Skin Organoid Research Progress and Potential Applications
Hong-Yang Li, Kun Ren, Cheng Wang, Wen-Bo Bu*
1Pharmacal Research Laboratory, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Hospital for Skin Diseases (Institute of Dermatology), Chinese Academy of Medical Science and Peking Union Medical College, Nanjing, Jiangsu 210042, China; 2Jiangsu Center for Pharmacodynamics Research and Evaluation, China Pharmaceutical University, Nanjing,Jiangsu 210009, China; 3Department of Dermatology, Zhongda Hospital Southeast University, Nanjing, Jiangsu 210009, China;4Department of Surgery, Hospital for Skin Diseases (Institute of Dermatology) Chinese Academy of Medical Science and Peking Union Medical College, Nanjing, Jiangsu 210042, China.
Abstract
Keywords: skin disease, organoid, three-dimensional culture, personalized treatment, drug screening
Introduction
At present, the culture systems contained epidermal cells and dermal cells are the main human skin-based in vitro methods used in laboratory research or clinical applications.1-3However,it has always been difficult to cultivate functional skin accessory structures in these models.Moreover, there are great difficulties in data collection and analysis and mechanism clarification because various factors,such as species differences and complicated in vivo environmental systems, making it impossible to conduct single-factor analysis using the traditional models.4
In recent years, as tissue engineering research has continued to progress, in vitro three-dimensional (3D)culture models have attracted increasing attention from researchers,and organoid technologies are the most rapidly advancing tools. In the construction of skin organoids,pluripotent stem cells(PSCs),adult stem cells,tumor tissues or precancerous tissues can be used to establish an in vitro 3D tissue culture model under specific liquid culture conditions that generate a tissue structure and biological characteristics that are similar to the source tissue,which provides a new in vitro model for simulating living organs.5
In 2009, the idea of organoids was proposed by Hans Clevers and colleagues. The team cultured adult Lgr5-positive stem cells derived from the intestine of mice in a 3D matrix gel containing R-spondin,epidermal growth factor, and Noggin factors to form a microstructure similar to the small intestine, namely, the crypt-villi complex, and successfully established an intestinal organoid culture system.6Subsequently, organoids derived from colon,7brain,8lung,9stomach,10-11gastroesophageal,12prostate,13-14pancreas,15-16liver,17breast,18kid-ney,19bladder,20endometrial,21ovary,22and other normal tissues and tumor tissue organoids have been studied and reported, and these models show huge potential applications in the study of tissue physiology,disease models and regenerative medicine.4It is possible to obtain samples to generate organoids through surgery or biopsy.23-24Compared with cell line models, organoids can replicate the features of patients and reproduce the original tumor heterogeneity.25Importantly, highthroughput drug screening and long-term subculture can be performed in organoids with stable phenotypes and genetic characteristics, which provides a powerful tool for tumor biology and stem cell biology research.12The schematic representation of application of skin organoid was shown in Figure 1.
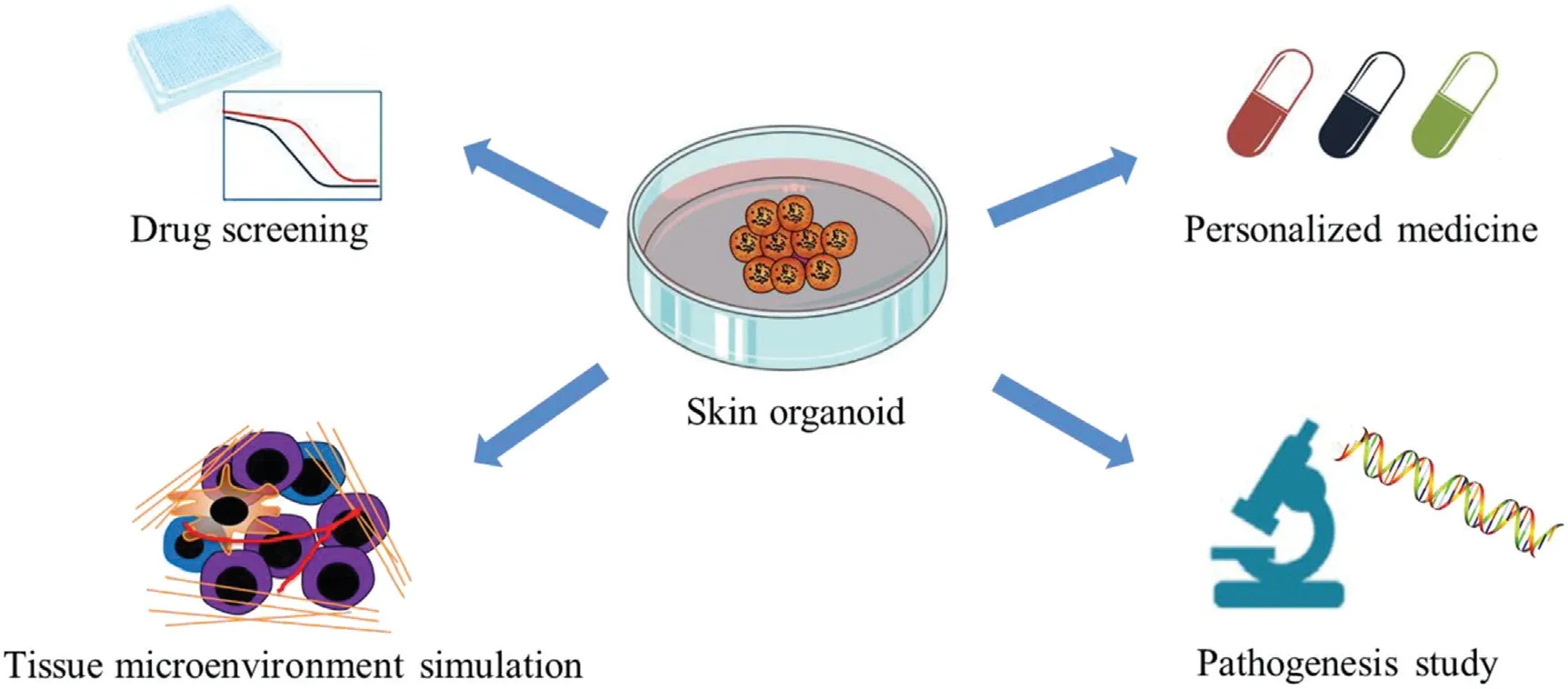
Figure 1. Schematic representation of application of skin organoid.
Here, we searched the literature published in PubMed database from January 1980 to December 2020 using“organoid [All Fields] AND skin cancer [All Fields]” and“organoid [All Fields] AND melanoma [All Fields] OR extramammary pagets disease [All Fields] OR keloid[All Fields]”, and summarized the papers that focused on organoids, microspheres, 3D culture for skin tissues or tumors, and discussed the construction of normal tissues and tumor tissues of skin diseases, and the potential application of skin organoids in scientific research.
Normal skin organoids
Skin is the largest multilayered organ of human body with various accessory structures inside, such as hair follicles,sweat glands, sebaceous glands, and nails, which play important roles in body temperature regulation,body fluid retention,resistance to external pressure,and regulation of touch and pain.26The epidermis and facial dermal require interactions with epithelial (epidermal) and mesenchymal(dermis) cells to ultimately develop various accessory structures to perform the various functions of skin.
The construction of skin organoids by epidermal and dermal cells
In 2017,Lei et al.2separated the epidermis and dermis of newborn mouse skin to generate a 3D culture system by mixing the epidermal cells and dermal cells at a ratio of 1:9.Skin organoids were formed in approximately 10days.The researchersanalyzedcellbehaviorchangesthroughdetailed 3D culture time-lapse imaging and observed that these cells passedthroughsixdifferentstages toformhairfollicles,i.e.,dissociated cells, cell aggregates, polarized cysts, cyst coalescence,planar skin,and hair-bearing skin.Importantly,transplanting the skin organoids into the backs of mice produced hair. Transcriptome profiling revealed that adhesion proteins,growth factors,Wnt protein,and matrix metalloproteinases played vital roles in the transformation of skin cells into hair follicles.This study presents a way to improve the ability of adult skin cells in the formation of fully functional skin in clinical applications, which can provide new strategies for stimulating hair growth for patients with hair loss and baldness.
The construction of skin organoids by PSC
In 2018,Lee et al.27first used mouse stem cells to cultivate skin tissue with hair follicles,which provided new research strategies for understanding hair growth and skin cancer development.C57BL/6 mouse embryonic fibroblasts were induced toproducePSCs.ThePSCs werethendigestedwith 1X TrypLE Express Enzyme, resuspended in ectoderm differentiationmedium,andsubsequentlyseededin96-well U-bottom plates. The samples were treated with transforming growth factor β(TGFβ)inhibitor SB431542(SB)and bone morphogenetic protein (BMP) to induce the formation of surface ectoderm on the third day,and FGF-2(FGF)andLDN-193189(aBMPinhibitor)werethenadded to induce the formation of basal epithelium on the 4th day.Finally, the skin organoids were cultivated with the combined self-assembled epidermis and dermis for approximately 20-30days, and these organoids spontaneously produced new hair follicles after implantation in mice.Importantly,the types of hair follicles were similar to those naturally grown on mouse skin. This in vitro skin developmentmodelwillenablethestudyofthemechanisms ofhairfollicleinduction,screeningfordrugsforhairgrowth or inhibition,and the simulation of skin diseases.
In 2020,Koehler’s team28reported an organoid culture model that could directly generate complex skin from human PSCs, this system enabled the self-assembly of nearly complete real skin in vitro and successfully rebuilt skin in the body for the first time. The researchers separated human embryonic stem cells(hESC)into single cells and placed them in U-bottom 96-well plates to form uniform cell aggregates, and the aggregates were then cultured in differentiation medium containing BMP4 and SB431542 (a TGFβ inhibitor). After approximately 75 days,the organoids entered the hair growth stage with the appearance of hair follicles.The hair follicle growth of the skin organoids featured a morphogenesis process that is similar to that seen in mammalian hair follicles, and the similar processes prompted the successful induction of skin organoids.When the skin organoids were implanted in a small incision on the back skin of nude mice, the epidermis fused with the host epidermis, and hair can grow vertically without ulcers or other types of uncontrolled growth.
In summary, the researchers established an organoid model that can be used to study the cell dynamics of the skin and its accessory structures and that almost perfectly reproduces the structure of the human skin system.In the future, the models can be used to study a variety of hereditary skin diseases and cancers to accelerate drug discovery.In addition,skin organoids can also be used to reconstruct the skin and accessory structures in patients with skin burns or other skin wounds.
The construction of skin organoids by immortalized cells
In 2020, Oulès et al.29constructed sebaceous gland organoids and used them in acne research.The researchers obtained the SebE6E7 sebocyte cell line through microdissection of adult facial skin and transduced the HPV16/E6E7 gene via a retrovirus to make the sebocytes immortal.The dissociated SebE6E7 sebocytes were resuspended in growth factor-reduced matrigel, seeded into 24-well plates, and cultured in keratinocyte serum-free medium to generate sebaceous organoids. Immunostaining showed that the sebaceous gland organoids contained ductal and sebaceous markers. The researchers used these sebaceous gland organoids to compare the therapeutic effects of tretinoin and RepSox(a TGFβ inhibitor).In addition,the researchers also performed mechanistic studies by constructing GATA6+and shGATA6+sebaceous organoids and suggested that GATA6regulatedtheupperpilosebaceousunitandmightbe a potential target for the treatment of acne. In summary,sebaceous gland organoids play important roles in the evaluation of drug efficacy,the exploration of pathological mechanisms, and the discovery of potential therapeutic targets.
Skin tumor organoids
In addition, it is difficult to predict the response of different patients to treatment using cell lines,cell-derived xenografts, or patient-derived xenografts models, which resulted in a large number of unnecessary adverse drug reactions and a huge economic burden.Furthermore,the lack of effective and feasible skin disease models in basic research hinders the progress of skin disease research to a certain extent, while the organoid technology might provide new opportunities and new hopes for the research of skin diseases and personalized treatment for patients.
Extramammary Paget’s disease organoids
Extramammary Paget’s disease (EMPD) is a rare malignant skin adenocarcinoma that originates from cells in the opening of the apocrine ducts or multipotent stromal cells that differentiate from the epidermis to the apocrine glands.30However,the treatment of EMPD has seen little improvement in recent years because of the lack of EMPDrelevant cell lines or effective research models.31
In 2020, Arita et al.31separated EMPD tissues inoculated in Matrigel and cultured in StemPro hESC medium containing basic fibroblast growth factor to generate organoids, and hematoxylin-eosin staining and immunohistochemistry staining showed that the pathological characteristics of the original tissues of the patient remained in the tumor tissue of the organoids and organoid-derived xenografts of the mouse model. The research team optimized the medium conditions and Matrigel concentration for growth of EMPD organoids and found that StemPro hESC medium and 5%Matrigel were the most conducive to the growth of EMPD organoids. They also explored the effects of different growth factors heregulin-β1/neuregulin 1(HRG),epidermal growth factor, insulin-like growth factor, basic fibroblast growth factor, and activin on the growth of EMPD organoids,and the results showed that the HRGHER3 pathway was related to cell proliferation in EMPD.Subsequently,an organoid model was used to evaluate the efficacy of chemotherapeutic drugs for the treatment of EMPD. This study was the first to establish an in vitro EMPD model, which will aid the development of new clinical treatment strategies and clarify the pathological mechanism of EMPD.
Keloid organoids
Keloids are common benign skin tumors caused by excessive proliferation of connective tissue in the skin and have the characteristics of a strong proliferative ability and a high recurrence rate.32However, the treatment options for keloids are still unclear because of the limited understanding of the pathogenesis of keloids.In addition,keloids are unique to human skin and cannot spontaneously develop in animals, which results in the lack of relevant animal models of keloids.Therefore,it is crucial to develop new animal or nonanimal models for the functional evaluation of keloid cells and tissues to explore the pathological mechanisms and develop targeted clinical treatment strategies.33
In 2013,Lee et al.34reported organotypic multicellular keloid spheroids, which were derived from 2mm perforated biopsies of patient keloid tissues. The spheroids were cultured in Iscove’s modified Dulbecco’s medium containing fetal bovine serum (FBS), penicillinstreptomycin (pen/strep), insulin, and hydrocortisone.The keloid organoids cultured in vitro maintained the main characteristics of keloids in patients, and the tissue structure, biological activity, and histological characteristics of the keloid organoids were stably maintained within a 7-day culture period. Subsequently, keloid organoids were used to evaluate fibrosis markers (such as apoptosis),the synthesis of collagen,and the efficacy of triamcinolone acetonide.35-36In 2017, Lee et al.37also used keloid organoids to conduct a gene knockout study.A Mortalin-specific shRNA-expressing adenovirus was transfected into keloid organoids to knock down Mortalin, which resulted in the downregulation of collagen I, collagen III, fibronectin, and elastin. In short,strategies to construct and apply keloid organoids have progressed well in recent years. In summary, the keloid organoids maintain a cultivation time for approximately 7days, which provide a crucial way to study the fibrosis mechanism of keloids and explore suitable clinical drugs.
Melanoma organoids
Melanoma is a highly malignant tumor derived from melanocytes accounting for 75%of skin cancer deaths.38There are approximately 132,000 new cases worldwide every year, and the incidence of melanoma is increasing each year.39Organoids can simulate the unique molecular characteristics and faithfully reproduce the features of human diseases in vivo to help study the development,heterogeneity, plasticity, and progression of humanmelanoma.
In 2018, Jenkins et al.40performed a combination of mechanical mincing and enzymatic digestion to treat fresh tumor tissue from melanoma patients and then used 100μ mand 40μm filters to produce S1(>100μm)and S2(40-100μm) and S3 (<40μm) spherical particles. Grade S2 particles were resuspended in type I rat tail collagen solution at a concentration of 2.5mg/mL.Subsequently,the spherecollagen mixture was injected into the central gel area of the 3D microfluidic culture device and cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% FBS,4.5mmol/L glucose, 100mmol/L sodium pyruvate, and 1:100pen/strep.In this way,a multicell melanoma organoid containing spheroids and autoimmune cells was constructed. Votanopoulos et al.41used a similar method to successfully construct melanoma organoids. They also isolated patient lymphocytes and developed them into organoids at a ratio of 1:1 to construct immune-enhanced patient tumor organoids.
Recently, Vilgelm et al.42proposed a simple and minimally invasive organoid culture technique based on fine-needle aspiration (FNA). They used FNA sampling technology to directly separate organoids from patients or resected tissues by using a small amount of tissue without a digestion processing step, which can better retain the complete cell population and the tissue growth pattern. The samples were then resuspended in Matrigel and cultured in DMEM/Hams F12/MCDB105 (2:1:1 ratio) containing FBS and B27 supplement. The FNAderived patient-derived organoids were cultured and passaged in solid or semisolid form without trypsinization. With the wide application of FNA in clinical practice, this technology can be widely used in the clinic to create organoid tissue banks for precision medicine and research.
Jenkins et al.40used flow cytometry and immunofluorescence staining to perform immunophenotyping of melanoma organoids and analyze the secreted cytokine profile and confirmed that melanoma organoids retained autoimmune cells, including key tumor-infiltrating T lymphocyte populations. Subsequently, they conducted drug sensitivity experiments with melanoma organoids to evaluate the response of melanoma organoids to PD-1 inhibitors. These experiments were the first to show that short-term melanoma organoid culture systems can simulate the response to PD-1 inhibitors. Similarly,Votanopoulos et al.41and Vilgelm et al.42used melanoma organoids to screen immunotherapy drugs, such as nivolumab, pembrolizumab, ipilimumab, dabrafenib,and trametinib, and their experiments proved that melanoma organoids are a feasible model for evaluation of drug efficacy and personalized patient treatments.Teresa Troiani et al.43used melanoma organoids to study tumor immune mechanisms. They cocultured organoids and patient autologous lymphocytes and proved that the interaction of tumor cells and T cells induced the expression of FKBP51s.In summary,melanoma organoids play important roles in drug screening, drug efficacy evaluation, and mechanistic research.
Limitations and prospects of organoids
This review summarizes the different methods for the establishment of skin organoids in skin disease research(Fig. 2), and the papers that focused onorganoids,microspheres, 3D culture for skin tissues ortumors, and summarized the construction of normal tissues and tumor tissues of skin diseases, and the potential application of skin organoids in scientific research (Table 1). Actually,the establishment of organoid culture is time-consuming,expensive and complicated, and reasonable medium formulations also need to be studied. Whether the consistency between the organoid and the original tumor reflects the main clone or partial subclones of the original tumor remains to be determined. Currently, two main limitations hampered the development of skin organoids.Firstly, the construction methods of skin organoids are diverse, which result in poor reproducibility and difficult operation. Secondly, the number of the study of skin organoids is less than tumor organoids, and the construction of organoids in most common inflammatory and immune skin diseases has not been reported,and rare skin diseases model of organoid are also lacking. Hence,skin organoids still have a long way from scientific research to clinical application.
However,skin organoid models have broad application prospects in disease modeling, tumorigenesis research,drug efficacy evaluation and high-throughput drug screening. The future challenge is to integrate the components of the microenvironment to develop more novel organoid models replicating physiological skin so that organoids can be cocultured with different cell types,such as immune cells or pathogens.44In short, culture of skin organoids in vitro is a good model for preclinical research and can accelerate the translation of basic experimental research into clinical practice. The use of such models will make it possible for patients to achieve personalized treatment and help humans overcome skin diseases and tumors in the near future.
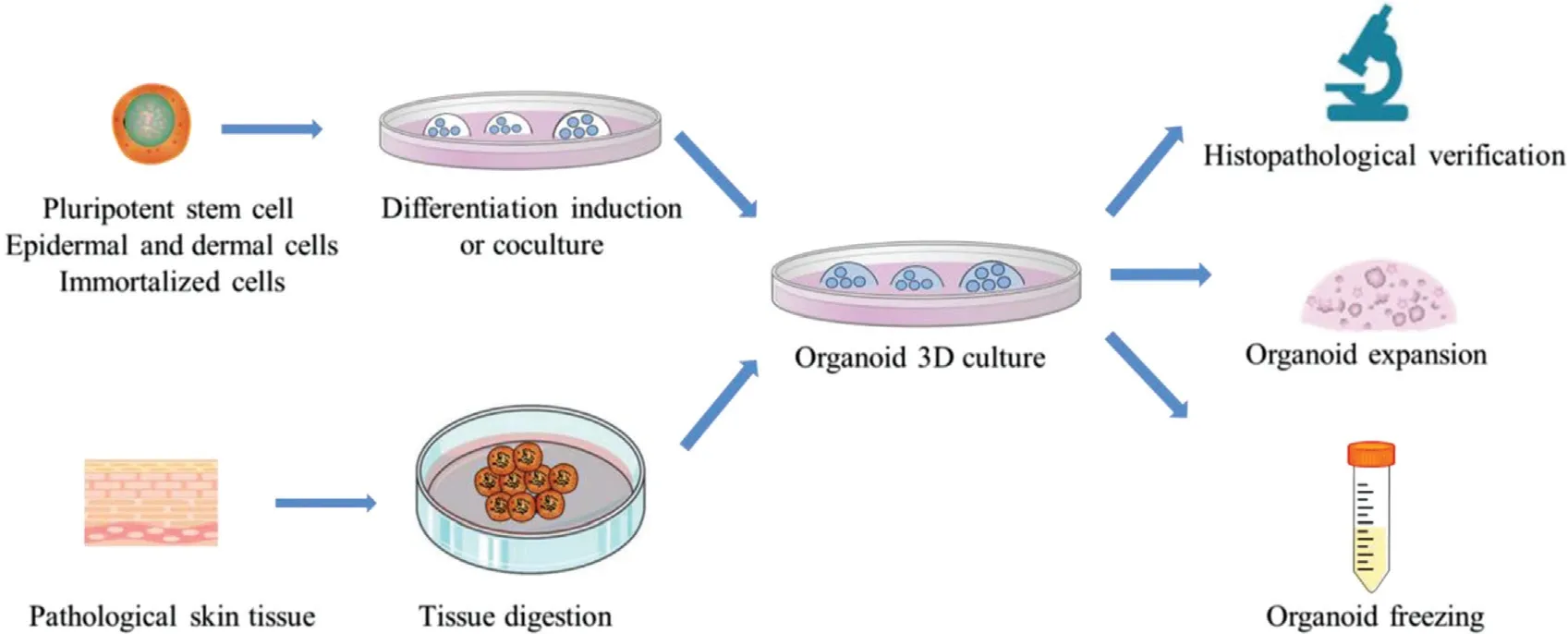
Figure 2. Schematic representation of the construction of skin organoid. 3D: three-dimensional.
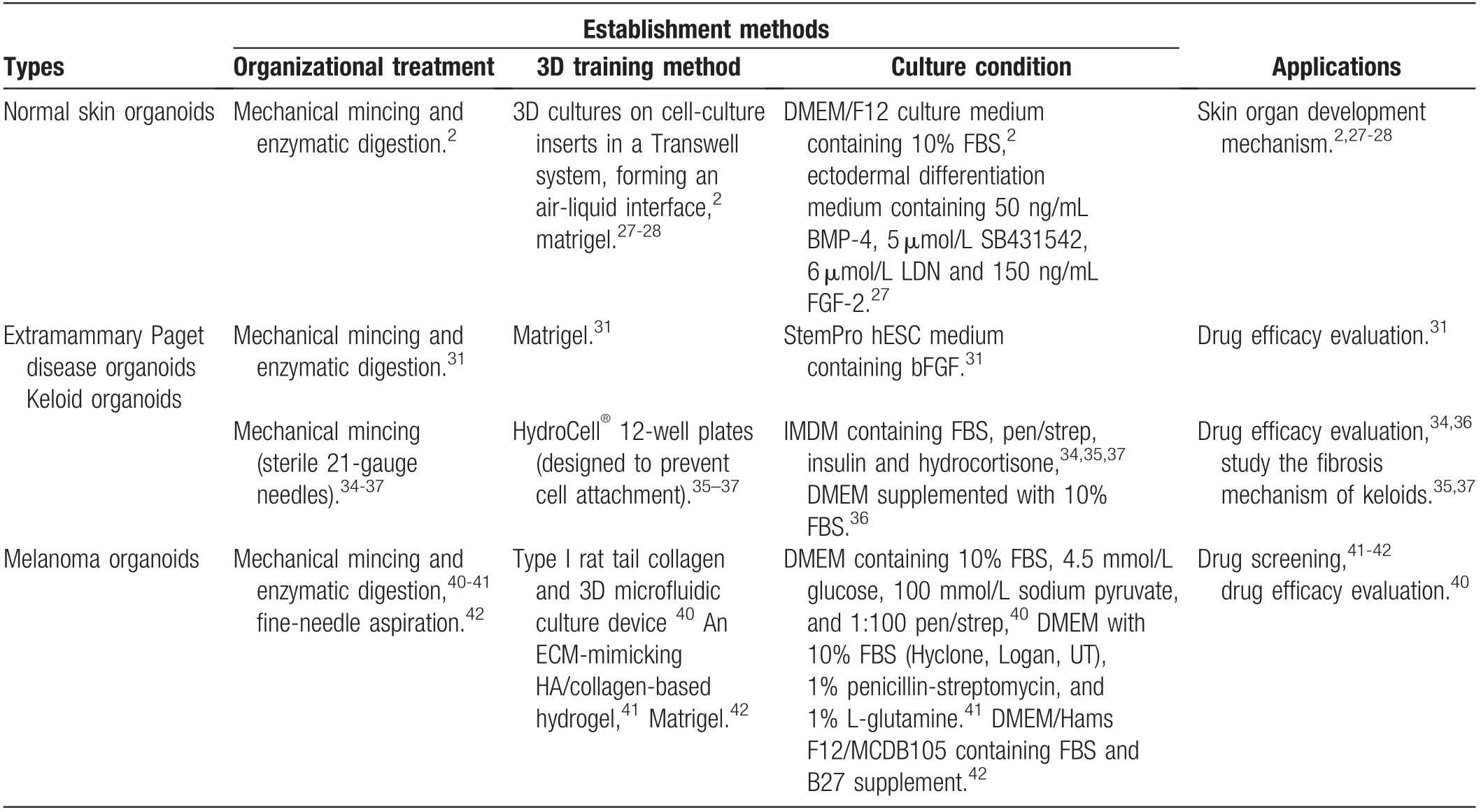
Table 1 The similarities and differences between the skin organoids.
Acknowledgements
Our research was financially supported by the National Natural Science Foundation ofChina(No.82003808)and the Natural Science Foundation of Jiangsu Province(BK20180157).
- 國際皮膚性病學(xué)雜志的其它文章
- Clinical Features and Corrected Factors with Neurosyphilis in HIV/Syphilis Co-Infected Patients Based on Stage of Syphilis
- Dermoscopic Features of Basal Cell Carcinoma and Their Association with Histological Types in A Chinese Population: A Perspective Study
- p62/SQSTM1 Participates in the Innate Immune Response of Macrophages Against Candida albicans Infection
- Frostbite in Southwest China: A Single-Center Retrospective Analysis
- Role of Epigenetics in the Pathogenesis of Systemic Sclerosis
- Dermatoscopy in the Diagnosis of Vulvar Basal Cell Carcinoma: A Case Report

