Adaptation of pine wood nematode, Bursap helenchus xylophilus,early in its interaction with two P inus species that differ in resistance
Yaqi Feng ·Lin Rui ·Xinyu Wang ·Xiaoqin Wu
Abstract Pine wilt disease (PWD) is one of the most devastating diseases of Pinu s spp.and is caused by the pine wood nematode (PWN), Bursaphelenchus xylophilus(Steiner & Buhrer) Nickle.To study adaptation of PWN to survive in hosts that differ in resistance, we examined the self-regulatory characteristics of PWN at the biological and molecular levels early in the interaction.Two-year-old susceptible Pinus thunbergii and resistant Pinus taeda were selected for this experiment, and changes in PWNs after inoculation were assessed.qRT-PCR was used to detect changes in genes related to PWN pathogenicity and detoxification.The results showed that the migration and reproductive abilities of PWNs in P.thunbergii were stronger than those of PWNs in P.taeda.After 7 d, the number of nematodes in P.thunbergii was approximately 3.2-fold higher than in P.taeda.After 15 d, the number of nematodes in P.thunbergii was approximately twofold higher than that in P.taeda.Because PWN can adjust its sex ratio after infection,we compared the sex ratio of uninoculated PWNs, to that in the two pine species.In P.thunbergii, the female to male ratio first decreased and then stabilized over time; in P.taeda first decreased and then increased.Relative fat accumulation in PWNs increased significantly after the PWNs entered the tree body; the accumulation rate in P.thunbergii was higher than in P.taeda at 7 d, but lower after 15 d.Scanning electron microscopy (SEM) showed significantly more bacteria on the surface of PWNs in P.taeda compared with PWNs in susceptible P.thunbergii.At 12 h after inoculation, the expression of genes related to cell-wall degradation ( BxBeta1-4 and Bxpel1), effectors ( BxCDP1, BxSapB1),and active oxygen metabolism ( Bxy-ctl-1 and BxGST3) was 2–6 × higher in the resistant pine than in the susceptible one.In contrast, in PWNs, the expression of autophagy-related genes BxATG1 and BxATG16 was 1.5–2 times higher in P.thunbergii than in P.taeda.These results indicate that the interaction between PWNs and pine trees with different resistance levels elicits a series of physiological and molecular adaptations that affect nematode reproduction and virulence.This study will help elucidate the adaptive mechanisms of PWN in different pine trees.
Keywords Pine wood nematode·Self-regulation·Pinus thunbergii · Pinus taeda · Pathogenicity and detoxificationrelated genes
Introduction
The pine wood nematode (PWN),Bursaphelenchus xylophilus(Steiner & Buhrer) Nickle, causes pine wilt disease(PWD), which can devastate forests.Native to North America, PWN is now also found in Asia and Europe.In Japan,China and South Korea, it can cause pine tree death and damage local forest ecosystems on a massive scale (He et al.2010; Ye 2019).Currently, it is listed as a quarantinable pest in 52 countries around the world (Mota and Vieira 2009).In China, PWD was first discovered in 1982 at the Sun Yat-sen Mausoleum in Nanjing.Subsequently, it rapidly spread to surrounding areas, killing large numbers of pine trees.In China, 42.7% of the forest area is occupied by conifers, and 58% of those conifers arePinusspecies, which are vulnerable to PWN infestations.In recent years, PWNs have successfully expanded beyond the average annual temperature line of 10 °C to middle-temperate-zone areas, such as Liaoning, and high-altitude areas, such as the Qinling Mountains(Li and Zhang 2018).
Pinusspecies are the main hosts of PWN.PWNs are spread to the hosts by longicorn beetles.The nematodes first enters the cortical resin tract, then feeds on parenchyma tissue near the resin channels (Yang 1995; Yang et al.2003),destroying the cells and invades the cortical tissue, then enters the xylem resin tract (Fukuda 1997).In the early stage, nematodes are mainly distributed in the cortex and xylem resin channels and subsequently become distributed throughout the trunk, from the cortex to the pith, in the late stage.They can also feed on fungi after the death of the host tree (Kobayashi et al.1974).Jin and Ye ( 2007) inoculated 2-year-oldPinus thunbergiiwith a nonpathogenic PWN and PWNs that differed in virulence and found that the virulence of PWNs and disease severity were positively correlated with the migration and propagation rate.If nematodes cannot survive and reproduce in pine trees, they do not cause death.
Previous studies have largely elucidated the morphological structure and epidemiology of PWN (Liu et al.2019a),and with the development of molecular biology techniques, an increasing number of studies have focused on proteins and genes associated with pine nematode pathogenesis, reproduction, and resistance.Cellulase (endo-β-1,4-glucanase) is a key enzyme for degrading plant cell walls (Smant et al.1998), and the silencing of the cellulase gene in the pine wood nematode resulted in significant changes in the PWN phenotype and in reduced reproduction, migratory rates, and virulence (Ma et al.2011).Studies have shown that the effector geneBxSapB1can trigger necrosis inNicotiana benthamianaand that silencing the pine wood nematode effector geneBxSapB1can delay the onset of pine necrosis (Hu et al.2019).In addition,PWN may secrete effectors from pharyngeal gland cells to suppress the expression of disease-resistance genes in pine cells and protect itself from host defense responses(Xie et al.2017; Margarida et al.2018).At the same time,detoxification genes in PWN can protect it from damage by defensive substances produced by pine trees.The peroxidase genesBxPrx,Bxy-ctl-1andBxy-ctl-2play important roles in the antioxidant process in pine nematodes (Li et al.2011; Fu et al.2014; Vicente et al.2014, 2015).According to Liu et al.( 2019b, 2020), in the early stages of pine pathogenesis, nematodes may respond to the complex defense response of the tree by increasing the expression of autophagy genes.PWN can resist the oxidative stresses generated by pine ROS metabolism through autophagy,which is essential for nematode feeding, reproduction, egg hatching and survival.
Previous studies have suggested that whether pine trees develop disease after infection is related to the survival of nematodes in pine trees and indicated that the speed of disease development is directly related to the rate of nematode dispersal within the tree and the ability to reproduce in large numbers (Ichihara et al.1999).However, studies on the adaptation of pine nematodes to different resistance levels in pines have not thoroughly described, which has to some extent hindered the in-depth study of resistance mechanisms in pines.Not all genotypes of the genusPinusare damaged by pine nematodes; even within the same species, some can be resistant to PWNs; therefore, the use of resistant genotypes may be a relatively cost-effective way to control the disease (Wei et al.2018).
In the present study, we evaluated relevant biological indicators in PWN, including migratory rate, reproduction, sex ratio, fat accumulation, and microorganisms on the body surface of PWNs in a susceptible and a resistant pine species.The expression levels of effector genes and genes related to cell-wall degradation, reactive oxygen metabolism, and autophagy were also quantified in the two pine species after infection with PWN.The study was carried out to obtain insight into how nematodes gradually expanded their host range and successfully colonized different pine species to provide a scientific basis for understanding the interactions between nematodes and hosts with different resistance.
Materials and methods
Culture of PWN
Nematode strain AMA3, reported as highly virulent,was isolated from diseasedP.thunbergiifrom Maanshan, China (Wu et al.2013) and preserved at the Forest Protection Laboratory, Nanjing Forestry University.The nematodes were cultivated on a necrotrophic fungus,Botrytis cinerea, grown on potato dextrose agar (PDA) plates at 25 °C.When the hyphae were completely consumed,PWNs were extracted overnight using the Baermann funnel technique (Viglierchio and Schmitt 1983) and washed three times with M9 buffer (30 g L-1KH2PO4, 60 g L-1K2HPO4, and 50 g L-1NaCl).
Plant material
We purchased 2-year-old seedlings ofP.thunbergiiandP.taeda,35–40 cm high approximately, for the experiments,which were grown from aP.thunbergiiseed source from Suqian, Jiangsu Province, and aP.taedaseed source from Yingde Forestry Academy, Guangdong Province.The seedlings were grown in 50% yellow soil and 50% nutrient soil in the greenhouse of Nanjing Forestry University and grown at room temperature (average relative air humidity 70%).
Inoculation and sampling method
A sterile blade was used to cut the bark on the stem at a height of 10 cm in the aboveground part of the plant to reach the xylem, and sterile cotton balls were inserted with forceps.Then 200 μL of a nematode suspension containing 3,000 nematodes, was dropped onto the cotton on each plant;200 μL of ddH2O was placed on cotton in wounds on control pine trees.The inoculated pine seedlings were placed in the incubation room after inoculation to ensure that the nematode solution did not leak out.The stem segments of the treated and control pine seedlings were collected at 0.5 d, 7 d and 15 d after inoculation.Whole stem segments were divided into five sections to assess the migration and distribution of PWNs (1 cm above and the inoculation point, 1 cm below and the inoculation point, 5 cm above the inoculation point, 5 cm or more below the inoculation point and 10 cm or more above the inoculation point), the PWNs in treated pine trees were then separated via the Baermann funnel method over 2 h.The stem segments and the obtained pine nematodes were snap frozen in liquid nitrogen and stored at - 80 °C.Each treatment was replicated three times.
Determination of sex ratios in seedlings
Samples were taken at 7 d and 15 d after inoculation to collect nematodes, and 10 μL of the nematode suspension was extracted before and after inoculation, respectively.Then,the nematode sex ratio was calculated in the different pine species and converted to a specific number.Each treatment was replicated three times.
Determination of relative fat accumulation in PWN
At 7 d and 15 d after inoculation ofP.thunbergiiandP.taeda, nematodes were collected in 10 mL centrifuge tubes using the Baermann funnel method.Uninoculated PWNs(CK) were used as controls for the staining and relative fat accumulation assays for each of the above groups using the oil red-O staining method (Zhao 2015).M9 buffer was used to wash the nematodes.One group was employed for staining observations, and the other group was washed three times with M9 buffer and then prepared for use.The dye was extracted with anhydrous ethanol, and the OD value at 510 nm was measured with a UV spectrophotometer.At sampling times ofn= 0, 7, 15, the number of test PWNs wasPn, the measured OD value wasBn, and the relative fat accumulation rate,Rn, was calculated as follows:

The significance of differences between treatments was analyzed using SPSS software (ver.17.0; IBM China Company Ltd., Beijing, China) for pairedt-tests of two samples(Wang et al.2017).
Scanning electron microscopy of PWN
At 7 d and 15 d after inoculation ofP.thunbergiiandP.taeda, the nematodes were collected in 10 mL centrifuge tubes and washed via the Baermann funnel method, while uninoculated PWN (CK) was used as the control.The nematode suspension was rinsed with PBS (pH=7.2) buffer,washed and fixed in 2 mL centrifuge tubes by adding 4%v/v glutaraldehyde in 0.5 M phosphate buffer, sealed with tinfoil and placed in a 4 °C refrigerator for storage.Later, the samples were sent to Nanjing Forestry University Analytical Testing Center and observed with a scanning electron microscope (SEM) (FEI Quanta 200, USA).
RNA extraction and cDNA synthesis from PWN
The PWN fromP.thunbergiiandP.taedawere collected in 1.5 mL centrifuge tubes at 0.5 d after inoculation, respectively, using CK as a control.The total RNA of the PWNs was extracted via the Trizol extraction method after f lashfreezing in liquid nitrogen, and the equipment used in the extraction process, such as the centrifuge tubes and pipette tips, were treated with 0.1% diethyl pyrocarbonate (DEPC)and sterilized.The purity of the total RNA was evaluated by electrophoresis, and the concentration was measured using a Nanodrop 2000C spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).cDNA was synthesized from 1 μg of total RNA using HiScript? II Q RT SuperMix for qPCR (+ gDNA wiper; Vazyme, Nanjing, China) and the manufacturer’s instructions.
Fluorescence quantitative PCR
The expression patterns of nematode pathogenicity- and detoxification-related genes were detected by qRT-PCR after 0.5 d ofinfestation in pine trees with differences in resistance.Pathogenicity-related genes were selected from cell-wall-degradation-related genes and effector genes, and detoxification-related genes were selected from among genes related to ROS metabolism and autophagy.
The qRT-PCR primers were designed according to the available PWN coding sequences (Table 1), and theActingene (EU100952) of PWN served as an internal control.The qPCR SYBR Green Master Mix kit (Novozymes, Nanjing, China) was used as instructed, and reactions were run on an ABI Prism7500 (Applied Biosystems,Foster City, USA) with a two-step thermocycling procedure: (1) 95 °C for 5 min, 95 °C for 10 s, and 95 °C for 5 min, then (2) 95 °C for 5 min; 40 cycles of 95 °C for 10 s and 60 °C for 34 s.For the dissolution curve acquisition program, the default program provided with the instrument was used: 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s.The analysis of each sample was repeated three times.The instrument software automatically set the threshold,and the 2-ΔΔCtmethod (Livak and Schmittgen 2001) was used to analyze the data.
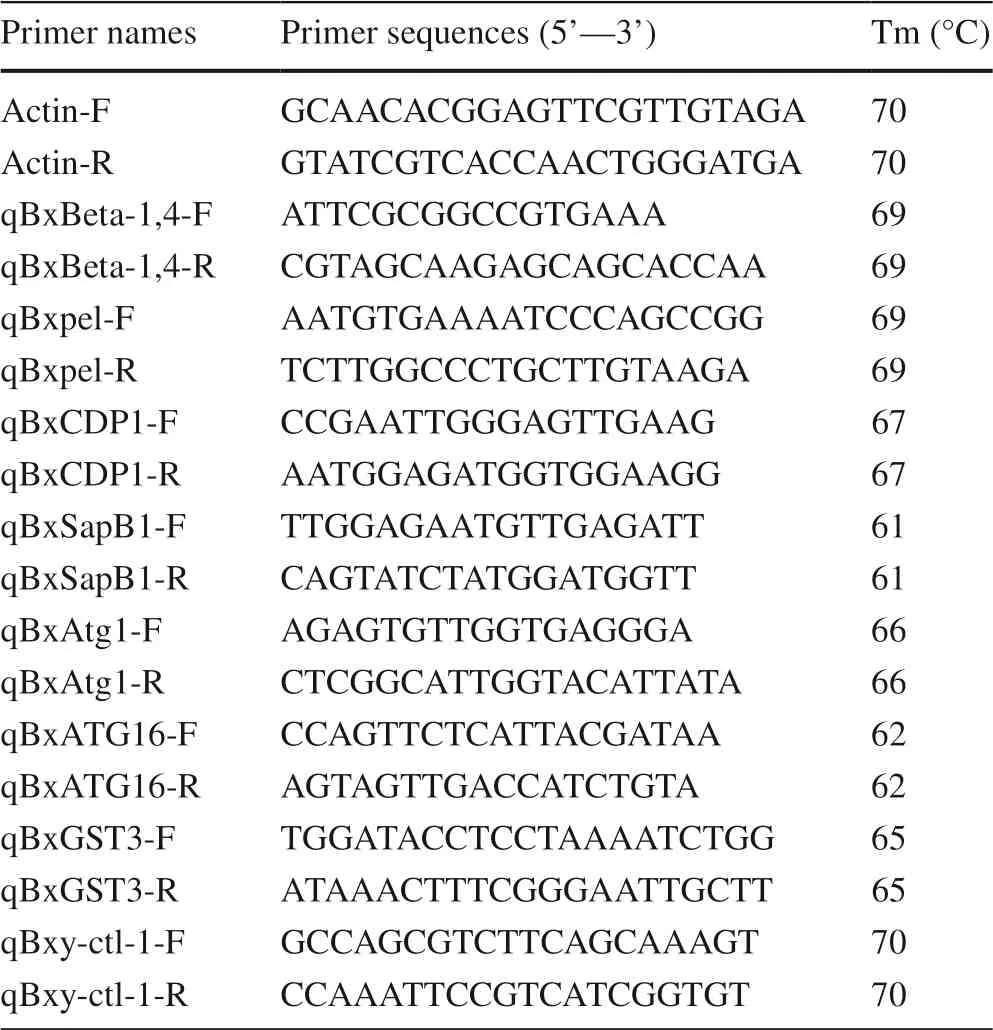
Table 1 Specific primers used in this study
Statistical analyses
All experiments were performed with three biological replicates and three technical replicates.Means and standard deviations (SD) were calculated using Excel (Microsoft,Redmond, WA, USA).Statistical significance of any differences was analyzed by a paired Student’st-test or Tukey’s HSD test using SPSS (ver.17.0; IBM China Company Ltd., Beijing, China) Statistics software.The threshold level of significance wasp≤ 0.05.
Results
Migratory and dispersal rates of PWN in the pine species
The results showed that the numbers of nematodes differed significantly between the two pine species.At 7 d after inoculation (dai), the nematodes were distributed throughout theP.thunbergiiandP.taedaplants, indicating successful nematode infestation.However, there were large differences in the numbers of PWNs in different parts of the trees, and more nematodes migrated downward than upward.At 7 dai, inP.thunbergii, the nematodes rapidly invaded the tree along mature resin tracts and migrated to other tree parts; the most nematodes were found 5 cm below the inoculation point (mean 950 nematodes/cm;approximately 1.6 times more than in the inoculum) InP.taeda, the nematodes aggregated near the infestation site and were less likely to migrate upward; 420 nematodes/cm (approximately 2/3 of the total in the inoculum) were found 0–5 cm below the inoculation point.At 15 dai ofP.taeda, at 5 cm or more below the inoculation point and 10 cm or more above the inoculation point, the number of PWNs was 1.5 and 1.3 times higher than in the inoculum,respectively.At 15 dai,P.thunbergiihad wilted, and the nematodes were concentrated 5 cm below the inoculation point; more than 13,000 nematodes were detected (approximately 4 times higher than in the inoculum) (Table 2).Thus, the PWNs inP.thunbergiimoved farther than inP.taeda.
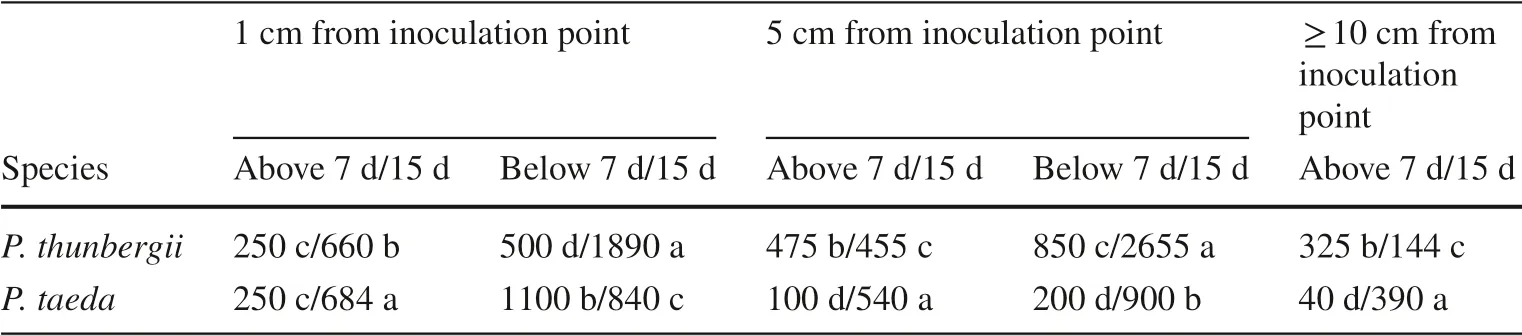
Table 2 Density (no./cm) of PWNs at different distances from inoculation point at 7 and 15 days after inoculation in susceptible Pinus thunbergii and resistant Pinus taeda
Changes of reproduction and sex ratios in PWN from two pine species
More nematodes were present inP.thunbergiithan inP.taedaat 7 d after inoculation, reaching more than 10,000 nematodes, while PWN inP.taedahad only approximately 3250, the same as that in the beginning of the inoculum.At 15 d after inoculation, the number of PWNs inP.thunbergiiwas twice than that inP.taedaand approximately 6 times the number in the inoculum; these results were consistent with the susceptibility level of the pine species (Fig.1 a).In addition, we counted the sex ratio of PWN.The female to male ratio before inoculation was approximately 3.3:1.At 7 d after inoculation, the female to male ratio of nematodes inP.thunbergiiandP.taedashowed a decreasing trend, to approximately 2.6:1.At 15 d after inoculation, the female to male ratio inP.taedashowed an increasing trend, reaching approximately 3.2:1, while the female to male ratio inP.thunbergiihad not changed significantly (Fig.1 b).
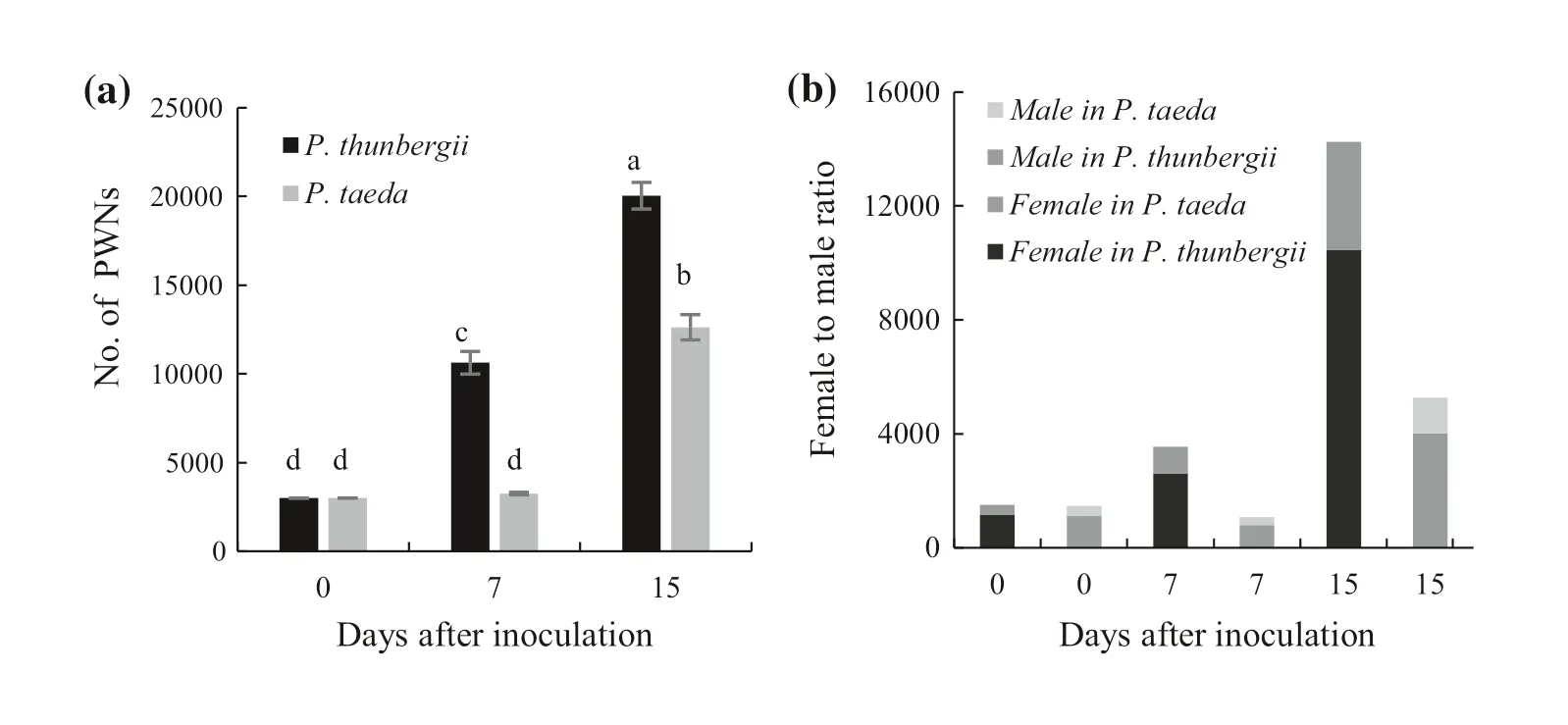
Fig.1 Reproduction and sex ratio changes of PWN in different pine species at different times after inoculation.a Number of PWNs in the tree.b Female to male ratio of PWN.Different letters indicate significant differences at the 5% level in Tukey’s HSD test
Body fat and number of bacteria on PWNs after coculture with the two pine species
As determined by oil red staining, fat accumulated relative to the control (before inoculated) occurred when PWN entered a tree (Fig.2 a), suggesting that fat accumulation may help nematodes maintain their reproductive capacity to varying degrees.Fat content of pine nematodes inP.thunbergiiwas significantly higher than inP.taedaat 7 d, but had decreased significantly by15 d.In contrast, the relative fat content of the nematodes inP.taedahad significantly increased by 15 d after inoculation and was four times higher than that of the nematodes inP.thunbergii(Fig.2 b).By 15 d after inoculation, SEM results showed that the body surface of the PWNs appeared to be wrinkled after coculture withP.thunbergiiandP.taedarelative to the control PWNs plate-cultured on fungal hyphae; the wrinkling of the body wall of nematodes inP.taedawas more obvious.In addition, few bacteria were observed on body surface of the control nematodes (CK) (Fig.3 a);slightly more bacteria were observed on PWN fromP.thunbergii(Fig.3 b) and substantially more fromP.taedaafter 15 d innoculation (Fig.3 d).
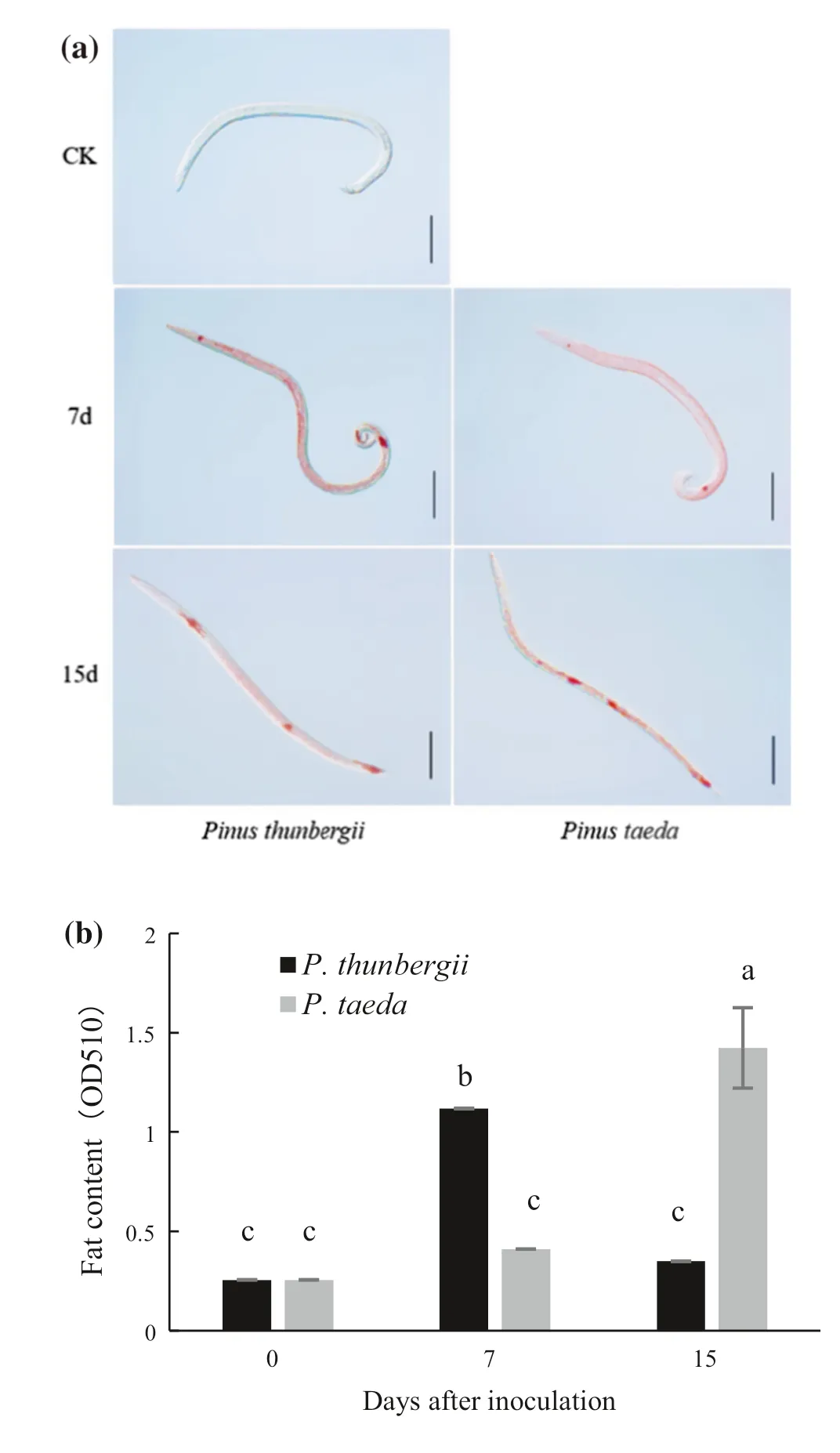
Fig.2 Fat content in PWNs after inoculation of two pine species.a Bright-field micrograph (scale bar: 100 μm) of fat (red) in PWN 7 d and 15 d after inoculation of two pine species.Control (CK) nematode was cultured on hyphae in a petri dish for 7 days.Fat was stained with oil red.b Relative fat content in PWNs.Absorbance at 510 nm was measured spectrophotometrically.Different letters indicate significant differences at the 5% level by Tukey’s HSD test

Fig.3 Scanning electron micrographs of surface of PWNs and bacteria before or after inoculation of two pine species.a PWN before inoculation.(b) PWN 15 days after inoculation (dai) of P.thunbergii.c P.thunbergii 15 dai.d PWN 15 dai of P.taeda.e P.taeda 15 dai
Expression of pathogenicity and detoxification-related genes in nematodes early in the interaction with the pine species
Compared with expression in the control nematodes (CK),expression ofBxBeta1-4andBxpel1after pine infestation was differentially upregulated (Fig.4 a).Both were significantly upregulated inP.taeda, and their expression levels were threefold and 4.5-fold higher, respectively,than those inP.thunbergii.In addition, the response ofBxpel1gene expression was stronger thanBxBeta1-4inP.taeda.Our results showed that the expression levels of the nematode pathogen-associated model effector genesBxCDP1andBxSapB1(encoding a sphingolipid) were significantly upregulated inP.taeda, 6.1-fold and 5.2-fold higher, respectively, than that inP.thunbergii,and 40–45 times higher than in the CK (Fig.4 b).Expression of autophagy genes in PWN (Liu et al.2019b) was higher inP.thunbergiithan inP.taeda, with expression ofATG11.5 times higher andATG162 times higher than that inP.taeda(Fig.4 c).The degree of upregulation of autophagy genes in PWNs differed between the two pine species.The expression of PWN autophagy genes in the early stage ofinfestation was inversely correlated with the susceptibility of the pine species (more resistant, lower expression).For genes related to reactive oxygen metabolism associated with nematode detoxification, glutathione transferase geneBxGST3was expressed at a significantly (2.6-fold) higher level inP.taedathan inP.thunbergii, whereasBxy-ctl-1was only slightly upregulated inP.thunbergiiandP.taeda(Fig.4 d).
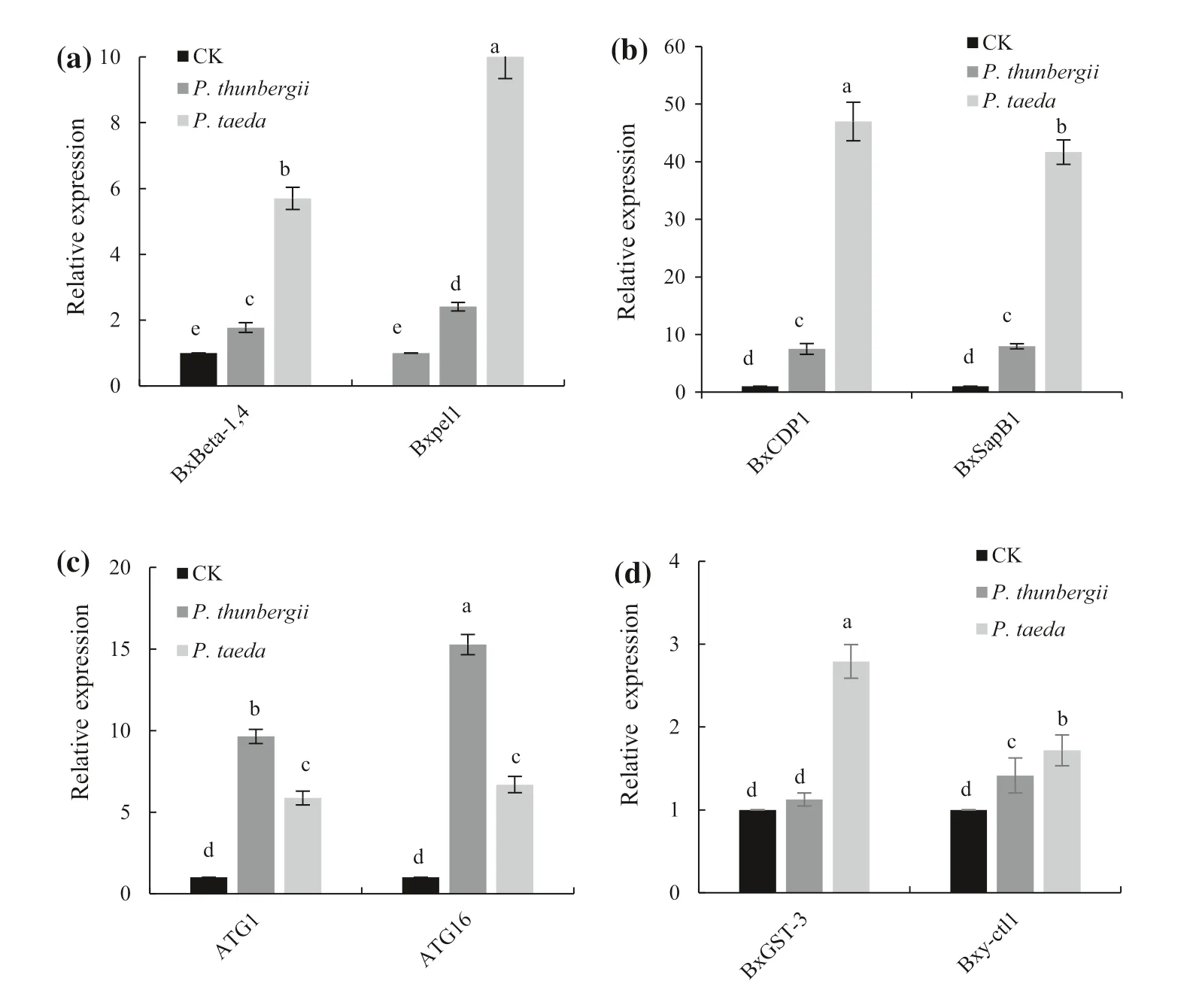
Fig.4 Relative expression levels of pathogenicity and detoxification-related genes in PWNs 12 h after inoculation of two pine species.a Expression levels of BxBeta-1, 4 and Bxpel1 after 12 h ofinfestation.b Relative expression levels of BxCDP1 and BxSapB1 after 12 h ofinfestation.c Relative expression levels of BxATG1 and BxATG16 after 12 h ofinfestation.d Relative expression levels of BxGST-3 and Bxy-ctl-1 after 12 h ofinfestation.The control (CK)nematodes were grown in petri dishes on hyphae for 7 days.Different letters above the bars indicate a significant difference in expression in Tukey’s HSD test at P < 0.05
Discussion
PWNs can cause the rapid death of pine trees within a short time.Numerous studies on the interaction between the PWN and pine trees have been reported (He et al.2010; Hu et al.2019; Liu et al.2020), but the mechanism the use to overcome the complex defense responses of pine trees to colonize the trees remains unclear.
Certain characteristics of pine trees, such as resin duct size and density, are closely related to the migration of PWNs in the branches and trunk (Son et al.2010; Zas et al.2015).The incidence of PWN infection is directly related to whether the nematodes can survive in the pine tree, and the rate of the incidence is related to the migratory rate and proliferation of the nematodes.Therefore, we first investigated migratory and dispersal rates, reproduction and sex ratio of PWN in the two pine species.PWN migrated farther inP.thunbergiithan inP.taeda, its reproduction inP.taedawas significantly inhibited early in the infestation.Reproduction of PWN inP.thunbergiiwas higher in the early and midlate stages ofinfestation.Ichihara et al.( 1999) also found that nematode survival and reproduction were differentially inhibited inP.taedaversusP.thunbergii.We also found that PWN adapted to different species by adjusting its sex ratio.The female to male ratio of PWN inP.thunbergiihad decreased by 7 dai then remained the same; the ratio inP.taedahad also decreased by 7 dai, but then increased.Some substances withinP.taedamay exert pressure on the reproduction of PWN, leading to a series of adaptive changes to continue its reproduction and survival (Wei et al.2018).
The pathogenicity of PWN is also affected by associated bacteria under certain conditions in vivo (He et al.2016; Fu et al.2020).Studies have shown that bacteria that remain below a certain threshold number exert no harmful effect on nematodes or contribute to their survival, while an excessive number of bacteria may inhibit the survival and reproduction of nematodes or even kill them (Li and Zhang 2018; Yin et al.2020).Huang et al.( 2009) found that a novel serine protease purified from Gram-negativeStenotrophomonason a G2 medium degraded the epidermis of the nematodes and killed them.After PWNs were treated for 24–48 h with the associated bacteriumSerratiasp.A88copa13, the bacterium accumulates in the cuticle of PWN, and eventually degrades it (Gabriel et al.2013).In our study, at 15 dai,substantially more bacteria were present on the body surface of the nematodes inP.taedawas significantly greater than on the control and PWNs inP.thunbergii, indicating that the PWNs encounter different conditions in the resistant and susceptible pine species that affect their survival.In general,the increase in bacteria on the body surface of PWNs inP.taedaindicated that the endophytic bacterial community might differ among pine trees with differences in resistance and also affect the virulence of nematodes to some extent.PWN can carry a variety of bacteria in nature, and there are a wide variety of nematode-associated bacteria among different countries and host pines (Nascimento et al.2015; Xue et al.2019).Additionally, various bacteria carried by PWNs may be closely associated with the host plant (Proen?a et al.2017).Therefore, further research on the interactions among pine trees with different levels of resistance, bacteria and PWNs is needed.
To better explain the differences in migration, reproduction, sex ratio, fat accumulation and interactions with other microorganisms of PWNs in the pine species with different resistances, we investigated changes in gene expression.During the infestation process, PWN pathogenicity-related genes are mostly secreted into the host via the nematode oral needle (Ma 2008; Wang et al.2009; Jones et al.2010;Kikuchi et al.2011; Santos et al.2012).In our study, the PWN cellulase geneBxBeta1-4and pectinase geneBxpel1showed higher expression levels after the infestation ofP.taeda, withBxpel1expression being particularly significant inP.taeda.In contrast, PWNs migrated farther and reproduced more inP.thunbergii, which suggested that the nematodes secreted more cell-wall-degrading enzymes to help them complete their colonization when enteringP.taeda.Effectors are another class of proteins secreted via nematode oral needles; these proteins include signal peptides and do not exhibit transmembrane structures, and they are mainly expressed specifically in nematode glandular cells and inhibit or stimulate host immune responses to promote self-infestation (Wu 2015; Li et al.2016; Hu et al.2020).In our study, expression ofBxCDP1andBxSapB1, two effector genes of PWN, was higher expression inP.taedathan in the control and inP.thunbergii.Thus, the nematodes may secrete greater amounts of effector proteins to counteract the stronger defense responses of resistant pines when they interact with resistant pines.In addition, PWN detoxification of host secondary metabolites such as terpenoids and cyclic aromatic products induced in response to the invasion is important for PWNs to adapt to the adverse stresses.As part of the PWN immune system, detoxification is essential for pathogenesis and colonization by PWN.Rui et al.( 2021)found that in vitro, when PWN was stressed by H2O2, the catalase genesBxy-ctl-1,Bxy-ctl-2and the peroxidase geneBxPrxshowed higher expression in highly virulent strains of nematodes than in low-virulence strains.In our study, a glutathione transferase gene was highly expressed in PWN inP.taedaat 0.5 d, while the expression ofBxGST3did not change significantly.These findings are consistent with the study by Nian et al.( 2017).
Autophagy is a resistance response of eukaryotes to adverse conditions (Boya et al.2013) to maintain metabolic homeostasis and stability by removing their own excess proteins and other biomolecular products, and damaged organelles.(Mizushima 2005; Yoshimoto 2012).Wu and Wu( 2018) noted ROS as among the factors that induce cellular autophagy, which PWN uses to resist the oxidative stress(Liu et al.2020).Specifically,Atg1, a serine/threonine protein kinase, is a major inducer of autophagy activation inArabidopsis thaliana(Deng and Wu 2016).The nematode homologAtg16.2is involved in the localization of theLC3/Atg8homologlgG-1-II(Zhang et al.2013).In our study,nematode cell-wall-degradation-related genes, effector genes, and reactive oxygen metabolism-related genes were all highly expressed inP.thunbergii, butP.taedastill had higher resistance.According to previous studies, the PWN autophagy geneBxATG16positively regulates the expression ofBxATG5; the silencing ofBxATG1andBxATG16significantly inhibits the autophagic activity of PWN, and the inhibition of autophagy causes a severe decrease in feeding rate, reproduction, egg hatching rate and survival rate of pine nematodes when they are exposed to oxidative stress generated by ROS metabolism inP.thunbergii, thus leading to a significant reduction in nematode pathogenicity (Deng and Wu 2016; Liu et al.2019b).Our experiments showed that the expression levels of autophagy-related genes were significantly higher in infestedP.thunbergiithan inP.taeda.Based on these results, we hypothesized that the self-repair ability of PWNs was suppressed inP.taeda, maintaining the high resistance ofP.taedaafter it was infected.In addition,both ROS and exoautophagy inducers induced nematodes to initiate autophagy, and exoautophagy inducers also caused an increase in the fat content in PWN (Wu and Wu 2018),consistent with our observation that nematodes inP.thunbergiiaccumulate more lipids early in infection.
Conclusions
PWNs migrated farther and reproduced more inP.thunbergiithan inP.taeda, and they adjusted their sex ratio in resistant pines to ensure sufficient reproduction.The number of bacteria on the body surface of PWNs was significantly higher in resistantP.taedathan in susceptibleP.thunbergii.The study further showed that within 0.5 d after inoculation,the expression levels of PWN cell-wall-degradation-related genes, effector genes, and reactive-oxygen-metabolismrelated genes were 2–6 times higher in resistantP.taedathan in susceptibleP.thunbergii.Autophagy-related genes of the nematodes, on the other hand, were expressed at higher levels inP.thunbergii.These results suggest that PWNs had significant upregulation of pathogenicity-related genes when infesting resistant pine trees, initiate the expression of detoxification-related genes to resist stress exerted by the tree and then adjust their own adaptations based on feedback to complete the infestation process.This study increases our understanding of the adaptive mechanisms of PWN and contributes to a theoretical basis for the control of PWD.
 Journal of Forestry Research2022年4期
Journal of Forestry Research2022年4期
- Journal of Forestry Research的其它文章
- Journal of Forestry Research
- Reversibly photochromic wood constructed by depositing microencapsulated/polydimethylsiloxane composite coating
- Surveillance of pine wilt disease by high resolution satellite
- Pine wilt disease detection in high-resolution UAV images using object-oriented classification
- Transcriptome analysis shows nicotinamide seed treatment alters expression of genes involved in defense and epigenetic processes in roots of seedlings of Picea abies
- Effects of enrichmemt planting with native tree species on bacterial community structure and potential impact on Eucalyptus plantations in southern China
