Reversibly photochromic wood constructed by depositing microencapsulated/polydimethylsiloxane composite coating
Zhen Jia · Wenhui Bao · Chengyun Tao ·Wenlong Song
Abstract Photochromic wood was fabricated by coating microencapsulated photochromic material (MP)/polydimethylsiloxane composites onto wood using a simple drop-coating method.Urea-melamine–formaldehyde resin was used to microencapsulate the photochromic material(PM) via in situ polymerization.The concentration of the MP affected the photochromic property of the wood surface.The total color change (Δ E*) reached 82.2 when the concentration of the composite coating is 8%.Adhesion tests confirmed that the composite coating adhered firmly to the wood.This method is potentially useful for the production of functional wooden products, such as anti-counterfeiting materials and aesthetic wood.
Keywords Wood·Photochromism·Microencapsulation ·Urea-melamine–formaldehyde resin·Drop-coating method
Introduction
Microencapsulation is used to enclose solid and liquid microparticles and gasses in inert shells, which can then be used for the sustained release of the materials within the core (Azagheswari et al.2015).Microcapsules are classified based on their functions (Lee et al.2016), including fire resistance (Ma et al.2013), thermochromism (Ma and Zhu 2009), photoresponsivity (Hu et al.2016), and energy storage (Chen et al.2015) (Fig.1).Microencapsulation has been used in the manufacture of several products including adhesives, agrochemicals, pharmaceuticals, and other advanced functional materials (Hu et al.2014; Xu et al.2016).Wood has been used by mankind for millennia owing to its excellent strength-to-weight ratio, aesthetic appearance, and low thermal expansion (Berglund and Burgert 2018).However, it is also susceptible to dimensional changes, biological attack,discoloration, and extensive checking.Hence, many modification methods have been developed to improve the functions or applications of wood products (Lang et al.2018).Although the number of wood modification methods has greatly expanded recently, wood functionalization is still a focus of both industry and academia (Perez et al.2018).
Fig.1 Combination of functional microcapsules and wood for multi-functional materials
Photochromic materials (PMs) undergo reversible color changes when illuminated.They are used in many applications (Saputro et al.2019), including building materials,solar cells, displays, and organic memory devices (Shallcross et al.2013).Li et al.( 2017) produced photoresponsive inorganic/organic hybrids by impregnating wood with phosphomolybdic acid/polyvinylpyrrolidone composites under pressure.Hu et al.( 2015) developed photochromic plywood by adding photochromic microcapsules to the plywood coating.Wang et al.( 2019) reported the fabrication of photochromic transparent wood by infiltrating a lignin-modified wood template with a mixture of photochromic materials to produce smart windows.Hui et al.( 2015) reported the fabrication of a photoresponsive smart coating on a wood substrate, the composite had a color index of 58.2 at a PM concentration of 3% and was hydrophobic (with a contact angle of 134°).Wood-based photochromic materials have developed rapidly, but they still have some shortcomings that limit their large-scale applications (Li et al.2010), such as complex preparation procedures, long reaction times, and poor long-term durability (Wang et al.2014a, b).
Among the many types of PMs, the spiropyrans have attracted attention owing to their ultrafast responses, fatigue resistance and stable photochromism (Choi et al.2003).Photochromism of spiropyran is due to photochemical conversion of the initial, the so-called closed form (colorless)or spiro (SP), form to the open form (colored) or isomeric merocyanine (MC).Upon UV irradiation such molecules are able to transform into the MC forms, which reverse back into the SP forms both thermally and photochemically (by irradiation with visible light).Urea-melamine–formaldehyde(UMF) resins are generally used as microencapsulation coatings because their preparation is controllable, and they have excellent water resistance and thermal stability (Mao et al.2013).The core–shell structure is tightly packed and tough,which prevents leakage of the core material during changes in the external environment.By encapsulation inside microcapsules, chemicals can be released at a desired rate only when and/or where the release is needed.The advantage of microencapsulated photochromic material (MP) is that the response time characteristics is significantly longer compared to the photochromic materials, mainly because of the UMF microcapsule shell act as a ref lective layer to mitigation the light absorption.Polydimethylsiloxane (PDMS)is used in microfluidic devices, self-powered devices, and electronic devices owing to its low cost, optical properties,biocompatibility, and water impermeability.
Herein, we report a facile method for fabricating low-cost,mechanically-durable organic photochromic surfaces with long-term coloration on solid wood.We began by synthesizing a microencapsulated photochromic material (MP) using a UMF resin, then dropped a MP-PDMS solution onto the wood surface.The process by which the photochromic wood was fabricated is illustrated in Fig.2.In this coating system,PDMS was used as an elastic material that provided resistance to external damage and ensured mechanical durability(Toepke and Beebe 2006).We demonstrated the large-scale production of photochromic wood, which was subsequently used as a photo-switchable and colorful smart wood surface.We carried out a series of characterizations and measurements to determine the properties of the films.The surface morphologies and chemical compositions were characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray photoelectron spectroscopy(XPS), and attenuated total ref lectance Fourier-transform infrared spectroscopy (ATR-FTIR).
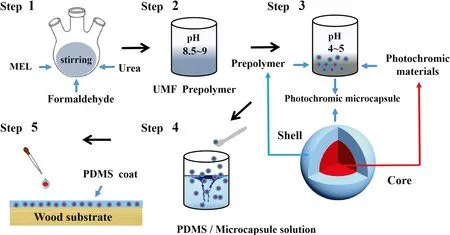
Fig.2 Schematic of the synthesis of photochromic wood
Materials and methods
Materials
Wood samples obtained from a forest in Harbin, P.R.China were cut into specimens measuring 50 mm × 25 mm × 5 mm(length × width × height).After ultrasonically rinsing in ethanol and deionized water for 10 min, the wood specimens were oven-dried (24 h, 103 °C ± 2 °C) to a constant weight, which was recorded.The photochromic material 3-(2,4-dimethoxyphenyl)-3-(4-methoxyphenyl)-3Hnaphthol[2,1-b]pyran (95.0%) was obtained from Sigma Chemical Reagent Corporation, Darmstadt, Germany.Urea,melamine, and formaldehyde were purchased from Tianjin Chemical Reagents Co., Ltd., China.PDMS (Sylgard 184)and the curing agent were provided by Dow Corning Co.,Ltd., USA.
Sample preparation
Preparation of the microencapsulated photochromic material.
Synthesis of prepolymer: Urea, melamine, and 37% formaldehyde solution were placed in a three-neck f lask with a stirrer.First, the pH of the mixture was adjusted to 8.5–9.0 with 20% NaOH solution.The mixture was then heated to 75 °C and held at that temperature for 10 min (Kandelbauer et al.2007).Second, 20 g of urea was added to the mixture,which was heated to 80 °C for 20 min and held at pH 7.5 at a constant temperature (No and Kim 2007).Finally, 40 g of urea was added to the mixture, which was cooled to 60 °C and held at a constant temperature for 30 min to obtain a prepolymer (Wang et al.2014a, b).
A mixture of the photochromic material (3 g) and ethanol(20 mL) was stirred at 1000 rpm at 30 °C for 10 min.Then,after adding the UMF prepolymer, the pH of the mixture was adjusted to 4–5 with 20% acetic acid (No and Kim 2004).The resulting mixture was heated at 80 °C for 3 h while stirring.After cooling to room temperature, the product was filtered, washed with distilled water, and dried at 105 °C to obtain the MP powder.
Preparation of the photochromic wood
First, a base coat solution of PDMS was prepared by dissolving 5 g of PDMS and 0.5 g of curing agent in 35 g of butyl acetate solution while stirring magnetically.Next, MP was added to the PDMS solution, which was magnetically stirred at 45 °C for 2 h, and ultrasonically dispersed at room temperature for 30 min.The percentage of MP was pre-set at 0, 2, 4%, 6 and 8%.Finally, the wood was dipped in the MP-PDMS solution for 10 min and cured at 80 °C for 5 h to obtain the PDMS pre-coated substrate.
Characterization
The morphologies of the as-prepared samples were investigated by SEM (Quanta 200, FEI, Eindhoven, Holland; 12.5 kV).The chemical compositions of treated and untreated wood were determined by FTIR (Magna-IR 560,Nicolet) and X-ray photoelectron spectroscopy (K-Alpha;ThermoFisher Scientific Company, Massachusetts, USA).Non-contact surface profilometry was used to determine the surface morphology.Thermogravimetric (TG) analysis was performed on a TA-50 instrument under an N2atmosphere.The solar simulator (XES-40S2-CE, Lamp L150SS, Japan,the wave length of solar light: 300–1100 nm) was used to examine the stable UV light and VIS light, and the irradiance was the standard level of 1000Wm-2.The UV light and VIS light were transformed each other by controlling the UV filter (300–380 nm) and VIS filter (380–780 nm),respectively.The distance between the lamp and samples was 20 cm.The sample color was determined using an optical spectrophotometer (CM-2300d; Konica Minolta Sensing,Inc.), and the color change was evaluated usingL,a, andbcolor parameter values (Gan et al.2015).The total color changes (ΔE) of the sample coatings were calculated using Eq.1:
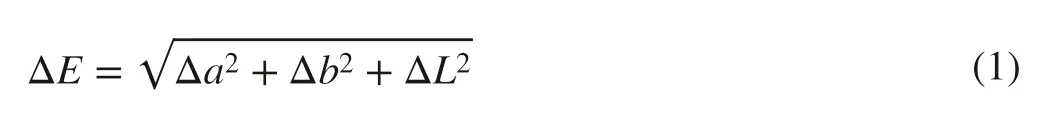
Results and discussion
Physical and chemical properties of the microcapsules
The structure of the microcapsules was determined by SEM.As shown in Fig.3 a, the PM comprised microspheres with diameters ranging from 1 to 5 μm with relatively smooth globular surfaces.The PM was completely covered with the cured UMF resin shell to form the MP.As expected, most of the visible MP was polypore, with highly heterogeneous particle sizes and shapes (Fig.3 b).
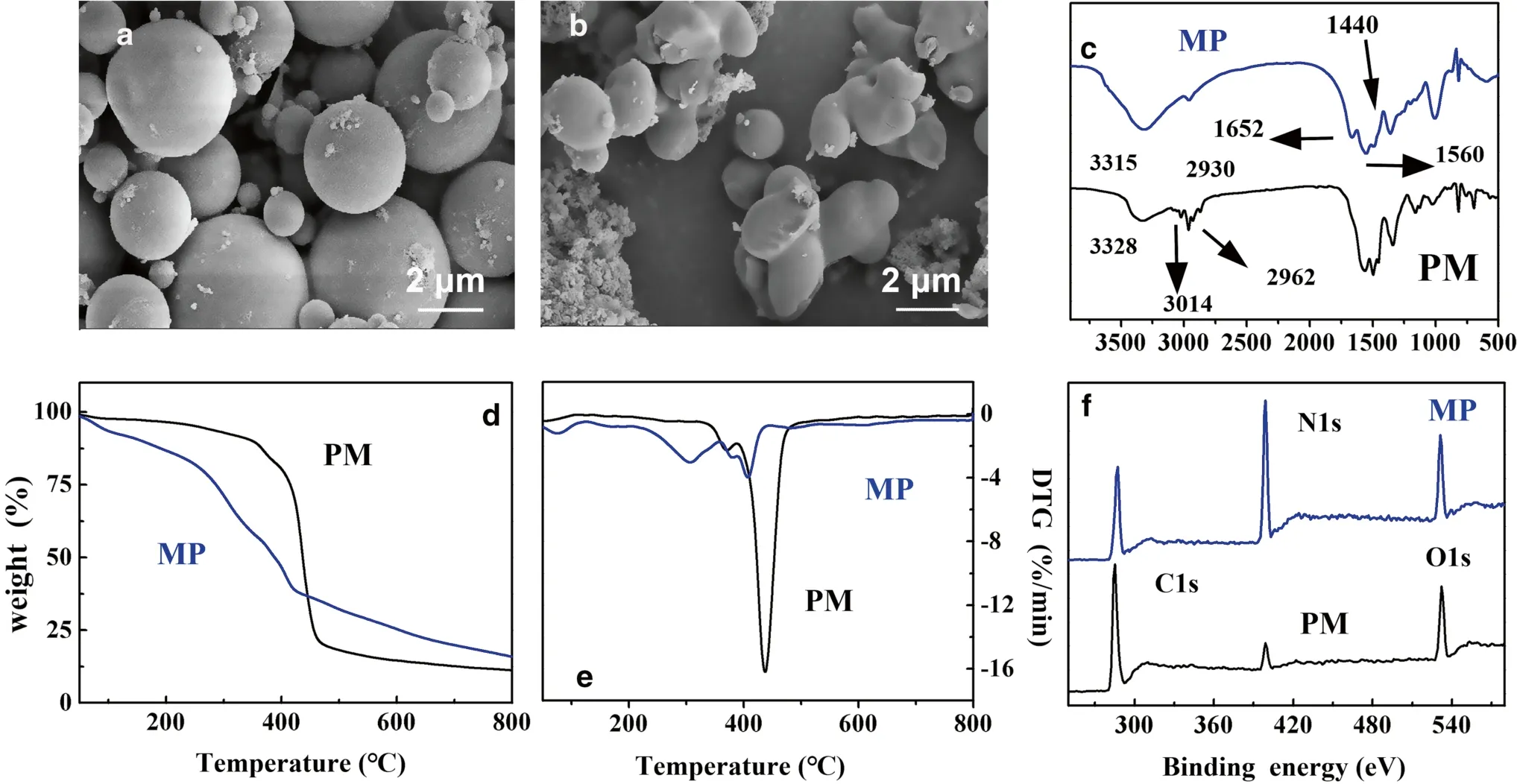
Fig.3 Scanning electron microscopy (SEM) investigation of surface morphology: ( a) photochromic material (PM), and ( b) microencapsulated photochromic material (MP); ( c) Fourier-transform infrared spectroscopy (FTIR) spectra of PM and MP; ( d) TG and ( e) DTG curves of PM and MP; (f) X-ray photoelectron spectroscopy (XPS)spectra of PM and MP
The FTIR spectra of the PM and MP are presented in Fig.3 c.The typical absorption peak of the PM at 3023 cm-1was attributed to the aromatic C–H stretching vibration (Kim et al.2008).The absorption peaks at 2962 and 2930 cm-1were attributable to alkane C-H stretching vibrations.The strong and wide absorption peak at 3346 cm-1was attributable to the ring vibration of melamine in the UMF resin (Pakdel et al.2007).The band at 1652 cm-1was associated with the C=O stretching vibrations of the amide group of methyl urea and the C=N stretching vibrations of the triazine ring (Chai et al.2018).The peak at 1560 cm-1was attributable to a combination of the vibrations of C–N and –NH2in the amide and melamine (Benson 2003).The band at 1440 cm-1was indicative of amide and triazine stretching vibrations (Marvel et al.1946).The surface elemental compositions of carbon, oxygen, and nitrogen in the PM and MP were determined by XPS (Fig.3 f).The peaks located at 294, 408, and 541 eV were attributed to C 1 s, O 1 s, and N 1 s, respectively.The MP showed a significant increase in the nitrogen concentration compared with the PM, whereas the oxygen content remained nearly constant.The MP N/C ratio determined by elemental analysis was 0.863, which was higher than the PM N/C ratio of 0.123.This indicated that that the PM was well-coated by the resin (Coullerez et al.2000).
The TG and differential thermogravimetric (DTG) curves of the PM and MP shown in Fig.3 d, e reveal that the PM began to decompose at approximately 310 °C and decomposed almost completely at approximately 440 °C.The residual weight of the PM was 11.6% at 800 °C.Compared with PM, MP has two main decomposition processes.The initial decomposition stage of the MP occurred more quickly than the decomposition of the PM owing to water evaporation and slow free formaldehyde emission (Wu et al.2008).During the second decomposition stage (above 425 °C),the MP was more stable than the PM because the UMF resin produced incombustible gases, such as NH3and CO2,which formed a thermally stable honeycomb char (Siimer et al.2009).TheTmaxvalues for the two MP decomposition stages were 305 and 410 °C (Németh et al.2018).After decomposition at 800 °C, the MP left approximately 23.5%residue, which was much more than the PM (Li et al.2007).Therefore, the MP was more effective at improving the thermal stability than the PM.As mentioned above, the results further indicated that the PM was completely covered with the cured UMF resin shell to form the MP.
Morphology of the photochromic wood surface
The surface morphologies of the pristine wood and the MP-PDMS nanocomposite-coated wood are presented in Fig.4 a, d.The original wood surface comprised a heterogeneous porous material with a micro-grooved surface (Fig.4 a).After dipping in the MP-PDMS coating solution, a continuous polymer film was formed on the surface of the wood (Fig.4 d).Micro-scale protrusions comprising the MP were distributed throughout the film.The MP played an important role in the photochromism,and the texture of the wood remained visible because the film was transparent.
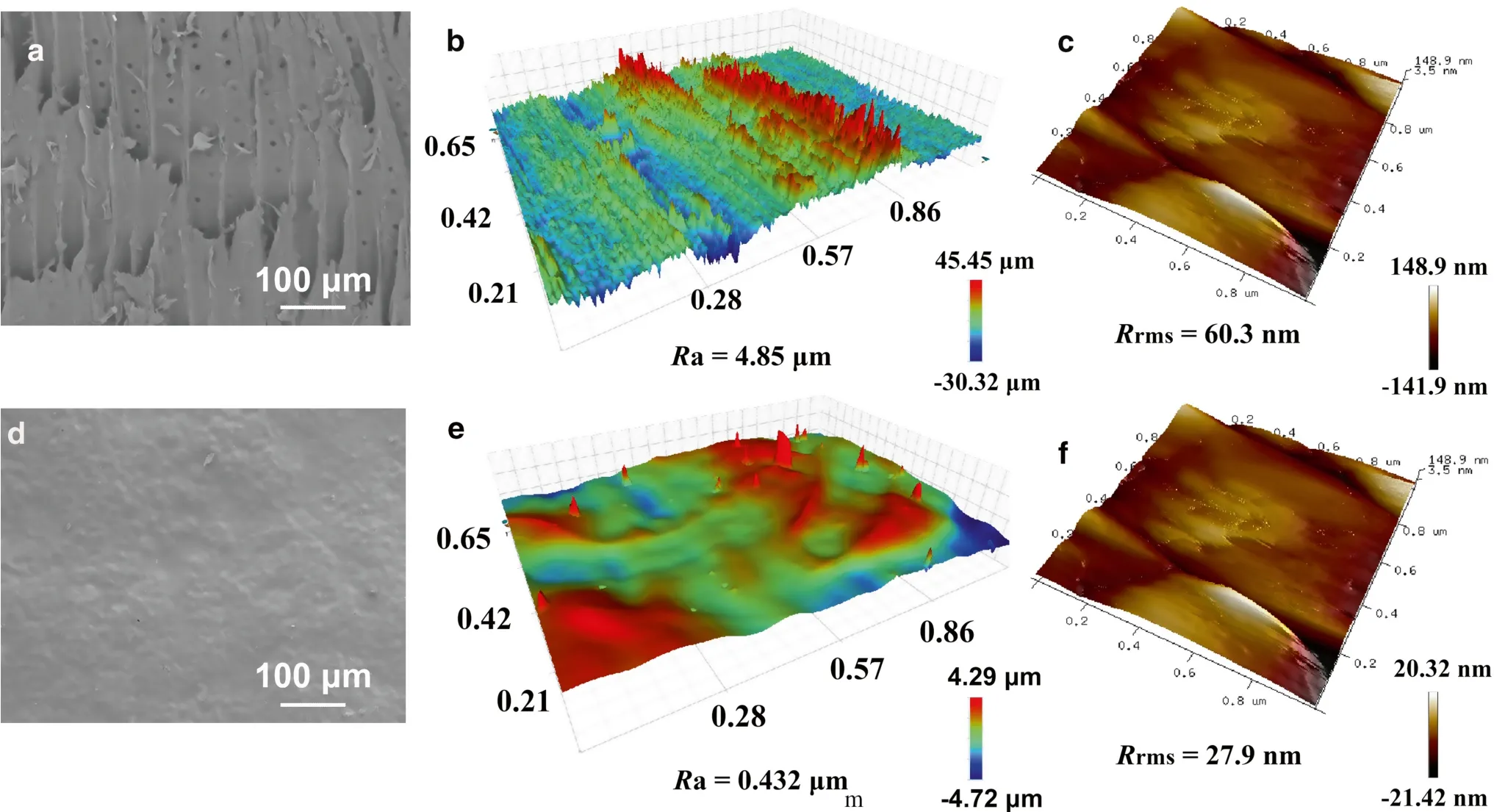
Fig.4 Scanning electron microscopy (SEM) images, 3D scans, and AFM images of the pristine wood ( a, b, c) and MP-PDMS nanocompositecoated wood ( d, e, f)
The samples were also investigated by non-contact surface profilometry and AFM to determine changes in the surface morphology.Figure 4 b, e presents 3D images of the as-prepared samples.As shown in Fig.4 b, the original wood surface was almost completely covered with a clear vertical gradient due to the complex surface texture.Compared with the original wood surface, the MP-PDMS nanocomposite-coated wood surface (Fig.4 e) was smooth and flat.The surface roughness (Ra) values were 4.85 μm(original wood) and 0.432 μm (MP-PDMS wood).
Tapping mode AFM images of the surface topography of the original wood and the MP-PDMS wood are shown in Fig.4 c, f.The different surface roughness values are reflected in theRrmsvalues (rms=root mean square=the standard deviation of theZvalue;Zis the total height range analyzed) of the two surfaces, which were 60.3 nm(original wood) and 27.9 nm (MP-PDMS wood).
Photochromic properties of samples with various MP concentrations
Figure 5 shows the color parameters of the pristine wood and the wood coated with MP-PDMS with MP concentrations of 2, 4, 6 and 8% in weight.As the MP concentration increased,the lightness factorL*of the samples under UV irradiation decreased from 60.1 to 12.2, indicating that the surface color darkened.Thea*value increased markedly from 5.2 to 10.3,implying the surface color turned a deeper shade of red.Theb*value decreased from 13.0 to - 22.3, indicating that the surface color changed from yellow to blue.Furthermore, the total color change (△E*) increased from 3.7 to 80.2 as the MP concentration increased from 0 to 8%, confirming the photoresponsivity of the MP-PDMS-coated wood.
As shown in Fig.6 a, the lightness and chroma of the wood coated with MP-PDMS with MP concentrations of 2%in weight was very similar compared to the pristine wood.With an increase in the MP concentrations from 4 to 8% in weight, the lightness of the treated wood surface is gradually increased due to the microcapsule shell (UMF) is white(Fig.6 b–d).However, the natural color texture of the wood surface is not affected by the composite coating.After UV light irradiation, surface color slightly darker (Fig.6 e).With increasing MP concentrations, the surface color turned dark red (Fig.6 f–h).Color changes of the photochromic coatings were related to the increasing number of chromophores.To further explore the relationship between the color change of the photochromic coating and the intensity of the UV irradiation, MP-wood samples with 8% concentration in weight under different UV intensity irradiation the digital images(Fig.6 a–d) were utilized.As a rule, high intensity UV irradiation leads to further increase of color intensity due to the accumulation of the open MC forms.
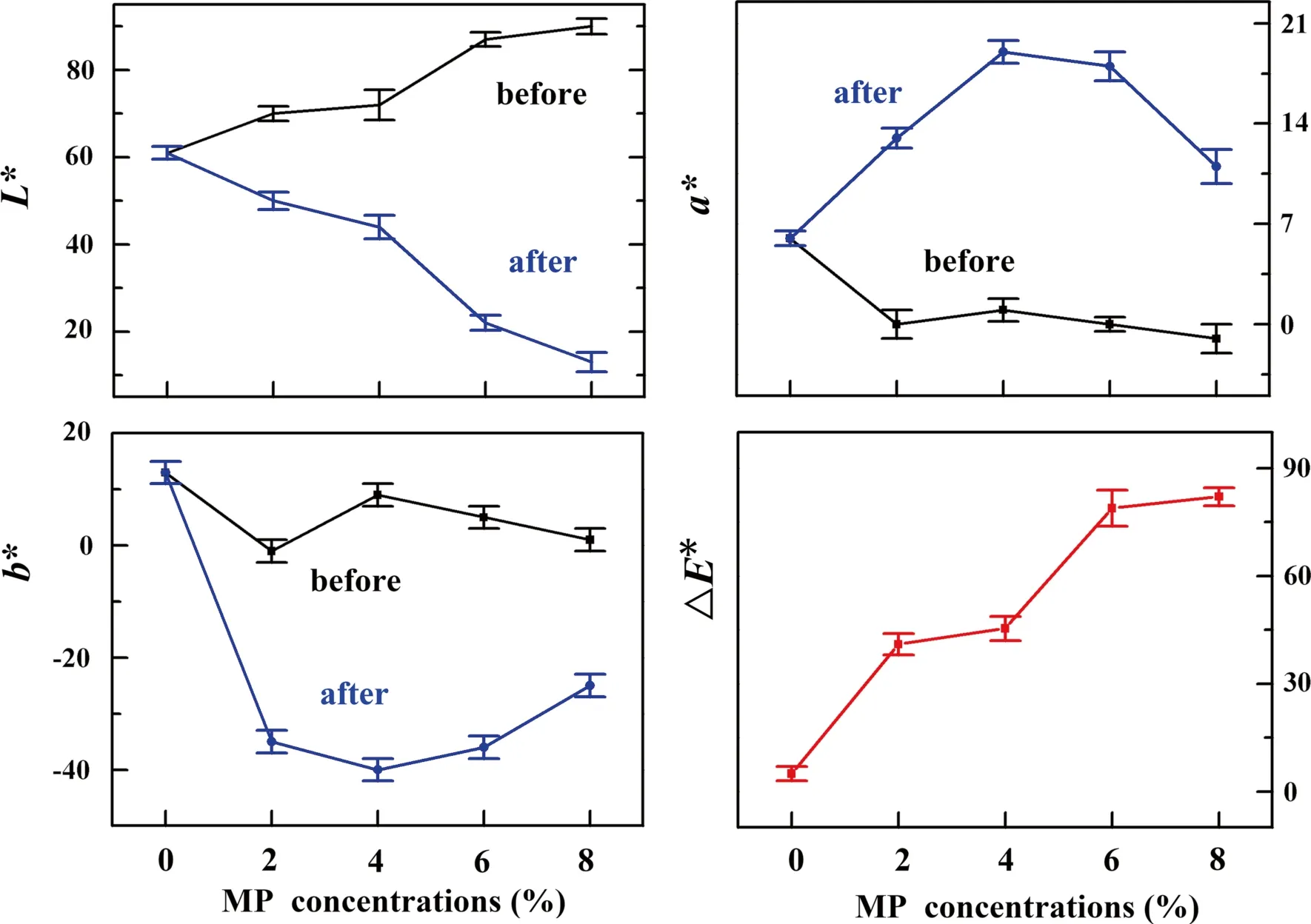
Fig.5 Changes in the sample surface chromaticity indexes L*, b*, a*, and △E* before and after UV irradiation
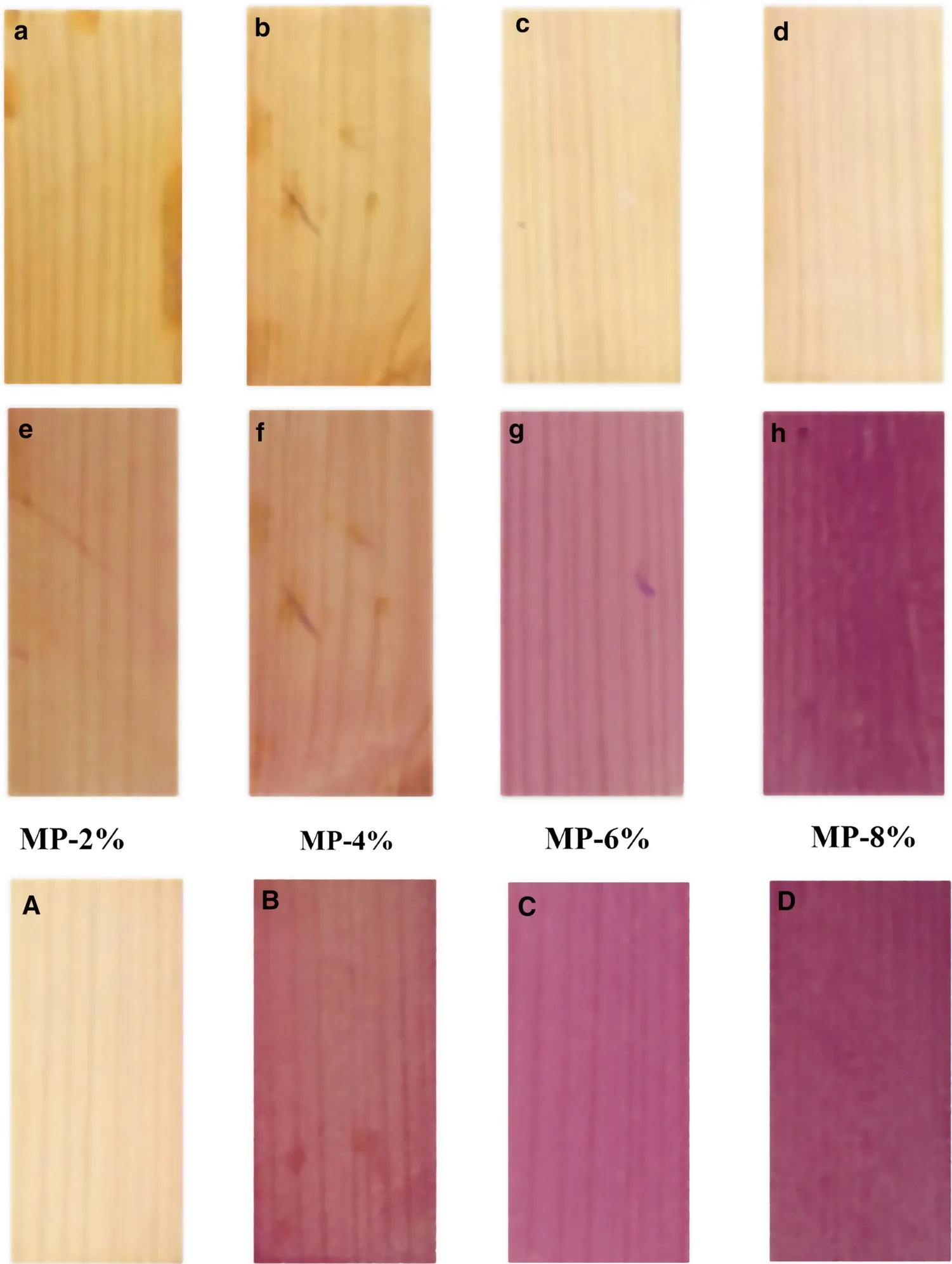
Fig.6 Digital images of MPwood with different concentration of 2, 4, 6, 8% before ( a - d)and after UV irradiation ( e - h),MP-wood with 8% concentration after different UV intensity irradiation for (A) 50Wm- 2 , (B)200Wm- 2 , (C) 500 Wm- 2 , and(D) 800Wm- 2
The practical applications of photochromic wood samples are directly affected by the response time, which includes the chromogenic time and the fading time.Figure 7 shows the response time curves of 2%–8% MP-wood.At a constant temperature, the response time of the sample gradually increased as the MP concentration increased, which is attributed to the fact that an increase in the MP concentration results in strong electrostatic interaction between the MP molecules and an increase in the degree of aggregation, as shown in Fig.7 a.As the concentration of the spiropyran increases, a transformation of the chemical structure is less likely because the closed form of the spiropyran increases steric hindrances; hence, there is a lag in the response time of MP-wood formed with higher MP concentrations.As a result, at high concentrations, the photochromic microcapsules samples are more stable under changing temperature conditions.As the temperature increased, the chromogenic time decreased.When the temperature exceeded 30 °C, the MP concentration exerted a minimal influence on the fading time (Fig.7 b).The result for the response time is attributed to the fact that an increase in the temperature favors the breakup and recombination of the C–O–C bond in the spiropyrane, thereby increasing the conversion rate of spiropyrane to merocyanine.
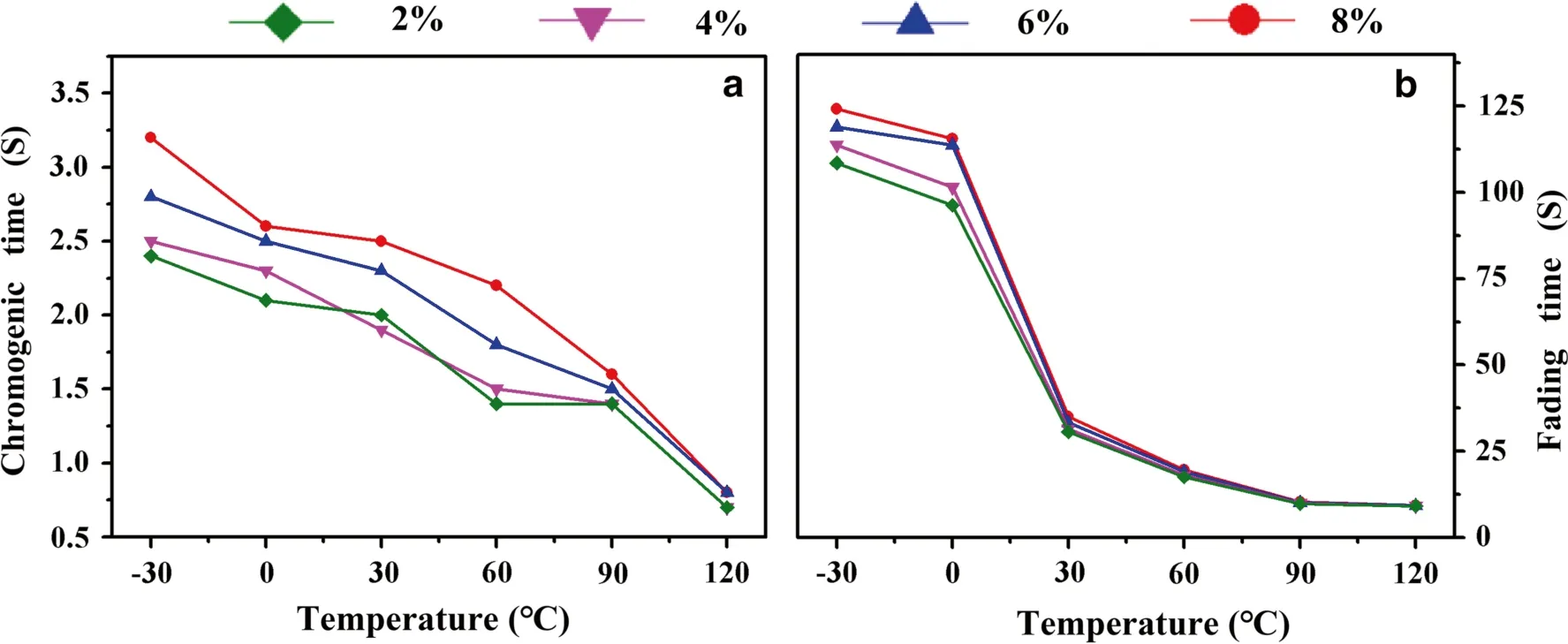
Fig.7 Response time–temperature curves of the samples: ( a) chromogenic time, and ( b) fading time
Color stability and adhesion properties of samples
Figure 8 a illustrates the △Ecycle characterization of samples with various MP concentrations.The results indicate that the photoresponsivity of the MP-wood was not affected by 100 UV irradiation cycles.To assess the mechanical resistance of the MP-wood, samples were abraded with sand and sandpaper.The surfaces of the MP-wood samples were subjected to impingement with 15 g of sand from a height of 25 cm, the sand grains had diameters in the range 200–250 μm.Figure 8 b shows that an as-prepared sample with a 100 g weight on its top was placed face-down on the sandpaper and moved 50 cm.After 100 sand impact tests,the surfaces of the films appeared smooth, with no signs of damage (Fig.8 e).In contrast, the SEM images showed that the film was broken and severely worn because the external load of the sandpaper exceeded the strength of the film (Fig.8 f).Figure 8 d shows the ΔE*values of the samples made with various MP concentrations (2, 4, 6 and 8%)before and after UV aging.The sample with an MP concentration of 8% had the highest ΔE*value during the aging test, indicating that UV aging resistance was proportional to the MP concentration.ΔE*decreased significantly from 100 to 150 h.The photochromic performances of the samples degraded with UV exposure time, but the samples with high MP concentrations still exhibited satisfactory photochromic performances after 250-h UV aging.
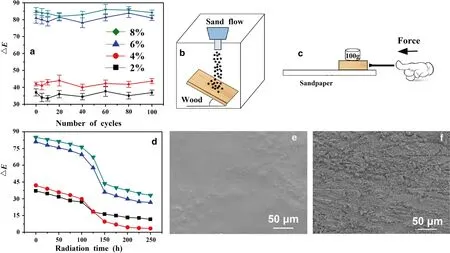
Fig.8 ( a) Cyclic UV aging tests and ( d) UV aging tests on the samples.Schematic drawing of the sand abrasion test ( b) and the sandpaper abrasion test ( c), SEM images of the surfaces after ( e) 100 cyclic impingements, ( f) 50 cm abrasion on sandpaper
Conclusion
Photochromic films were deposited on the surface of wood by drop-coating a solution containing MP particles and PDMS.The ΔE*values of the as-generated wood increased with increasing MP concentration, and 8.0%MP produced a photochromic surface with a ΔE*of 82.2.The chromogenic time and fading time of the photochromic coatings decreased with the increasing of temperature.The sand abrasion test revealed that the film had high mechanical resistance; however, the sandpaper abrasion test destroyed the film when the external force was higher than its own load.The present study provides an effective and convenient method for obtaining novel photochromic wood products.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
 Journal of Forestry Research2022年4期
Journal of Forestry Research2022年4期
- Journal of Forestry Research的其它文章
- Journal of Forestry Research
- Surveillance of pine wilt disease by high resolution satellite
- Adaptation of pine wood nematode, Bursap helenchus xylophilus,early in its interaction with two P inus species that differ in resistance
- Pine wilt disease detection in high-resolution UAV images using object-oriented classification
- Transcriptome analysis shows nicotinamide seed treatment alters expression of genes involved in defense and epigenetic processes in roots of seedlings of Picea abies
- Effects of enrichmemt planting with native tree species on bacterial community structure and potential impact on Eucalyptus plantations in southern China
