Manipulated precipitation regulated carbon and phosphorus limitations of microbial metabolisms in a temperate grassland on the Loess Plateau, China
HAI Xuying, LI Jiwei, LIU Yulin, WU Jianzhao, LI Jianping, SHANGGUAN Zhouping,DENG Lei,*
1 State Key Laboratory for Soil Erosion and Dryland Farming on the Loess Plateau, Institute of Soil and Water Conservation,Northwest A&F University, Yangling 712100, China;
2 Institute of Soil and Water Conservation, Chinese Academy of Science and Ministry of Water Resources, Yangling 712100,China;
3 School of Agriculture, Ningxia University, Yinchuan 750021, China
Abstract: Manipulated precipitation patterns can profoundly influence the metabolism of soil microorganisms. However, the responses of soil organic carbon (SOC) and nutrient turnover to microbial metabolic limitation under changing precipitation conditions remain unclear in semi-arid ecosystems. This study measured the potential activities of enzymes associated with carbon (C: β-1,4-glucosidase (BG) and β-D-cellobiosidase (CBH)), nitrogen (N: β-1,4-N-acetylglucosaminidase (NAG) and L-leucine aminopeptidase (LAP)) and phosphorus (P: alkaline phosphatase (AP)) acquisition, to quantify soil microbial metabolic limitations using enzymatic stoichiometry, and then identify the implications for soil microbial metabolic limitations and carbon use efficiency (CUE) under decreased precipitation by 50%(DP) and increased precipitation by 50% (IP) in a temperate grassland. The results showed that soil C and P were the major elements limiting soil microbial metabolism in temperate grasslands. There was a strong positive dependence between microbial C and P limitations under manipulated precipitation. Microbial metabolism limitation was promoted by DP treatment but reversed by IP treatment. Moreover, CUE was inhibited by DP treatment but promoted by IP treatment. Soil microbial metabolism limitation was mainly regulated by soil moisture and soil C, N, and P stoichiometry, followed by available nutrients (i.e., NO– 3,NH+ 4, and dissolved organic C) and microbial biomass (i.e., MBC and MBN). Overall, these findings highlight the potential role of changing precipitation in regulating ecosystem C turnover by limiting microbial metabolism and CUE in temperate grassland ecosystems.
Keywords: carbon use efficiency; ecoenzymatic stoichiometry; microbial metabolic limitations; semi-arid ecosystems;soil organic carbon
1 Introduction
Arid and semi-arid ecosystems, where the availability of soil moisture is the main limiting factor of biological metabolism and activity, cover one-third of the Earth's land area (Collins et al.,2008). Altered patterns of precipitation distribution (IPCC, 2014) lead to large changes in soil moisture (SM), and have important impacts on the turnover and circulation of soil organic matter(SOM) and nutrients (Ru et al., 2018; Deng et al., 2021). Variable precipitation patterns affect the microbial biomass and composition (Cregger et al., 2012; Nielsen and Ball, 2015; Deng et al.,2021), and further influence extracellular enzyme activity, leading to disproportionate enzymatic stoichiometry (Peng and Wang, 2016; Li et al., 2022). Therefore, it is crucial to understand the response of soil microbial metabolic activity to variations in precipitation patterns in temperate grassland ecosystems.
Soil microorganisms play critical roles in the carbon (C) and nutrient cycles through the degradation and mineralization of SOM (Sinsabaugh and Follstad Shah, 2012). These procedures are affected by soil microbial biomass and activities that are restricted by soil C, N, and P contents (Chen et al., 2018; Deng et al., 2019). Soil microbial C, N, and P metabolisms are strongly influenced by soil nutrient content and physical-chemical properties (Deng et al., 2019).Stoichiometry is one of the key drivers of microbial metabolism (Sinsabaugh et al., 2009; Cui et al., 2018), and is vital for regulating the main processes in soil ecosystems (Zechmeister-Boltenstern et al., 2015). Water availability is one of the dominant limiting resources for microbial metabolism, and variations in precipitation patterns greatly influence microbial metabolism in drought areas (Ru et al., 2018). Some studies have reported that soil microbial metabolism is limited by P in areas with high precipitation (Xu et al., 2017), however, continuous water restriction limits microbial metabolic activity, and uncouples the growth of microbial communities from respiration (Feng et al., 2019). In particularly, seasonal redistribution of precipitation potentially plays a more significant role than the amount of precipitation in regulating microbial metabolism and ecosystem C cycling (Ru et al., 2018). These studies indicate that manipulated precipitation has different impacts on microbial resource acquisition and metabolism limitations. Therefore, it is necessary to understand how limitations of soil microbial C and nutrients are mediated under manipulated precipitation throughout seasons.
Decomposition of SOM through the secretion of extracellular enzymes is the main process by which microorganisms obtain energy and nutrients (i.e., C, N, and P) (Sinsabaugh et al., 2009; Ru et al., 2018). A substantial part of SOM is of microbial origin as plant inputs or microbial products are cycled through the soil microbial community (Takriti et al., 2018). C taken up by heterotrophic microorganisms is partitioned between biomass production and respiration, which is described by microbial carbon use efficiency (CUE) (Takriti et al., 2018). That is, some of C is used to sustain microbial growth, and some is used for the production of energy (Manzoni, 2017),which leads to C partly remaining within the system, while part is lost (Sinsabaugh et al., 2013).Limitation of microbial metabolism can strongly influence the decomposition of SOM, circulation of soil nutrients, and soil C sequestration through affecting microbial CUE (Deng et al., 2019; Cui et al., 2020). The potential mechanisms, especially the poorly understanding of connection between SOM variations and microbial metabolic limitation, will be revealed by measuring soil C pool changes by linking microbial metabolic limitations to manipulated precipitation patterns in temperate grasslands. It will be useful to explore the microbial metabolic characteristics under manipulated precipitation to better understand their functions and the dynamics that are connected to the turnover of soil organic C (SOC). In addition, ecoenzymatic stoichiometry reflects the ability of microorganisms to maintain their function by adjusting their own elemental balance(Sistla and Schimel, 2012), and is used to reveal the nutrient limitations of microbial metabolism(Sinsabaugh et al., 2009). The findings can reveal the relative nutrient requirements of microorganisms, and provide a distinct metric of relative limitation of C and nutrients (N or P)(Moorhead et al., 2016). Hence, this method can help understand the characteristics of microbial metabolism and the connection between microbial metabolic limitations under manipulated precipitation patterns.
As an important subsystem of terrestrial ecosystems, grasslands play a critical role in global change and ecosystem functioning. Temperate grasslands are one of the most vulnerable ecosystems, and are typical arid and semi-arid ecosystems. Due to water deficiency, soil microbial metabolism is sensitive to manipulated precipitation in temperate grasslands, which strongly influences soil nutrient cycle and decomposition of SOM, and further influences C sequestration by soil, and ecosystem stability (Jia et al., 2017). We hypothesized that microbial metabolism could be more limited by decreased precipitation and relieved by increased precipitation, but microbial CUE shows an opposite response to manipulated precipitation. The aims of this research were to investigate the effect of microbial metabolism limitation and microbial CUE on soil C turnover under manipulated precipitation conditions.
2 Materials and methods
2.1 Study area
The study was performed in a temperate grassland in the region of Yunwu Mountains, Ningxia
Hui Autonomous Region, China (36°16?44?N, 106°17?49?E), which has a typical continental arid and temperate climate. The mean annual temperature (MAT) and mean annual precipitation (MAP)of the study area are 7.1℃ and 439 mm, respectively, and 60% of rainfall falls from June to September (Hai et al., 2022). The soils are gray and loessial (pH 8.2). The dominant species areStipa grandisP.A. Smirn.,Stipa bungeanaTrin.,Artemisia gmeliniiWeb. ex Stechm., and their main companion species areAgropyron cristatum(L.) Gaertn. andHeteropappus altaicus(Willd.)Novopokr.
At the end of 2017, a long-term precipitation-manipulation experiment was conducted on a typical temperate grassland. The experiment included three precipitation gradients: ambient precipitation (control), decreasing precipitation by 50% (DP), and increasing precipitation by 50%(IP). Each gradient was set for five replicates, and there were a total of 15 plots with an area of 6 m×6 m and an interval buffer zone with a width of 1 m between the plots. Transparent plastic rain sheltering racks were adopted to intercept precipitation. Each precipitation-shelter was fixed by steel pillars, and transparent plastic plates were fixed in ''V'' shapes to the ground. Rain shelters intercepted 50% of the ambient precipitation to set a manipulated decrease in precipitation, and the intercepted precipitation was piped to adjacent plots to increase their precipitation (Fig 1a).
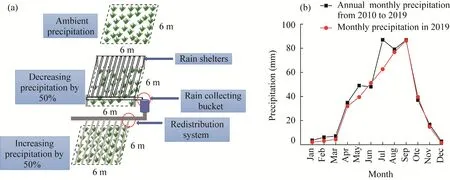
Fig. 1 Experimental design (a) and monthly precipitation of the study area (b)
The research was carried out in mid-May (initial growth stage), mid-July (middle growth stage),and mid-September (final growth stage) 2019. The distributions of MAP for each month (from 2010 to 2019) and monthly precipitation in 2019 of the study area were shown in Figure 1b.
2.2 Soil sampling
Three soil cores (5-cm inner diameter) were collected from each plot in the top 0-20 cm layer,and were homogenized to form one sample from each plot. All samples were sieved through a 2-mm sieve to remove roots and other debris. Then, all soil samples were separated into two parts.One part was used for physical-chemical analysis (air-dried), and the other was immediately stored in an ice box for analysis of enzymatic activity and microbial biomass.
2.3 Analysis of soil physical-chemical properties
SM (%) and soil temperature (ST, °C) were determined using a soil moisture meter. SOC content was analyzed using calorific boiling and combustion methods (Nelson and Sommers, 1982). Total N (TN) content was measured using the Kjeldahl method (Brookes et al., 1985). The molybdenum blue method and an ultraviolet spectrophotometer were used to determine total P(TP) and available P (AP) contents in the soil (Olsen and Sommers, 1982). Dissolved organic C(DOC) content was measured using a TOC analyzer (Li et al., 2015). Total dissolved nitrogen(TDN) was measured using alkaline digestion/ultraviolet (Liu et al., 2021). Available soil N content (NO-3-N and NH+4-N) was measured using a continuous flow analytical system, and the dissolved organic N (DON) content was calculated as TDN-(NH+4+NO-3). Total dissolved phosphorus (TDP) content was measured using the method of ammonium molybdate spectrophotometric (Galhardo and Masini, 2000). Soluble reactive phosphorus (SRP) content was measured using the phosphor-molybdenum blue method, and the dissolved organic P (DOP)content was calculated as the TDP-SRP.
2.4 Measurements of microbial biomass and extracellular enzymatic activity
Microbial biomass carbon (MBC), nitrogen (MBN), and phosphorus (MBP) contents were analyzed using the chloroform fumigation-extraction method (Vance et al., 1987). The specific experimental methods have been described by Li et al. (2019).
The activities of enzymes associated with C (BG: β-1,4-glucosidase and CBH: β-Dcellobiosidase), N (NAG: β-1,4-N-acetylglucosaminidase and LAP: L-leucine aminopeptidase),and P (AP: alkaline phosphatase) were measured using fluorometric techniques (Sinsabaugh et al.,2009), and the specific methods were described by Deng et al. (2019).
2.5 Quantification of microbial metabolic limitation and CUE
To quantify the C and nutrient (N or P) limitation of soil microbial metabolism, we performed vector analysis of the relative activities of C- vs. N-acquiring enzymes, and C- vs. P-acquiring enzymes (Sinsabaugh et al., 2008). Actually, this model shows the potential and relative limitation rather than the actual limitation of resource for soil microorganisms. Vector lengths (C limitation)and angles (N limitation: angles<45°; P limitation: angles>45°) of enzymatic activity were used to calculate the microbial metabolic limitations. All the data were based on untransformed proportional activities. Vector length was calculated as the square root of the sum ofx2andy2(Eq.1) (Moorhead et al., 2016):

wherexis the relative activity of C- vs. P-acquiring enzymes;yis the relative activity of C-vs.N-acquiring enzymes.
Vector angle was calculated as the arctangent of the line extending from the plot origin to the point (x,y) (Eq. 2):
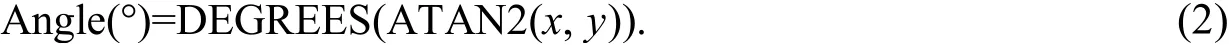
Soil microbial C limitation showed a trend of increasing with increasing vector length. Vector angles <45° denote soil microbial N limitation, and angles >45° denote soil microbial P limitation(Moorhead et al., 2012).
A biogeochemical equilibrium model was used to calculate the microbial CUE (Eqs. 3-5)(Sinsabaugh and Follstad Shah, 2012):
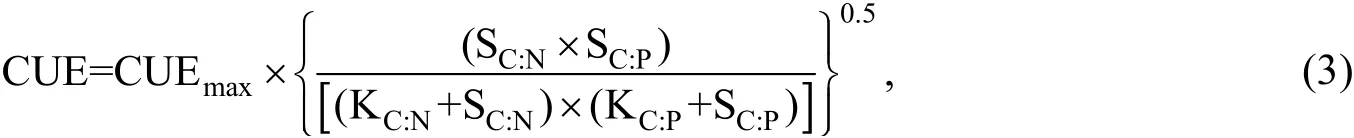
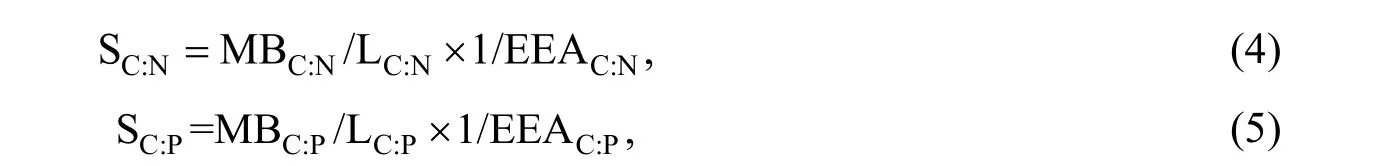
where EEAC:Nand EEAC:Pwere calculated as (BG+CBH)/(NAG+LAP) and (BG+CBH)/AP,respectively. The molar ratios of SOC to soil total N (SOC:TN) and the ratio of SOC to soil total phosphorus (SOC:TP) were used as estimates of LC:Nand LC:P. Similarly, microbial biomass C:N and C:P (MBC:Nand MBC:P) were calculated as molar ratios. KC:Nand KC:Pare the half-saturation constants for CUE based on C, N, and P availability, respectively. The model assumed that the growth rates were maximal when the proportion of absorbable nutrient supply matched the stoichiometry of microbial biomass, and that growth efficiency was proportional to geometric mean of N and P supplies relative to C. In this study, we assumed that the values of KC:Nand KC:Pwere 0.5, for all model scenarios, and that CUEmaxwas 0.6 (Sinsabaugh et al., 2013).
2.6 Statistical analysis
One-way analysis of variance was applied to identify the differences in response variables (i.e.,soil properties, microbial biomass, metabolic limitation, and their CUE) of different precipitation manipulations and growth stages, and mean values were compared using Duncan's multiple comparisons test (P<0.05) using SPSS v.20.0. Linear regression was used to determine the relationships between microbial C and P limitations using Origin v.2021. Pearson's correlation was used to examine the relationships among all variables assessed in the study using Origin v.2021. We fitted a path analysis framework to evaluate the direct and indirect relationships between soil microbial C limitation and P limitation with all significant environmental variables and the fixed driving factor. The model was constructed using Amos v.22.0.
3 Results
3.1 Response of soil extracellular enzyme stoichiometry to manipulated precipitation
There were significant variations in AP, (BG+CBH)/(LAP+NAG), and (BG+CBH)/AP (P<0.05)at different growth stages (Table 1). Throughout the growth stages, C-acquiring enzyme activities(BG+CBH) under DP treatment significantly increased in the middle growth stage and then decreased (Fig. 2a). However, under IP treatment, an opposite trend was observed, showing that P-acquiring enzyme activities (AP) under ambient precipitation and IP treatments decreased significantly with growth stage (Fig. 2c). However, the ratios of C:P ((BG+CBH)/AP) under ambient precipitation and DP treatments generally increased with growth stage (Fig. 2e).
Manipulated precipitation also had a significant impact on (LAP+NAG) and (LAP+NAG)/AP(P<0.05; Table 1). In the initial growth stage, C-acquiring enzyme activities (BG+CBH) showed the following decreasing order of IP>ambient precipitation>DP, whereas an opposite trend was observed in the middle of growth stage (Fig. 2a). Additionally, at the end of growth stage, C:P ratios ((BG+CBH)/AP) under DP treatment were significantly lower than those in the other stages(Fig. 2e).
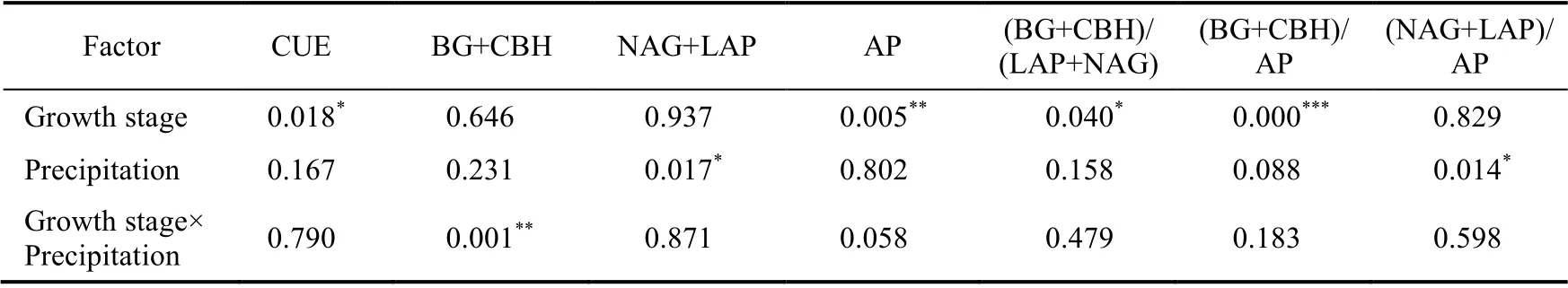
Table 1 Results of ANOVA of the effects of growth stages and precipitation manipulation on soil extracellular enzymes and their stoichiometric characteristics
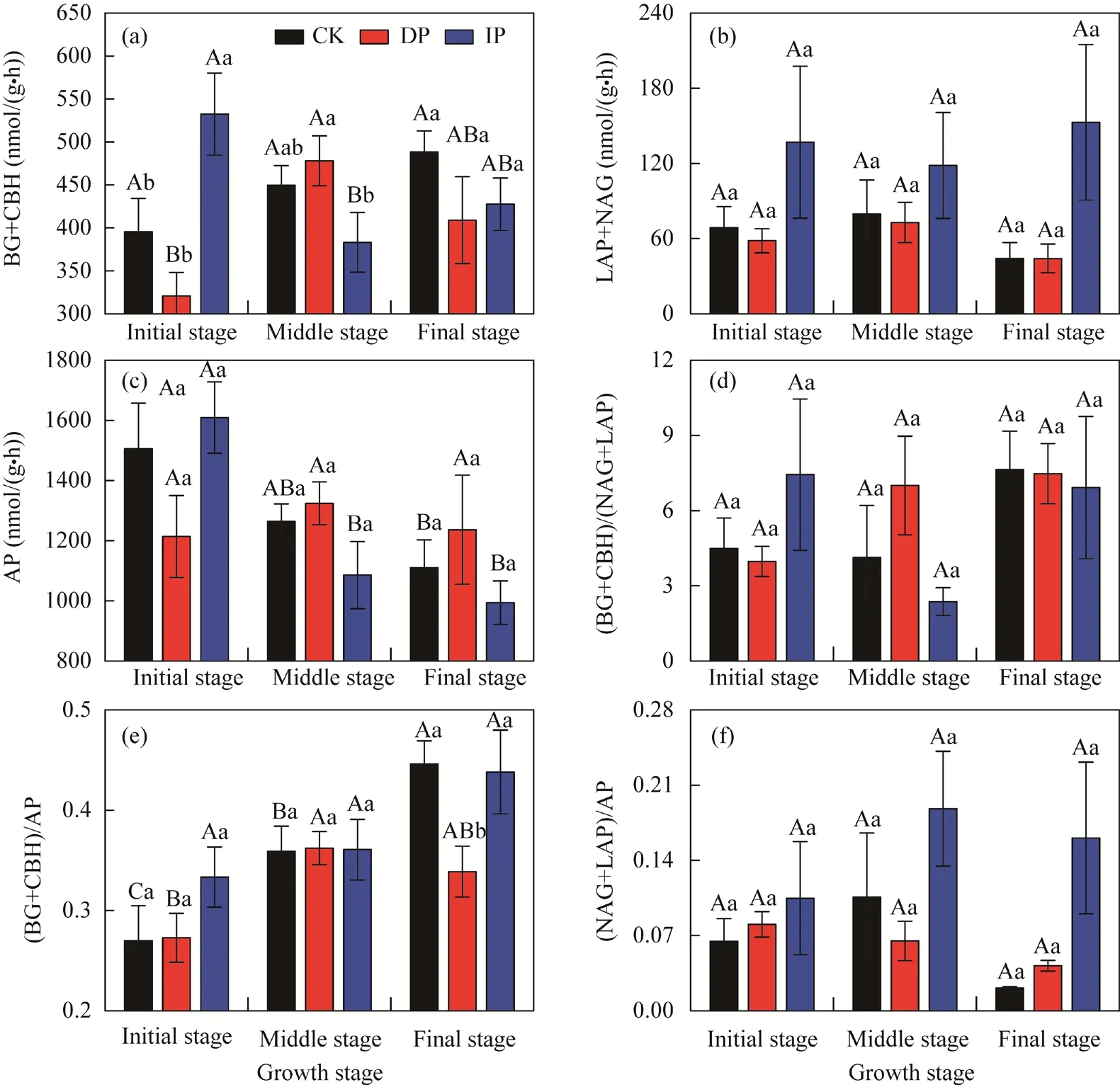
Fig. 2 Variations of soil enzymatic activity (a-c) and enzymatic stoichiometry (d-f) under manipulated precipitation at different growth stages. CK, ambient precipitation; DP, decreasing precipitation by 50%; IP,increasing precipitation by 50%. BG, β-1,4-glucosidase; CBH, β-D-cellobiosidase; LAP, L-leucine aminopeptidase; NAG, β-1,4-N-acetylglucosaminidase; AP, alkaline phosphatase. Different lowercase letters indicate significant difference among different treatments within the same growth stage at P<0.05 level. Different uppercase letters indicate significant difference among different growth stages within the same treatment at P<0.05 level. Bars are standard errors.
3.2 Response of soil microbial metabolism limitation and CUE to manipulated precipitation
Vector angles were all >45° (Fig. 3a), indicating that the availability of P to the soil microbial community was limited. Microbial C limitation decreased under IP treatment, but increased under DP treatment in the middle growth stage. This trend did not vary significantly between different growth stages and precipitation treatments. However, the microbial P limitation under ambient precipitation treatment decreased significantly with growth stage. DP treatment enhanced microbial P limitation, but IP treatment reduced it, particularly in the final growth stage (P<0.05).Correlation analysis indicated that soil microbial C was positively correlated with P limitation over the entire growth season (Fig. 3d).
Plant growth stage had a significant effect on microbial CUE (P<0.05; Table 1). Microbial CUE of all precipitation treatments showed the following trends: middle growth stage>initial growth stage>final growth stage (Fig 4). Microbial CUE under ambient precipitation and DP treatments varied significantly with growth stages (P<0.05). Moreover, DP and IP treatments had negative and positive effects, respectively, on microbial CUE, but the variations were not significant.
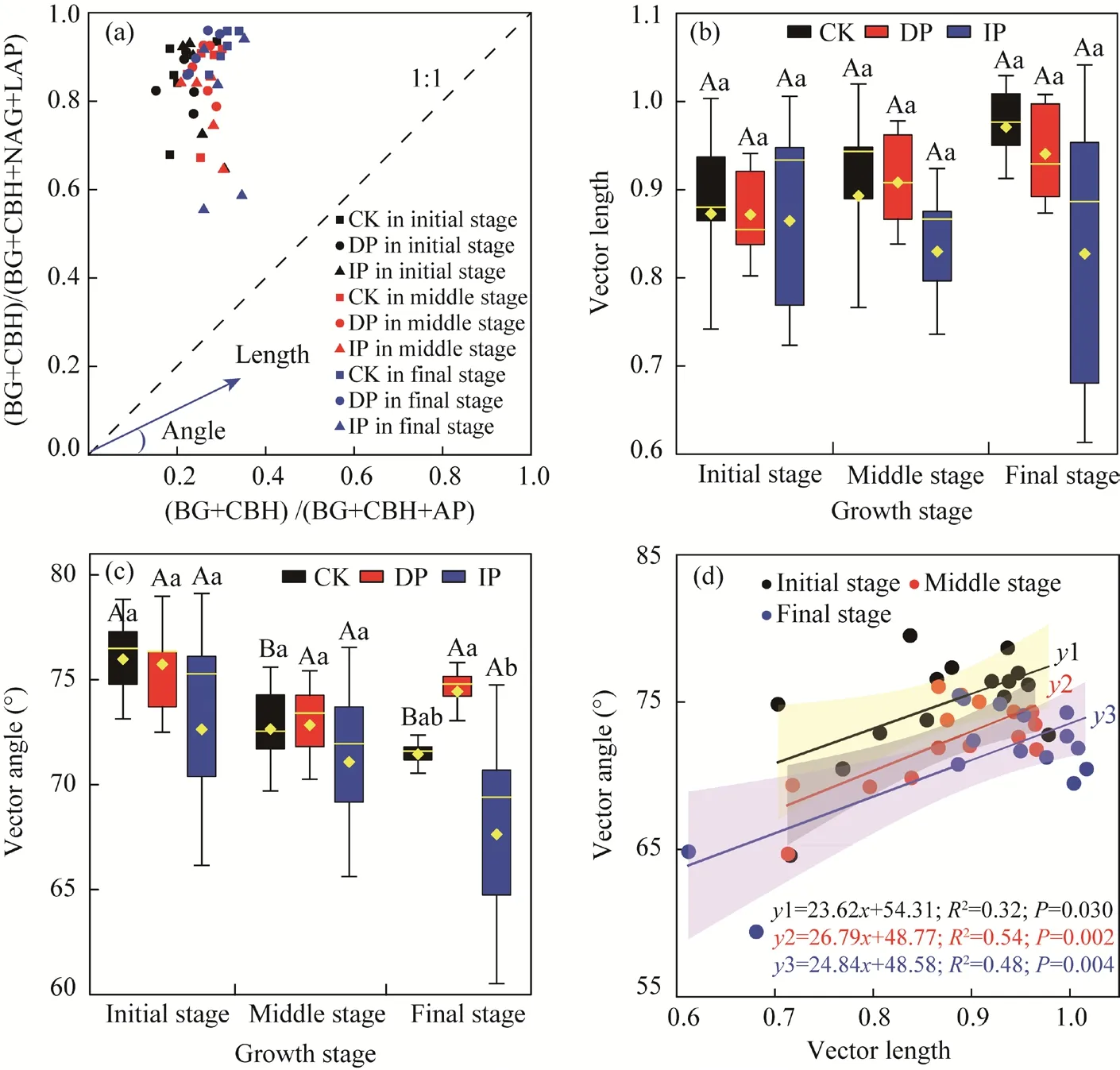
Fig. 3 Enzymatic stoichiometry of relative proportions of C to N acquisition versus C to P acquisition (a), the variation of vector length and angle (b and c) and their relationships (d). CK, ambient precipitation; DP,decreasing precipitation by 50%; IP, increasing precipitation by 50%. BG, β-1,4-glucosidase; CBH,β-D-cellobiosidase; LAP, L-leucine aminopeptidase; NAG, β-1,4-N-acetylglucosaminidase; AP, alkaline phosphatase. In the box plot, black boxes show 25% and 75% quantiles, yellow point is the mean value, and yellow horizontal line is the median. Different lowercase letters indicate significant difference among different treatments within the same growth stage at P<0.05 level. Different uppercase letters indicate significant difference among different growth stages within the same treatment at P<0.05 level. Bars are standard errors.
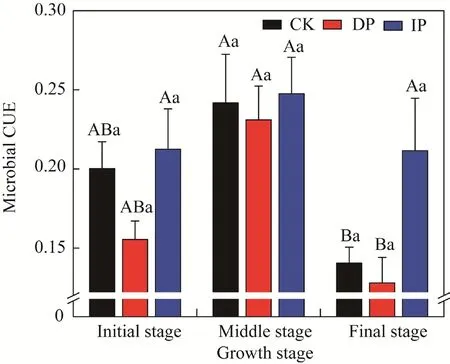
Fig. 4 Microbial carbon use efficiency (CUE) under manipulated precipitation in different growth stages. CK,ambient precipitation; DP, decreasing precipitation by 50%; IP, increasing precipitation by 50%. Different lowercase letters indicate significant difference among different treatments within the same growth stage at P<0.05 level. Different uppercase letters indicate significant difference among different growth tages within the same treatment at P<0.05 level. Bars are standard errors.
3.3 Factors affecting soil microbial metabolism limitation under manipulated precipitation
Soil microbial C limitation was related to TN, TN:TP, DOC, NO-3-N, and MBN, and microbial P limitation was related to SOC, SOC:TN, SOC:TP, MBC, and MBC:MBP (Fig. 5). The above influencing factors and the fixed driving factor SM were combined to conduct a multiple regression linear regression analysis (Table 2) and to verify the relationship between each factor,and C and P limitation. The regression coefficients of DOC and MBN that affect C limitation were all zero; therefore, they did not appear in the equation, but were used in the subsequent analysis. Path analysis showed that changes in SM and nutrient stoichiometry induced by manipulated precipitation at different growth stages affected soil available nutrients and microbial biomass, ultimately influencing the characteristics of microbial metabolism (Fig. 6). The final model explained 42% and 22% of the changes in soil microbial C limitation (Fig. 6a and b) and P limitation (Fig. 6c and d), respectively.
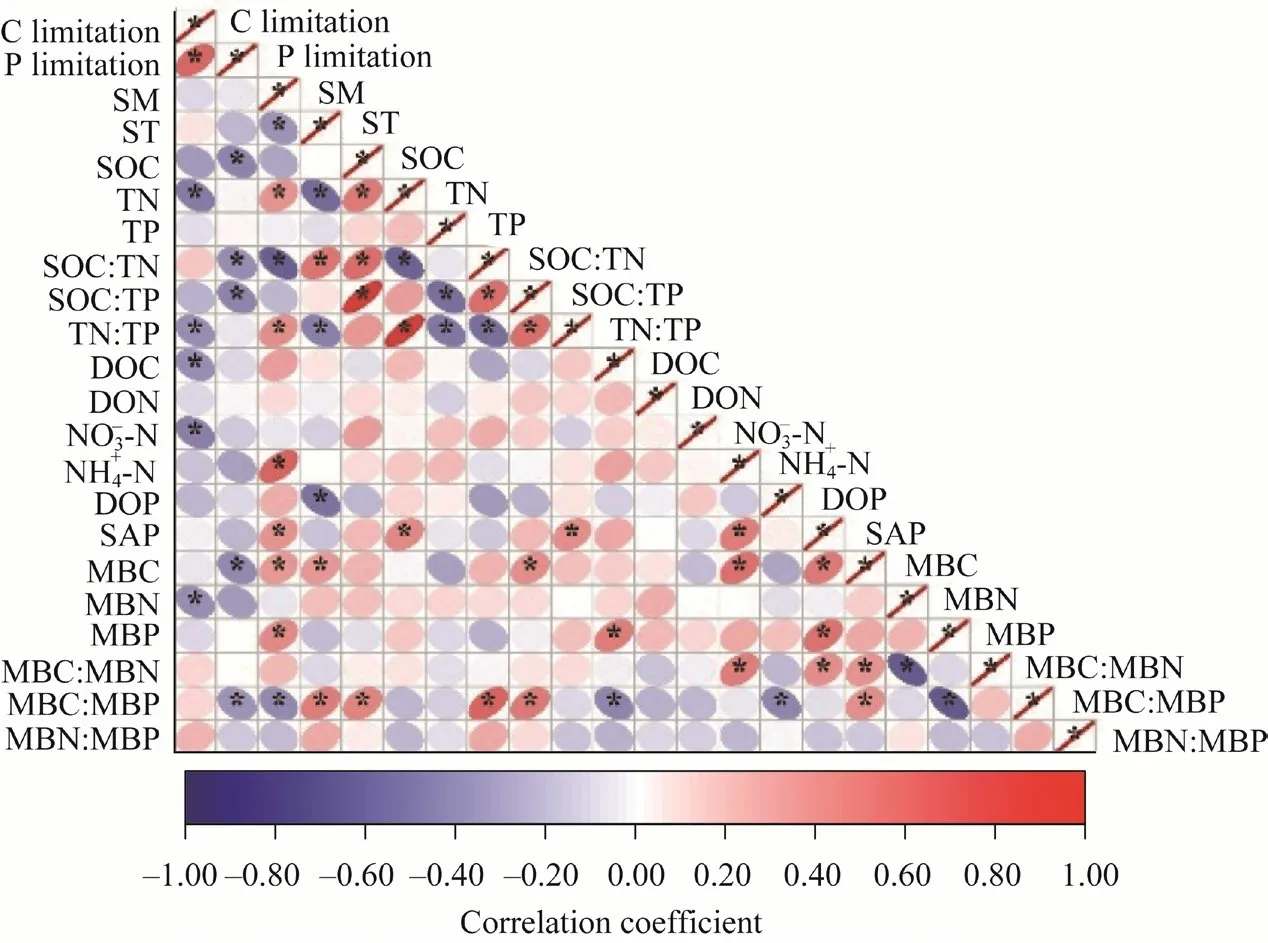
Fig. 5 Correlation analysis of factors affecting microbial C limitation and P limitation under precipitation manipulation. SM, soil moisture; ST, soil temperature; SOC, soil organic carbon; TN, soil total nitrogen; TP, soil total phosphorus; DOC, dissolved organic carbon; DON, dissolved organic N; DOP, dissolved organic P; SAP,soil available phosphorus content; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; MBP,microbial biomass phosphorus; *. P<0.05 level.
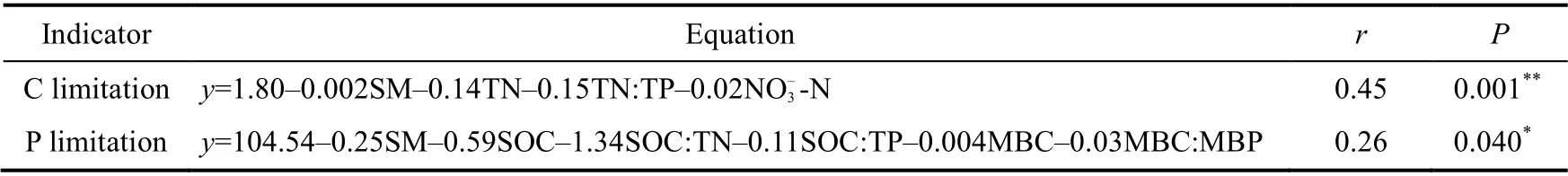
Table 2 Multiple regression equation of microbial C limitation and P limitation with influencing factors
4 Discussion
4.1 Effects of manipulated precipitation on soil microbial metabolic limitations
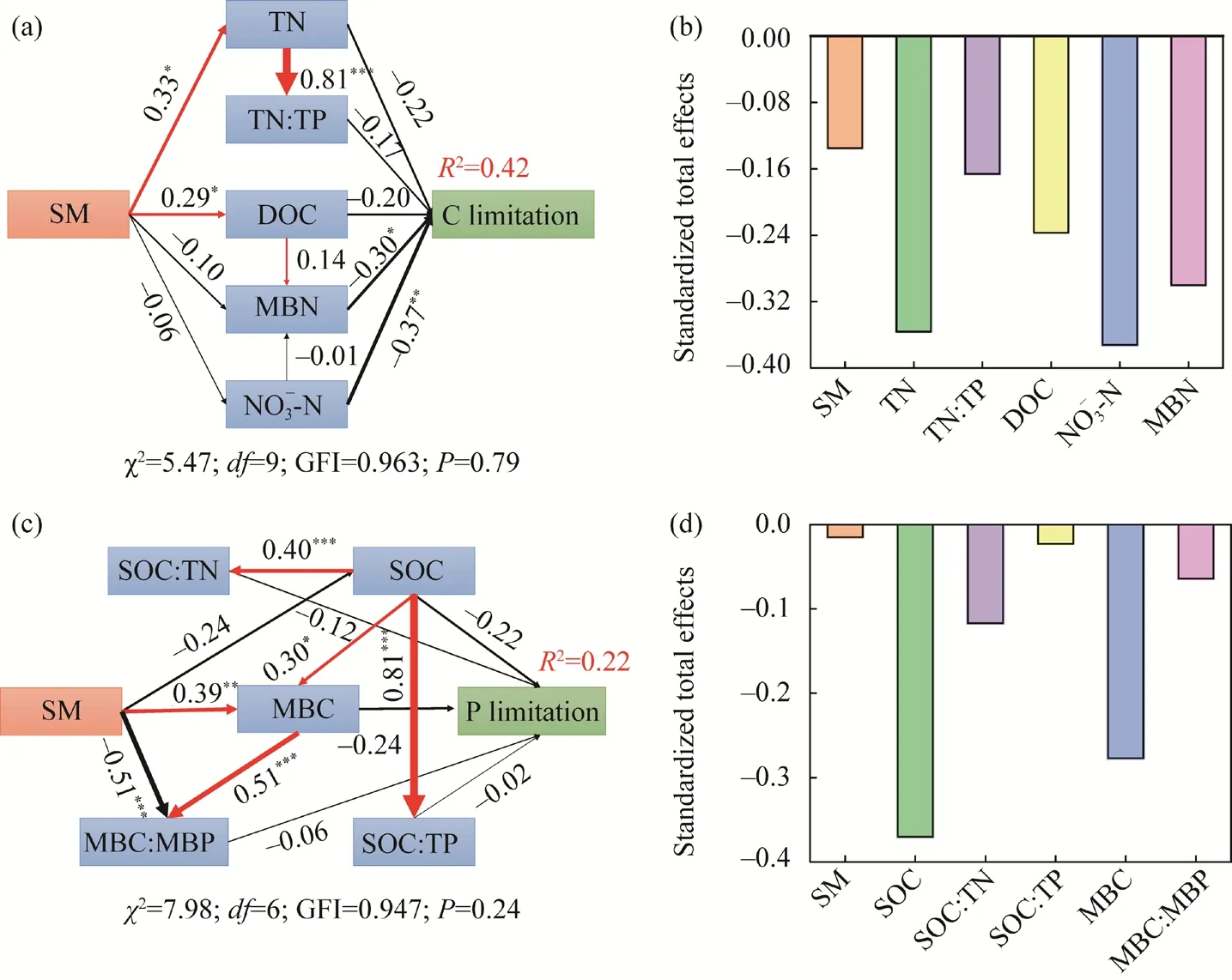
Fig. 6 Conceptual model of the effects of soil microbial properties on microbial C (a and b) and P (c and d)limitations under manipulated precipitation. SM, soil moisture; SOC, soil organic carbon; TN, soil total nitrogen;TP, soil total phosphorus; DOC, dissolved organic carbon; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen. MBP, microbial biomass phosphorus. Numbers at arrows are standardized path coefficients. *,P<0.05 level; **, P<0.01 level; ***, P<0.001 level.
Manipulated precipitation determines soil moisture, which directly influences nutrient availability,microbial metabolism, and function (Borken and Matzner, 2009) by affecting the input of SOM in plants (Hai et al., 2022), litter decomposition, and nutrient release (Waring et al., 2014; Ru et al.,2018). In our study, the extracellular enzymatic stoichiometry model revealed that soil microbial C and P limitations existed in the temperate grasslands (Fig. 3). C limitation is prevalent in most soil microbial communities (Moorhead et al., 2016), which could be explained by the local climate (i.e., temperature), vegetation, and soil properties collectively (Jing et al., 2020). A previous study suggested that microbial C limitation decreased significantly as precipitation increased (Jing et al., 2018), and the same decreasing trends were observed in our results (Fig. 3).A possible explanation for this is that the alleviation of stressful conditions by the higher water and nutrient availability enhanced soil microbial metabolic activity. Furthermore, DP treatment enhanced microbial C limitation in the middle growth stage. In water-deficient areas, plants may restrict their photosynthetic capacity and C uptake to limit water loss from the open stomata(Peters et al., 2018). Therefore, soil microbial C limitation is exacerbated by decreasing C uptake and allocation belowground, along with increased organo-mineral stabilization (Huang and Hall,2017). However, our study found that soil microbial C limitation was statistically insignificant with manipulated precipitation and growth stages, which was unexpected as there were some significant changes in C-acquiring enzyme activities (Fig. 2). This may be because the constitutive C-acquiring enzymes, which were less affected or not sensitive to the environmental changes, may have accounted for a larger proportion of soil enzymes in the subsoils (Sinsabaugh and Follstad Shah, 2012).
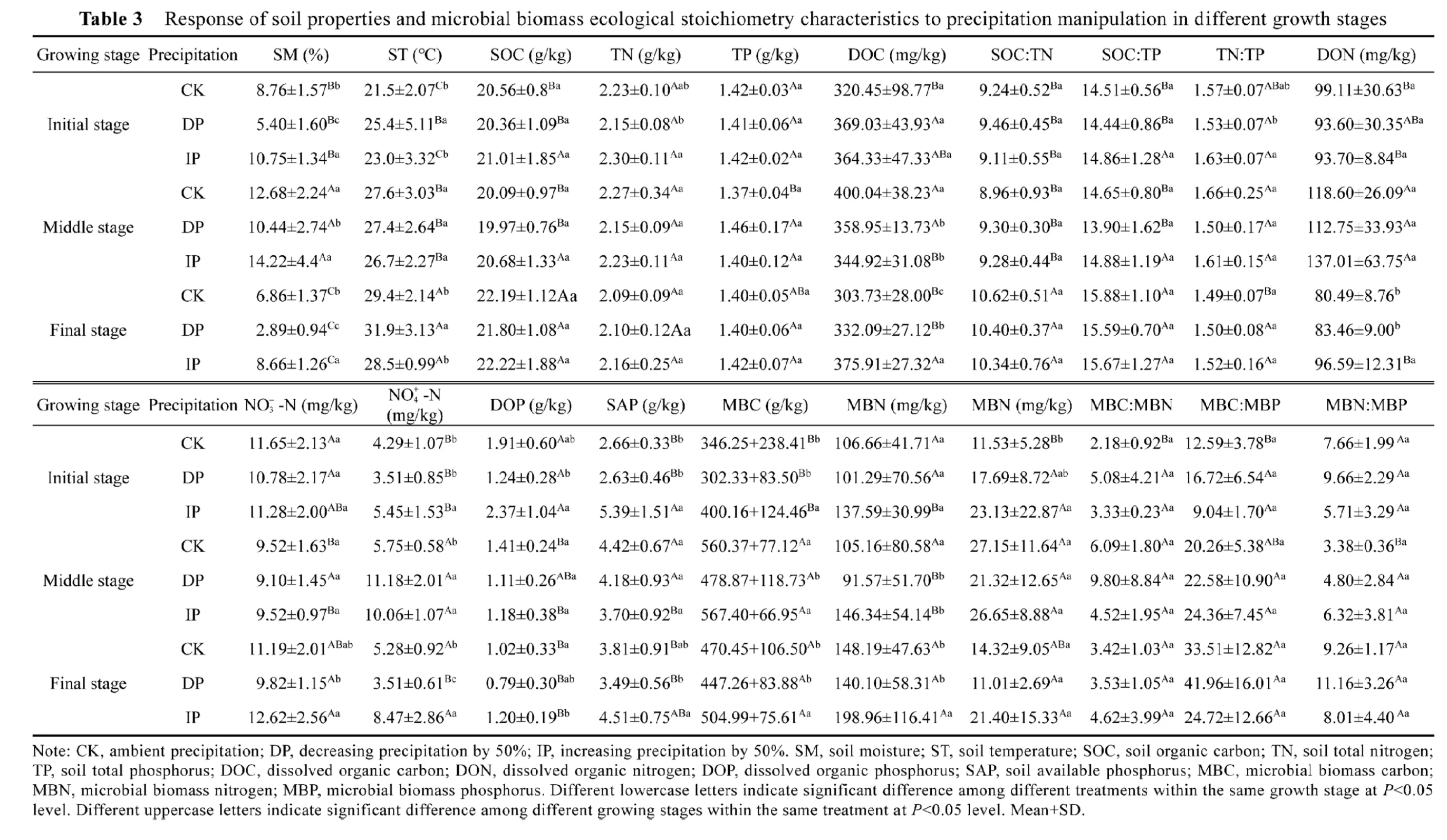
Phosphorus limitation is common in natural grassland ecosystems (Waring et al., 2014; Peng and Wang, 2016), and is strongly mediated by precipitation variation (Li et al., 2022). This may be attributed to the soil available P being deficient in loessal soil regions, leading to microbial P limitation (Liu et al., 2013; Deng et al., 2019), and causing intense competition between microorganisms and plants during all growth seasons. Soil microbial P limitation decreased with the increase in soil moisture (Fig. 3). Soil moisture is a crucial parameter affecting the decomposition of litter, microbial structure, metabolic function (Bell et al., 2008), extracellular enzyme stoichiometry, and the available nutrient content of soil (Waring et al., 2014). Generally,water deficiency inhibits soil microbial metabolism by reducing microbiological activity (Borken et al., 2006), consequently, microbial P limitation is alleviated by promoting microbial metabolism under IP treatment (Cui et al., 2019; Li et al., 2022). Moreover, our results indicate that soil microbial P limitation tended to decrease as growth stage changed (Table 3). This may be explained by the amount of nutrients that plants put into the soil continuously increasing to meet the growth and metabolism of microorganisms during the growth season.
4.2 Drivers of variance in soil microbial metabolic limitation
Soil properties have been reported to have the strongest effect on soil microbial metabolic limitation, which is mainly due to the imbalance in soil nutrient supply caused by nutrient stoichiometric changes (Sinsabaugh et al., 2009). The results showed that soil C, N, and P stoichiometric ratios were negatively correlated with microbial C and P limitation (Figs. 5 and 6),indicating that soil nutrient stoichiometry had a strong influence on nutrient acquisition by microorganisms. Soil C pool was increased by increasing plant C inputs (Deng et al., 2018),which strongly affected the supply of C available to microorganisms (Zhang et al., 2018), and the microbial metabolic limitation was subsequently reduced by feeding soil microorganisms with more C sources (Deng et al., 2019). Soil TN improves the availability of N and increases the input of SOC by increasing plant biomass and microbial nutrient access, thereby affecting soil enzyme and metabolic activities. In addition, the contents of SOC, TN, and TP indirectly affected microbial C and P limitation by determining soil C:N:P stoichiometry (Figs. 5 and 6). Previous studies have also found that SOC:TN, SOC:TP, and TN:TP were negatively correlated with microbial metabolic limitation (Deng et al., 2019). Several potential mechanisms can be proposed to suggest that stoichiometry ratio in resources mediates soil microbial resource limitation: (1)manipulated precipitation may lead to an elemental stoichiometry imbalance, owing to the regulation of microbial community homeostasis that could affect microbial C and P limitations;and (2) microbial community composition and diversity are mainly controlled by resource stoichiometry (Li et al., 2022). Therefore, soil C:N:P stoichiometry has significant effects on the activity, function, and structure of microbial communities, and the nutrient requirements of microorganisms are determined by elemental stoichiometry of microbial biomass relative to available nutrients in the environment (Sinsabaugh et al., 2009). Furthermore, the enhancement of nutrient availability decreases microbial metabolism limitations (Deng et al., 2019). In our study,DOC and NO-3-N were negatively related to microbial C limitation (Figs. 5 and 6), which may be partly explained by the changes in soil extracellular enzyme activity and enzymatic stoichiometry by affecting soil substrate (C and N) availability.
Soil microorganisms play a significant role in soil C cycles through SOM decomposition;however, microbial growth and activity are often limited by resource availability (Luo et al.,2021). There were negative correlations of microbial metabolism limitation with MBC, MBN,and MBC:MBP, which may reveal a trade-off between C, N, and P acquisition and microbial biomass. In view of the fact that microorganisms investing more in one metabolic pathway would invest less in other processes (Ehrlich et al., 2017), there must be trade-offs to ensure a balance of resource allocations between biomass accumulation and biosynthesis of enzymes associated with energy and nutrient acquisition, affecting the microbial metabolism limitation (Liao et al., 2021).
4.3 Implications for soil microbial metabolic limitations and CUE under manipulated precipitation
?
There was a strong dependence between microbial C and P limitations (P<0.05; Fig. 3d) under manipulated precipitation throughout the growth season, which indicated that microbial C metabolism was coupled with P metabolism. Previous studies have shown that relative C and P limitations of microorganisms play crucial roles in nutrient cycling and soil turnover, especially in microbial SOM decomposition (Sinsabaugh et al., 2009). As mentioned above, low soil moisture and C availability resulted in strong microbial C limitation. Microorganisms capture more C when C resources are input via plant-derived residues following digestion by soil microorganisms with increased precipitation, which increases P requirements of microorganisms (Cui et al., 2019; Li et al., 2022). Thus, microbial P limitation will consequently exacerbate if the increase in P does not satisfy microbial needs. In addition, if P becomes relatively limited, microorganisms may excrete P-acquiring enzymes by investing more C and N, which are related to P metabolism (Marklein and Houlton, 2012). Therefore, high P limitation could enhance relative C limitation in microorganisms. Simultaneously, microbial C and P limitations may limit plant growth and aboveground productivity in arid and semi-arid regions. This study suggested that research on temperate grasslands should not only explore the effects of manipulated precipitation on microbial resource limitation but also discuss their influence on the stability of C pool.
Manipulated precipitation influences microbial resource limitations through its effect on metabolic processes. Microbial metabolism limitation can greatly affect microbial growth and metabolism because decomposer cells need to maintain a balanced composition of C, N, and P and homeostasis of microbial biomass. The increase in microbial C and P limitation induces a microbial metabolism switch from synthetic metabolism (growth) to maintenance respiration,which could affect soil C sequestration by decreasing the assimilation of SOC by microorganisms(Manzoni et al., 2012). Soil microbial metabolism limitation has some effect on ecological balance, and can accelerate SOM decomposition, promote the availability of C, N, and P, and further stimulate the release of C from soil into the environment (Deng et al., 2019). Moreover,the Earth system model showed that resource limitation reduced soil C sink to a certain extent,making some regional sinks into net sources (Exbrayat et al., 2013). To evaluate the ecological impact of microbial metabolism limitation on soil C cycle quantitatively, we used soil microbial CUE as a quantitative index linking soil microbial metabolism with SOM decomposition.
Soil microbial CUE is a pivotal ecological parameter in the sequestration and release of soil C,which directly affects the retention time, turnover rate of C in ecosystems, and storage capacity of soil C (Xu et al., 2014). High microbial CUE indicated increased investment in the amounts of microbial products and the stability of C, whereas a lower CUE may reveal a lower C storage in the soil (Manzoni et al., 2012; Zechmeister-Boltenstern et al., 2015). Previous studies have shown that water stress inhibits microbial growth and CUE (Tiemann and Billings, 2011). In our study,microbial CUE was inhibited by DP treatment, but promoted by IP treatment (Fig. 4), which is consistent with the fact that microbial metabolism limitation was promoted by DP treatment, but decreased by IP treatment (Fig. 3). Investments in enzyme synthesis increased during decomposition progresses (Xu et al., 2018). Therefore, microorganisms increased the potential decomposition of SOM by secreting C-acquiring and P-acquiring enzymes (Moorhead et al.,2012), to obtain energy and nutrients under low water and nutrient availability conditions, which resulted in higher microbial C and P limitations, and lower microbial CUE, further causing SOC loss. However, when water and nutrients were sufficient to meet the needs of microorganisms,soil microorganisms reduced the demand for sources and decreased the secretion of corresponding C-acquiring and P-acquiring enzymes, leading to a decreased microbial metabolism limitation and higher microbial CUE. Therefore, soil microorganisms could fix C as their biomass and promote C sequestration (Malik et al., 2020).
5 Conclusions
Soil microbial metabolism was limited by C and P in temperate grassland, and there was a strong dependence between microbial C and P limitation under manipulated precipitation throughout the growth season. Variations in soil C, N, and P stoichiometry, nutrient availability, and microbial biomass ecological stoichiometry characteristics mainly contributed to the limitations. Microbial metabolic limitations and CUE reflect the response strategies of microorganisms to precipitation changes in temperate grassland ecosystems. These results suggested that decreased precipitation aggravated microbial metabolism limitation, and then causing C loss, however, increased precipitation relieved microbial metabolism limitation, and was beneficial to C sequestration in soil. These findings provide a referenced basis for understanding relative responses of microbial metabolic limitation and CUE to manipulated precipitation, contributing to improved predictions of the regulation of microbial metabolic limitation and CUE on soil C turnover.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41730638), the Key Research and Development Program of Shaanxi Province, China (2021ZDLSF05-02), the Scientific and Technological Innovation Project of Shaanxi Forestry Academy of Sciences, China (SXLK2021-0206), the Funding of Special Support Plan of Young Talents Project in China (2021), and the National Forestry and Grassland Administration in China (20201326015). We thank the data support from "National Earth System Science Data Center, National Science & Technology Infrastructure of China (http://www.geodata.cn)".
- Journal of Arid Land的其它文章
- Antelope adaptations to counteract overheating and water deficit in arid environments
- Leaf morpho-physiology and phytochemistry of olive trees as affected by cultivar type and increasing aridity
- Competition, spatial pattern, and regeneration of Haloxylon ammodendron and Haloxylon persicum communities in the Gurbantunggut Desert,Northwest China
- Leaf stoichiometry of Leontopodium lentopodioides at high altitudes on the northeastern Qinghai-Tibetan Plateau, China
- Effects of native and invasive Prosopis species on topsoil physiochemical properties in an arid riparian forest of Hormozgan Province, Iran
- Contents and spatial distribution patterns of heavy metals in the hinterland of the Tengger Desert, China

