateau, China
In the laboratory, leaves were dried in an oven for 48 h at 65°C to a constant weight, ground by ball milling, packed with tin foil, and marked. After air drying at room temperature and removing stones, roots, and other debris, soil samples were split in two; one part of soil samples were passed through a 10-mesh sieve for the determination of soil available nitrogen (AN) and pH, and the other part of soil samples were passed through a 100-mesh sieve for the determination of soil organic carbon (SOC), soil total nitrogen (STN), and soil total phosphorus (STP). SOC and leaf C were determined by the potassium dichromate oxidation-external heating method. STN and leaf N were analyzed using the Kjeldahl method (Nelson and Sommers, 1982) following digestion with H2SO4and K2SO4-CuSO4-Se accelerator, distill by Kay's nitrogen analyzer, and titration with dilute sulfuric acid. STP and leaf P were obtained by the H2SO4-HClO4, and estimated by molybdenum antimony colorimetry (Olsen et al.,1954). Soil pH was measured by the Sartorius PB-10 meter with a ratio of air-dried soil to distilled water of 1.0:2.5. Soil ammonium nitrogen(NH+4-N) and nitrate nitrogen (NO-3-N) were determined by the SmartChem Discrete Auto Analyzer 200 (AMS/Westco, Italy). In brief, 5 g of soil was mixed well in 50 mL of 2 mol/L KCl and shaken for 1 h. The extracted liquid after filtration was placed in the machine to measure NH+4-N and NO-3-N.
2.3 Data analysis
Studies have shown that the formula for MAT and MAP are as follows (Zhao et al., 2005, 2006):
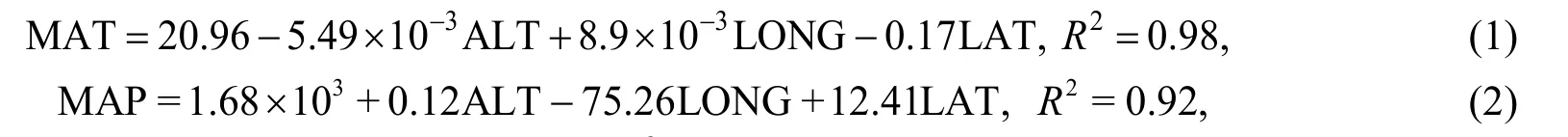
where MAT is the mean annual temperature (℃); MAP is the mean annual precipitation (mm);ALT is the altitude (m); LONG is the longitude, LAT is the latitude; andR2is the regression coefficient.
To verify the independence of the data, we first tested the linear mixed model (LMM) for the fixed effect of altitude as well as the random effect of plots in R v.4.1.1 software (Liu et al., 2018).After all data have been normalized, SPSS v.22.0 software (SPSS Inc., Chicago, IL) was mainly used fort-test, which was used to determine the difference in parameters between altitudes.Redundant analysis (RDA) in R software with ggrepel, vegan, and ggplot2 packages (Yang et al.,2018) was used to determine the dominant environmental factors affecting leaf stoichiometry ofL.lentopodioides. Structural equation modeling (SEM) of Amos v.24.0 software (Smallwaters Corporation, Chicago, IL, USA) was applied to further estimate the direct and indirect effects of dominant factors on leaf C, N, and P. The chi-square (χ2) test (P>0.05), normed chi-square (NC)in the range of 0-2, goodness-of-fit index (GFI) (>0.9), and root mean square error of approximation (RMSEA) (<0.05) demonstrated the model fit (Schermelleh-Engel et al., 2003).The data in the figures are represented as mean±standard error at the significance level ofP<0.05.
3 Results
3.1 Effects of altitude and plot on soil properties and leaf stoichiometry of L.lentopodioides
LMM results showed that except STP, altitude had a fixed effect on all leaf stoichiometry parameters ofL. lentopodioidesand soil properties, while plot had a random effect on a few parameters such as STN, NH+4-N, SOC:STN, pH, leaf N, and leaf C:N (Table 2), indicating that altitude, not plot, mainly affected leaf stoichiometry ofL. lentopodioides.
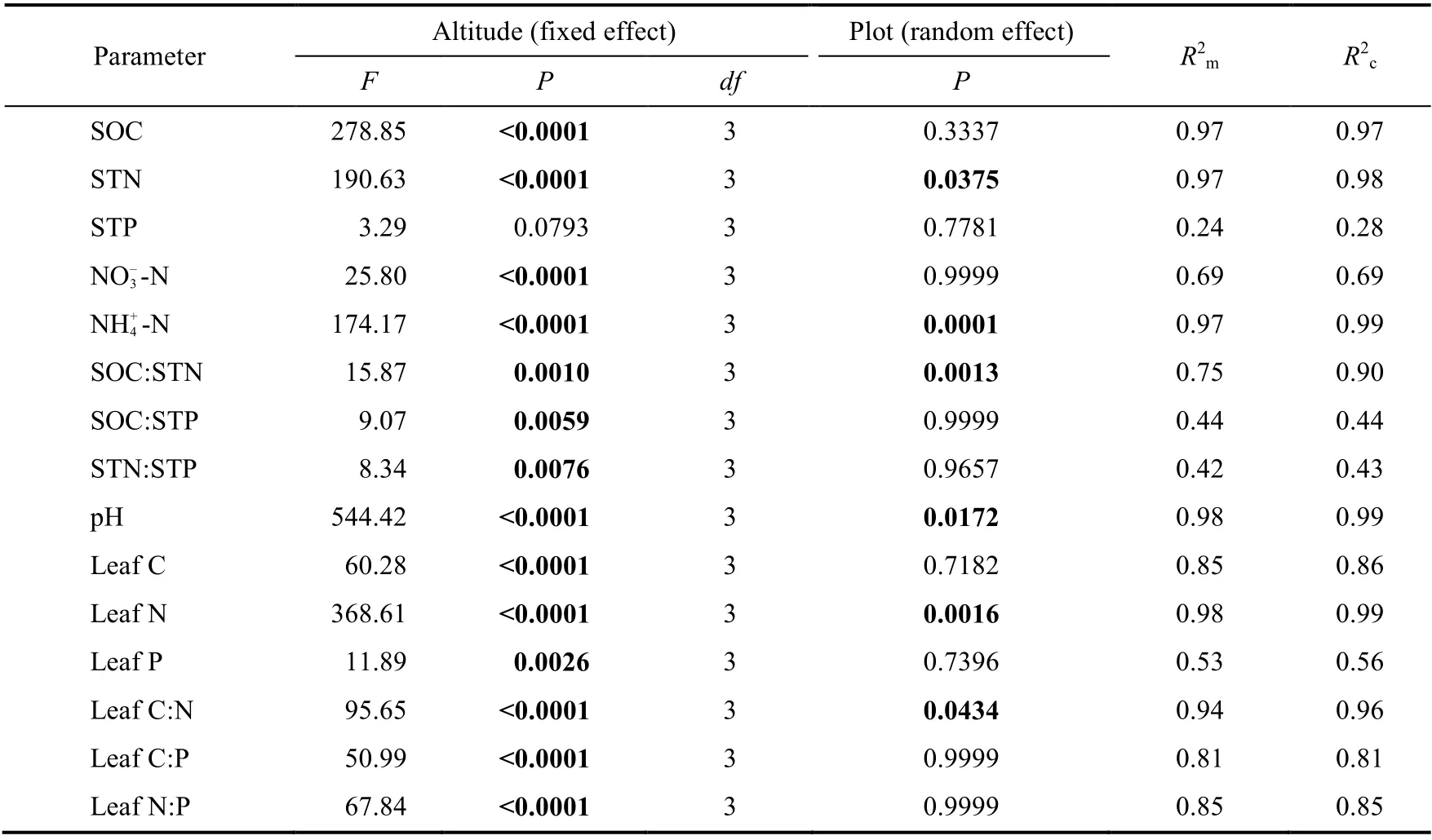
Table 2 Effects of altitude and plot on soil properties and leaf stoichiometry of L. lentopodioides
3.2 Variations in soil properties at different altitudes
Variations in SOC, STN, NH+4-N, and NO-3-N with increasing altitude were similar, and their values were higher at 3500 and 3800 m than those at 3000 m and below, suggesting that the combination of low temperature and high precipitation at high altitudes was contributed to the accumulation of SOC and soil nutrients. SOC:STP and STN:STP showed a sharp increase at 3500 m, while SOC:STN showed a relatively slow increase with increasing altitude, indicating that the capacities of soil N and soil P mineralization represented by soil stoichiometries were different among altitudes. Soil was alkaline at 2500-3000 m, and was weakly acidic at 3500 m and above(Table 3) due to high SOC.
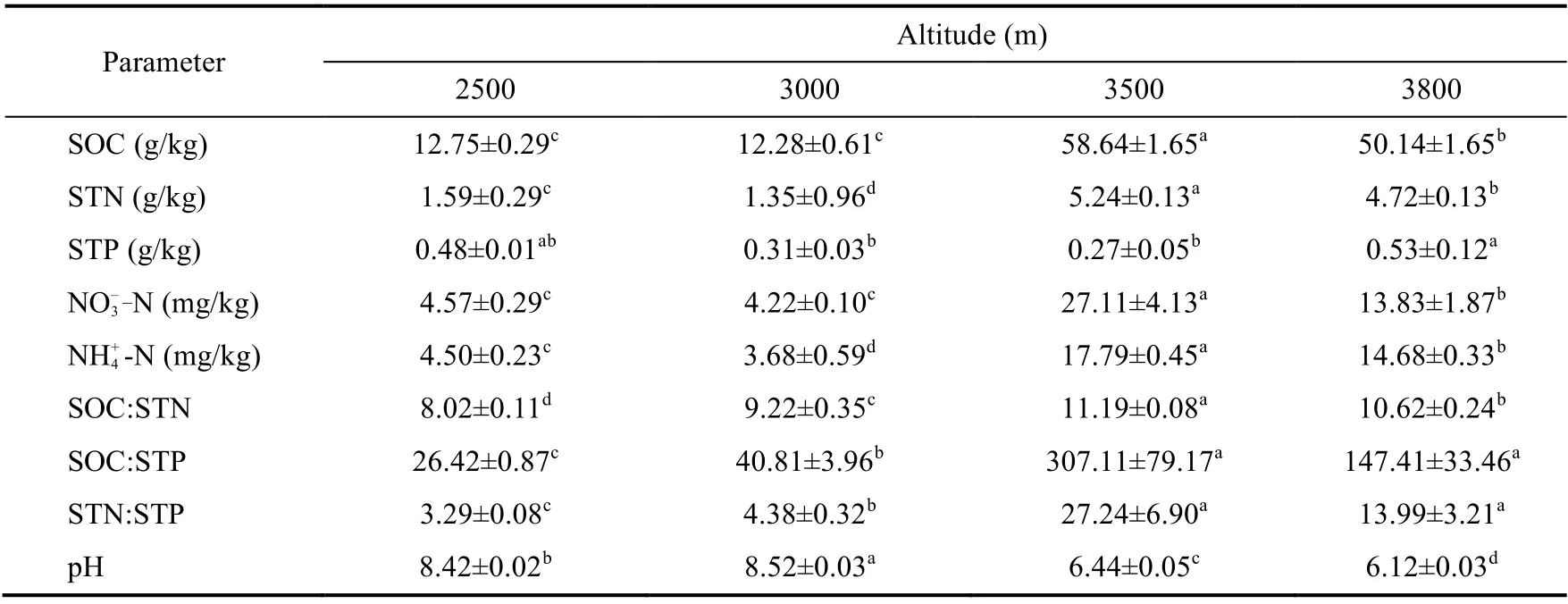
Table 3 Soil properties at different altitudes in the northeastern Qinghai-Tibetan Plateau, China
3.3 Variations in leaf stoichiometry of L. lentopodioides at different altitudes
With increasing altitude, leaf C ofL. lentopodioidesvaried from 343.74 to 397.54 g/kg with significant differences among altitudes (Fig. 2a), implying thatL. lentopodioidesat high altitudes had the potential for carbon dioxide uptake and climate change mitigation. Leaf N and P ofL.lentopodioidesshowed certain stability at 2500-3000 m, but sharply increased by 32.33% and decreased by 66.16% at 3500 m, respectively. Both elements showed an increasing trend at 3500-3800 m (Fig. 2b and c), indicating that high leaf nutrient may help plants to adapt to harsh environments such as low temperature.
Leaf C:N ofL. lentopodioidessignificantly declined with increasing altitude, ranging from 10.81 to 14.32 (Fig. 2d), indicating thatL. lentopodioidesimproved nutrient utilization efficiency for N in response to low temperature stress at high altitudes. The trend of leaf C:P was highly similar to that of N:P, and their values were the highest at 3500 m, 2-3 times higher than at other altitudes (Fig. 2e and f), suggesting thatL. lentopodioidesgrew slowly due to low soil TP at this altitude.
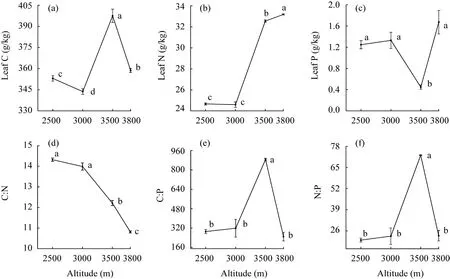
Fig. 2 Leaf C (a), N (b), P (c), C:N (d), C:P (e), and N:P (f) ratios of L. lentopodioides at different altitudes.Different lowercase letters indicate significant differences among different altitudes at P<0.05 level. Bars are standard errors.
3.4 Effects of environmental factors on leaf stoichiometry of L. lentopodioides
The results of RDA showed that soil properties and climatic elements explained 77.87% of the total variation (Fig. 3). NH+4-N, pH, STP, MAT, and MAP were the dominant factors that contributed to the variation of leaf stoichiometry ofL. lentopodioidesat different altitudes, and their contributions were in the following order: NH+4-N>pH>STP>MAT>MAP (Table 4),indicating that leaf stoichiometry ofL. lentopodioideswas mainly determined by edaphic environments and climatic conditions but not by others such as plant community composition and interspecific competition. And soil property had larger effects on it than climate.
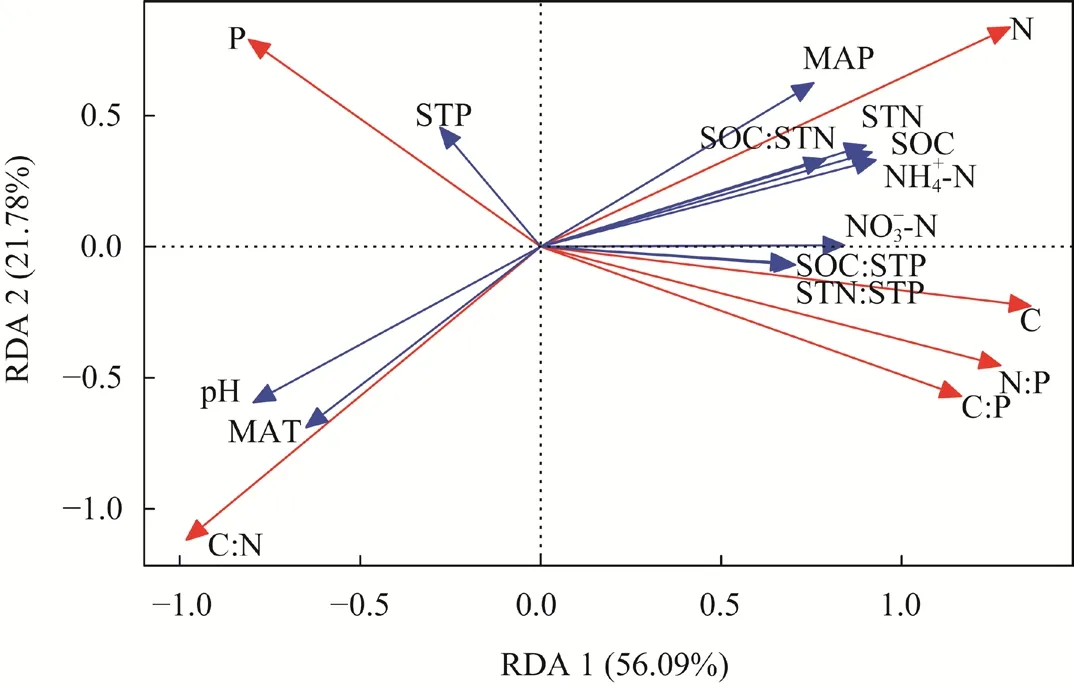
Fig. 3 Redundancy analysis (RDA) result for the leaf stoichiometry of L. lentopodioides and environmental factors. SOC, soil organic carbon; STN, soil total nitrogen; STP, soil total phosphorus; MAT, mean annual temperature; MAP, mean annual precipitation.
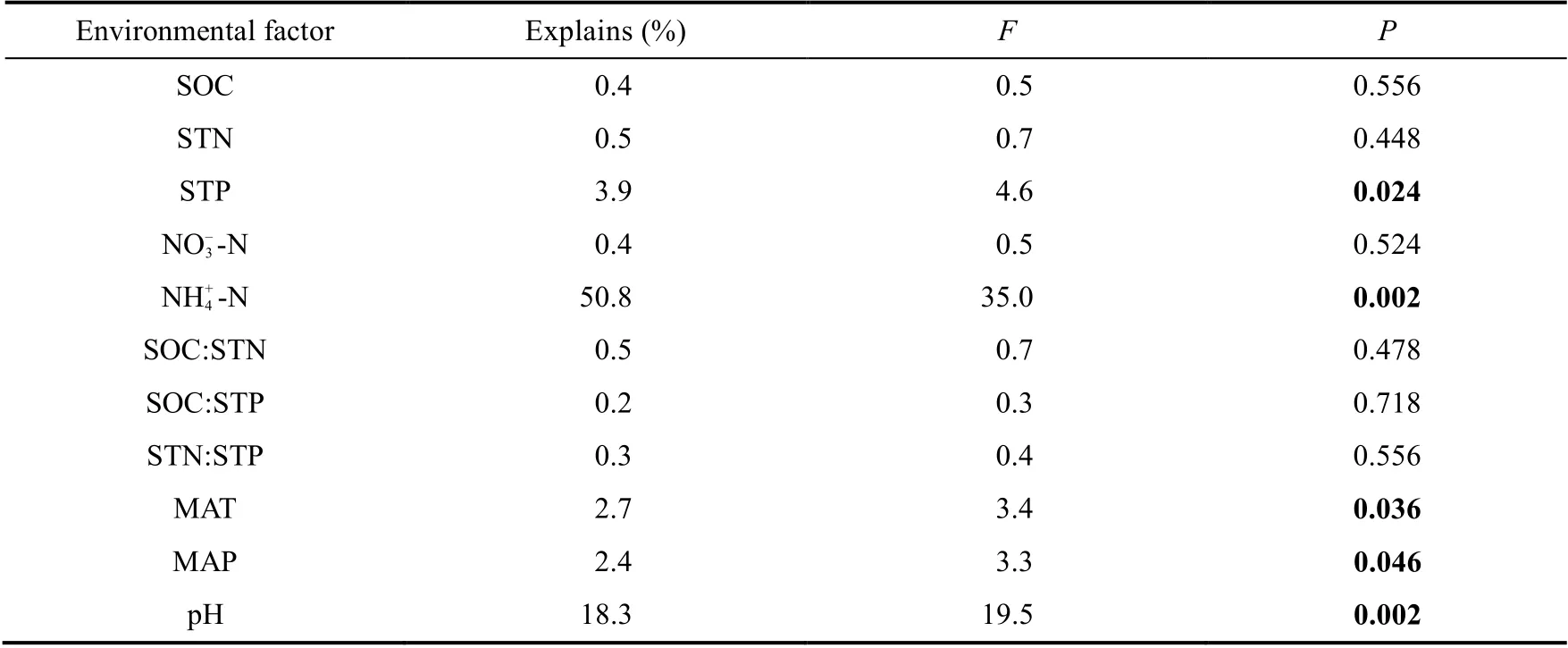
Table 4 Dominant environmental factors influencing leaf stoichiometry of L. lentopodioides at different altitudes
SEM results further revealed that environmental factors explained 71%, 98%, and 34% of the variations in leaf C, N, and P, respectively (Fig. 4). MAT affected leaf C directly, and soil pH affected leaf C indirectly through two paths: soil pH-NH+4-N and soil pH-STN:STP (Fig. 4a).MAT exerted a direct negative influence on leaf N. Soil pH affected leaf N mainly through three paths: soil pH, soil pH-NH+4-N, and soil pH-NO-3-N-NH+4-N (Fig. 4b). MAP not only directly affected leaf P but also indirectly affected leaf P by affecting NO-3-N and STN:STP, and STP indirectly affected leaf P (Fig. 4c). Results from SEM imply that each of these environmental factors had a different role in shaping leaf stoichiometry ofL. lentopodioides.
4 Discussion
Although the element concentrations and their ratios ofL. lentopodioideshad no clear variation trends with increasing altitude (Fig. 2), LMM results showed that the fixed effect of altitude on leaf stoichiometry was significant as reported in other studies (Du et al., 2017; Su et al., 2022),indicating that plants can actively adjust their nutritional requirements to maintain better growth and development in response to environmental changes brought about by altitude (Cernusak et al.,2010).
Generally, altitude has a substantial impact on leaf stoichiometry of plants by controlling MAT and MAP, and affecting soil properties (Zhang et al.,2019). For example, the variation of leaf stoichiometry ofO. ochrocephalaat different altitudes in the Qilian Mountains was mainly determined by SOC:STP, SOC, and MAT (Cao et al., 2020), andS. chamaejasmewas mainly determined by MAT, MAP, SOC:STN (Su et al., 2021). In the present study, the impact of altitude on leaf stoichiometry ofL. lentopodioideswas mainly driven by NH+4-N, soil pH, STP, MAT, and MAP, supporting our hypothesis (Table 4; Fig. 3). Among them, NH+4-N was the most dominant environmental factor. Results found that NH+4-N can influence a range of growth metabolic processes, such as photosynthetic and respiration by affecting the synthesis of chlorophyll, protein,and enzyme in plants (Pandey et al., 2015; Fu et al., 2021). In summary, we found that the crucial factors influencing leaf stoichiometry of each plant species are different even along the similar altitude in the Qilian Mountains. BecauseL. lentopodioides,O. ochrocephala(Cao et al., 2020),andS. chamaejasme(Su et al., 2021) belong to the Compositae, Leguminosae, and Thynchophyceae families, respectively, and their mechanisms for nutrient capture and storage may differ (Aerts and Chapin, 1999). Furthermore, differences in community composition and soil nutrient status at the sampling sites (Tables 1 and 3) (Cao et al., 2020; Su et al., 2021) may also be partly responsible for it (Treseder and Vitousek, 2001; Wang et al., 2014). Therefore, to understand the adaptation strategies of each plant species to environmental changes, studies at the level of single species are required.
4.1 Dominant factors influencing leaf C, N, and P of L. lentopodioides
Climatic variables (MAT and MAP) were dominant factors driving the changes in leaf C, N, and P ofL. lentopodioidesat different altitudes. In particular, leaf C and N were mainly sensitive to MAT, while leaf P was sensitive to MAP (Fig. 4). MAT directly and positively affected leaf C ofL.lentopodioides(Fig. 4a), because MAT can influence the activity of enzymes in plants, and photosynthesis and C sequestration capacity of plants (Wu et al., 2011). On the contrary, MAT directly and negatively affected leaf N ofL. lentopodioides(Fig. 4b). Leaf N ofL. lentopodioideswas generally lower at low (vs. high) altitude in this study (Fig. 2b), being consistent with the hypothesis that low temperatures could reduce plant enzyme efficiency and ribonucleic acid(RNA) synthesis efficiency, and that plants might increase leaf N to compensate for the effects of their reduction in physiological efficiency (Reich and Oleksyn, 2004). MAP directly and positively affected leaf P ofL. lentopodioides(Fig. 4c), and similar results have been reported by Su et al. (2022). Because in high-altitude areas, precipitation is high, and temperature is low(Table 1),L. lentopodioidesmay absorb more P into the leaves and promote the synthesis of soluble proteins and proline, to prevent cell freezing and dehydration and improve its cold resistance (Puhakainen et al., 2004; Reich and Oleksyn, 2004; Patton et al., 2007). However, the results of Ding et al. (2012) were contrary to ours, because of low MAP (230.12-360.18 mm) in their study area, plants are not necessary to store large amounts of P in leaf non-photosynthetic tissues to resist drought stress with increasing precipitation.
Soil pH, as an important chemical property of soil, had an important effect on leaf C and N. For example, soil pH directly and negatively affected leaf N (Fig. 4b), being consistent with Gong et al. (2017). Generally, in alkaline soils, salt content shows a colinear relationship with soil pH(Zhao et al., 2018). In our study area, soil at 2500-3000 m was alkaline (pH=8.42-8.52) (Table 3),and high soil pH represented high soil salinity (Sun et al., 2017), which would directly impair N uptake byL. lentopodioidesroots, and result in leaf N decline (Cramer et al., 1986; Rong et al.,2015). In addition, both soil pH and MAP indirectly affected leaf nutrient concentrations ofL.lentopodioidesby affecting soil properties (Fig. 4). In our study, soil pH affected leaf C by influencing NH+4-N and STN:STP, and leaf N by NH+4-N and NO-3-N, while MAP affected leaf P by affecting NO-3-N and STN:STP (Fig. 4). It is well known that soil pH and MAP are often related to the activities of many microorganisms (Chen et al., 2021), which can affect STN replenishment by influencing litter decomposition and affect SAN by regulating the rate of soil organic matter decomposition and N mineralization (Fierer et al., 2009; Hou et al., 2018; Huang et al., 2018; Liu et al., 2019; Li et al., 2020). Moreover, increasing MAP can increase litter yield and promote the accumulation of STN by facilitating plant growth and plant biomass accumulation (Bai et al., 2008; Nakagawa et al., 2019). In contrast, STP is mainly derived from parent rock weathering, and influenced by soil parent material, and it is much less sensitive to the environment than STN (Aerts and Chapin, 1999), as shown in our results (Table 1). Therefore, the variation of STN with changes in soil pH and MAP was responsible for STN:STP variation.
Soil nutrients and soil stoichiometry also had a significant effect on leaf nutrient concentrations.NH+4-N was a critical factor influencing leaf C and N ofL. lentopodioides(Fig. 4a and b), because N had a significant effect on plant chlorophyll synthesis as the main component of chlorophyll,directly affecting plant photosynthesis (Fu et al., 2021). Although soil TN is usually used to measure the basic fertility of soil N concentration, SAN (NH+4-N and NO-3-N) is more closely related to plant growth (Fois et al., 2009; Pandey et al., 2015). Compared with NO-3-N, NH+4-N may be more easily absorbed and utilized by plants, because plants can use less ATP and energy to absorb NH+4-N and assimilate it into amino acids (Ruan and Giordano, 2017). Therefore,L.lentopodioidesgrown in alpine environments may prefer to absorb NH+4-N, thus saving growth costs in favor of plant growth (Miller and Bowman, 2002), but it needs further research. The conversion of mobile NO-3-N in soil to non-fluid NH+4-N (Fig. 4b) is a protective mechanism for soil N to prevent soil N loss, which would provide more NH+4-N forL. lentopodioidesgrowth, and thus promote its growth (Cheng et al., 2022). NO-3-N also affected plant nutrient uptake (Fig. 4c),because that NO-3-N, as an anion, can promote plant uptake of cations such as K, Ca, and Mg, and inhibit the uptake of P and other anions (Ruan et al., 2000). Moreover, studies have shown that NO-3-N acts as a signaling molecule to regulate plant growth (Crawford and Glass, 1998; Medici et al., 2019), which may also account for the effect of NO-3-N on LP (Fig. 4c). STN:STP, as one of the soil nutrient limitation indicators, not only affects plant N and P nutrient supply and soil microbial activities (Fanelli et al., 2008; Tang et al., 2018; Zhao et al., 2018), but also affects plant photosynthesis, because plant C fixation cannot be achieved without the involvement of the protease, i.e., N, and the assembly of protease also demands a large amount of nucleic acid replication, i.e., P (An et al., 2011). Therefore, STN:STP significantly affected leaf C and P (Fig.4a and c).
4.2 Variations in leaf stoichiometric ratios and nutrient limitation of L. lentopodioides
Leaf C:N ofL. lentopodioidesdecreased, while leaf C:P increased, and then decreased with increasing altitude. Both of them reached the minimum value at 3800 m (Fig. 2d and e). Since leaf C:N and C:P reflect the N and P utilization efficiency and growth rate of plants (Elser et al.,2003; Sun et al., 2017), N and P nutrient utilization efficiency and growth rate ofL.lentopodioidesmay be relatively high at 3800 m.
Compared with a single nutrient concentration, the nutrient ratio can more truly reflect the nutrient supply status of the environment for plant growth (Yan et al., 2016). Koerselman and Meuleman (1996) proposed that plants were restricted by P when leaf N:P>16, by N when leaf N:P<14, and by both N and P when14≤N:P≤16. In this study, the ratios of leaf N:P ofL.lentopodioidesat all altitudes were greater than 16. However, leaf N:P not only reflects soil nutrient limitation, but also relates to the genetic characteristics of plants (Reich and Oleksyn,2004). Accordingly, there is a great deal of uncertainty to determine the nutrient limitation ofL.lentopodioidesgrowth by leaf N:P alone (Du et al., 2020; Hou et al., 2021). Therefore, it is essentical to assess its growth limitation in conjunction with soil nutrient status. In this study, the mean SOC:STN was 9.76, lower than 25.00, indicating a high soil N mineralization capacity and abundant supply of SAN for plant growth in this area (Jiang et al., 2019). The mean STP (0.39 g/kg) was lower than that of the Chinese average (0.65 g/kg), while the mean SOC:STP (130.45)and STN:STP (12.23) were higher than the national average (52.70 and 5.10, respectively) (Han et al., 2005; Tian et al., 2010). Considering that plants need more P at high altitudes than at low altitude to resist low temperature (Cao et al., 2020; Niu et al., 2021), and in combination with the positively indirect effect of STP on leaf P (Fig. 4c), the growth ofL. lentopodioidesin the study area may be mainly limited by STP. In recent years, studies have shown that N deposition on the QTP is continuously increasing (Liu et al., 2013). In this case, N-induced plant demand for P will likely further exacerbate the P limitation ofL. lentopodioidesgrowth (Li et al., 2016).
5 Conclusions
This study investigated the changes in leaf stoichiometry ofL. lentopodioidesat 2500, 3000, 3500,and 3800 m on the northeastern QTP. Variations of leaf C and N were high, but leaf P changed little with increasing altitude. Except for leaf C:N having a decreasing trend with increasing altitude, altitude had little effect on both leaf C:P and N:P. These results demonstrated thatL.lentopodioidescould adjust its leaf C and N in response to changes in altitude to ensure its normal growth, and in terms of leaf N:P,L. lentopodioidesgrowth in this area was mainly limited by STP.
In the study area, the effects of soil properties on leaf stoichiometry ofL. lentopodioideswere larger than that of climatic conditions. Specifically, leaf C and N were mainly determined by NH+4-N, followed by soil pH and MAT, while leaf P was mainly determined by STP and MAP.C sequestration capacity ofL. lentopodioidesleaves may be elevated under future warming and increased N deposition in the study area.
Acknowledgements
This work was supported by the Science and Technology Planning Project of Gansu Province, China(18JR4RA002), and the Qilian Mountains Eco-Environment Research Center in Gansu Province, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences (QLS202002).
- Journal of Arid Land的其它文章
- Antelope adaptations to counteract overheating and water deficit in arid environments
- Leaf morpho-physiology and phytochemistry of olive trees as affected by cultivar type and increasing aridity
- Competition, spatial pattern, and regeneration of Haloxylon ammodendron and Haloxylon persicum communities in the Gurbantunggut Desert,Northwest China
- Manipulated precipitation regulated carbon and phosphorus limitations of microbial metabolisms in a temperate grassland on the Loess Plateau, China
- Effects of native and invasive Prosopis species on topsoil physiochemical properties in an arid riparian forest of Hormozgan Province, Iran
- Contents and spatial distribution patterns of heavy metals in the hinterland of the Tengger Desert, China

