Can small-scale altitudinal gradients predict spatial and temporal patterns in tropical forests?
Mariana Caroline Moreira Morelli·Cléber Rodrigo de Souza·Jean Daniel Morel·Vinícius Andrade Maia·Alisson Borges Miranda Santos·Kaline Fernandes Miranda·Rubens Manoel dos Santos
Abstract In tropical montane forests,compositional and structural changes are commonly driven by broad-scale altitudinal variation.Here,given the lack of knowledge on small-scale vegetation changes and temporal dynamics,we address the effects of small-scale variations in soil and altitude on tree community structure,temporal dynamics and phylogenetic diversity in a semi-deciduous tropical forest of the Atlantic Forest Domain,southeastern Brazil.In 2010 and 2015 we sampled thirty plots of 400 m2,set up along an altitudinal gradient between 1000 and 1500 m a.s.l..In each plot,we collected soil samples for chemical and textural analyses.We fitted linear models to test the effects of altitude and soil on community dynamics and phylogenetic parameters.Altitude and soil explained the spatial variation in number of individuals and phylogenetic diversity metrics.From lower to higher altitudes,we found decreasing fertility,increasing tree density and decreasing phylogenetic diversity.Altitude significantly influenced the increases in total biomass (from 240.9 to 255.4 t ha?1) and individual biomass (from 0.15 to 0.17 t) recorded in the interval.And while community temporal dynamics had rates of 1.96% for mortality,1.02% for recruitment,1.61% for biomass loss and 2.81% for biomass gain,none of them were explained by altitude or soil.Temporal species substitution averaged 0.1 in the interval.Altogether,these results suggest that the small-scale variations in altitude and soil likely determine the conditions and resources that drive community assembly and structure,which are expressed by spatial variations along the altitudinal gradient.At the same time,temporal patterns were not influenced by altitude-related environmental variation,resulting in a similar dynamic behaviour across the gradient,suggesting that broad-scale factors may play a more important role than local ones.
Keywords Fine-scale·Semideciduous forests·Montane forests·Dynamic patterns
Introduction
Tropical forest ecosystems are known for harboring a significant fraction of total global biodiversity (e.g.,nearly 47,000 tree species) and for storing in its living biomass approximately 66% of the terrestrial carbon (Pan et al.2013;Slik et al.2015).Tropical forest ecosystems function through the stock and flow of matter and energy driven by biological activity and biodiversity over time and space (Midgley 2012).The functional dynamics of tropical forest ecosystems are shaped by factors that act simultaneously:climate,soil,topography,initial biomass and vegetation status,such as species richness and functional characteristics (Quesada et al.2012;Fine et al.2015;Lohbeck et al.2015).Abiotic factors that influence tropical forest function may also vary depending on spatial and temporal scales (Malhi 2 012).At regional scales spanning multiple habitats able support a metacommunity (Leibold et al.2004),climate is the main ecological driver of species distribution,adaptive and evolutionary patterns (Jiang et al.2017).At local scales consisting of a single habitat with multiple microsites capable of maintaining a local community (Leibold et al.2004),soil and microclimate are the main ecological drivers of plant community composition and structure (van der Putten et al.2016;Fyllas et al.2017).
Altitude is one of the main drivers of variation in tropical forest function,for synthesizing ecologically relevant microclimate variables (e.g.,temperature,humidity,precipitation,cloud cover,solar radiation,wind speed) and soil variables (e.g.,nutrient availability) (K?rner 2007;Malhi et al.2010;Gastauer et al.2020).Even at small spatial scales,diurnal microclimate variation coupled with sharp soil fertility decline with increasing altitude can produce environmental gradients that influence spatial community patterns (John et al.2007;Crausbay et al.2016).Tropical forests along mountain slopes display high beta diversity and peculiar ecological characteristics following altitudinal change,related to community structure,taxonomic and phylogenetic composition (Crausbay et al.2010;Martin et al.2011;Unger et al.2012;Girardin et al.2014).In the Brazilian Atlantic Forest domain,for example,altitude change drives variation in community composition,plant diversity,biomass,proportion of endemic species,physiology and patterns of phylogenetic relatedness among co-occurring species (Oliveira-Filho and Fontes 2 000;Alves et al.2010;Rezende et al.2015;Silva and Souza 2018).However,altitude effects in interaction with spatial factors on forest temporal dynamics and productivity need further investigation(Chisholm et al.2013).
Community variation along environmental gradients may owe to deterministic processes of habitat filtering and niche differentiation (Wiegand et al.2012;Baldeck et al.2012).The uneven distribution of environmental conditions and resources interacts with evolutionary processes and results in niche differentiation among species (Bose et al.2019).Species have different environmental preferences and may be better competitors under conditions that contemplate their niches,as long as there is enough environmental variation (Paine et al.2011).On a temporal scale,information on community dynamics is needed to test whether there are differences in community demography and growth parameters in different habitats provided by environmental heterogeneity.Although parameters of community dynamics bring relatively little information if isolated,when linked to community phylogenetic diversity they may provide a better understanding of whether a community’s temporal patterns are set by its own“evolutionary clock”;i.e.,whether community dynamics is similar or different among communities with contrasting phylogenetic diversity.
Some of these issues have been separately addressed in several contexts.However,the investigations about the interaction between local environmental conditions and community dynamics and phylogenetic diversity are lacking for semi-deciduous forest tree communities along altitudinal gradients.Since past diversifications and adaptations drive patterns of ecological strategies for current species (Bose et al.2019),phylogenetic diversity ref elcts both the influence of adaptations inherited over evolutionary time and current environmental restrictions (Finegan 2015;Gerhold et al.2015).Therefore,the questions related to this topic may be relevant to understand broad ecological patterns and also community responses to environmental changes.
Thus,this study aimed to investigate the following question:What are the effects of small-scale environmental heterogeneity (i.e.,altitude and soil variation) on tree community evolution,dynamics and structure in a tropical forest in southeastern Brazil? We hypothesized that altitude and soil heterogeneity drive phylogenetic and temporal differentiation in tropical forest tree community dynamics.
Materials and methods
Study area
The study area is located in the Atlantic Forest Domain in Minas Gerais state,southeastern Brazil (latitudes 21°36′25″S and 21°37′27″ S,and longitudes 44°33′25″ W and 44°36′26″ W) Fig.1.The studied forest is the largest element of a 20-km long native vegetation mosaic along the region’s mountainous relief.It spans 1000 ha (10 km2) in an altitudinal gradient varying between 1000 and 1500 m.Mean annual temperature is 25 °C and mean annual precipitation is 1539.2 mm,with rainfall concentrated between November and March.
Data collection
In 2010 (first inventory),we set up 30 sample units (or plots)of 400 m2(10 × 40 m) in the study area (total sampled area of 1.2 ha).The plots were distributed aiming to capture the entire altitudinal gradient,which varies between 1000 and 1500 m a.s.l.(Fig.1).In each plot,we recorded,measured and marked with aluminum tags all living trees with circumference at breast height (CBH,at 1.30 m from the ground) ≥ 15.7 cm.Trees with multiple stems were included when the square root of the sum of squares of each stem’s circumference reached the inclusion criterion (Scolforo and Melo 2006).In 2015 (second inventory),we remeasured all surviving trees in each plot and recorded the dead trees and recruits (i.e.,trees that reached the inclusion criterion of CBH ≥ 15.7 cm).Plant identification followed Angiosperm Phylogeny Group IV (2016).
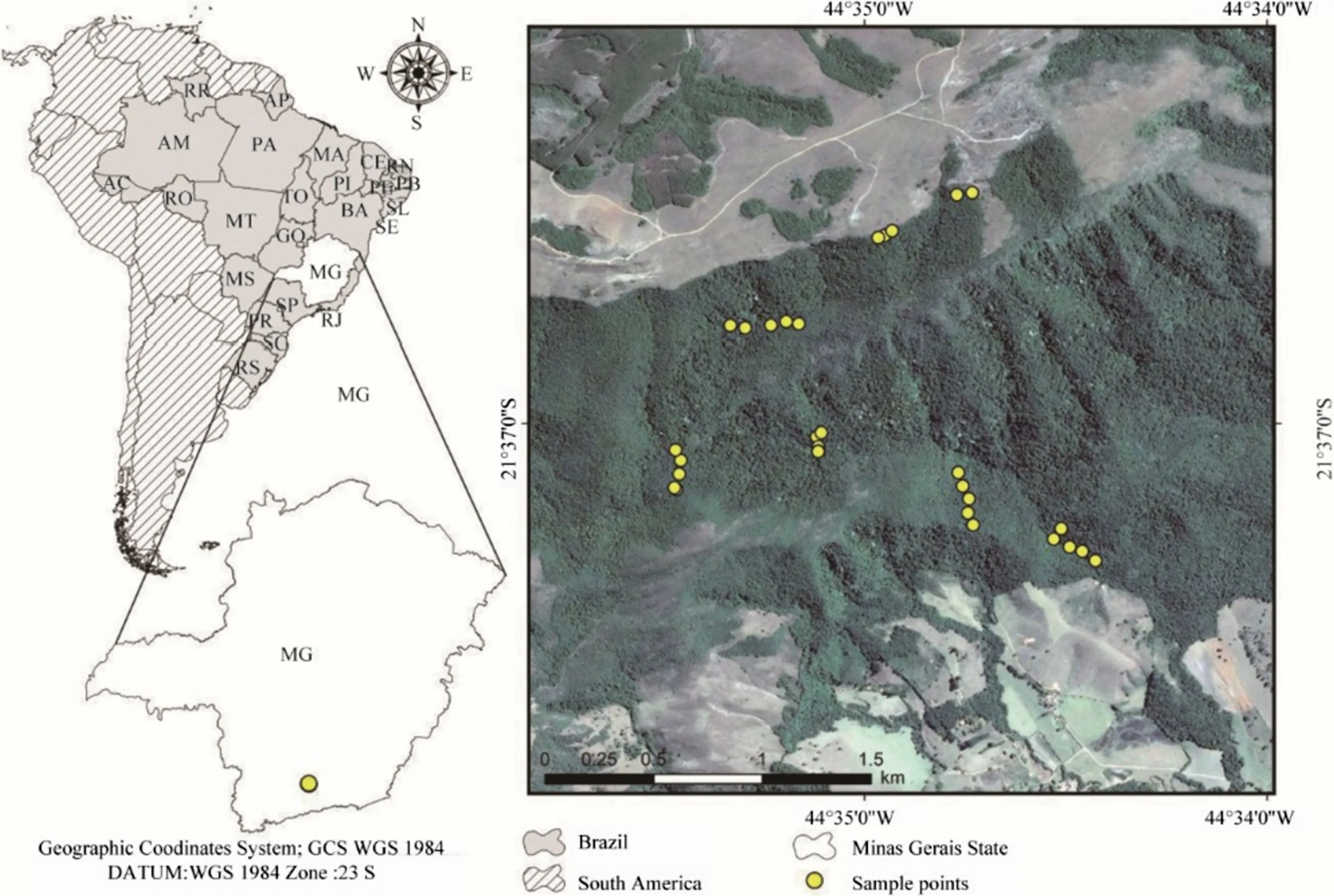
Fig.1 Location of the study area in South America and plot distribution in a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil
For the soil analyses,we collected one-liter composite soil samples from three points in each plot (0 ? 20 cm depth).Following the protocol by Embrapa (1997),we obtained the following soil chemical and textural variables:pH,phosphorus (P,mg cm?3),potassium (P,K,mg cm?3),calcium (Ca,cmol dm?3),magnesium (Mg,cmol dm?3),aluminum (Al,cmol dm?3),organic matter of soil (OMS,× 10 g kg?1),sand percentage (× 10 g kg?1),silt percentage (× 10 g kg?1) and clay percentage (× 10 g kg?1).To reduce data complexity,we synthesized these variables through a principal component analysis (PCA).
Data analysis
To assess the influence of altitude and soil (axis 1 of the soil PCA) on tree community spatial and temporal patterns,we divided the analyses into two parts:first,focusing on spatial and punctual observations from the temporal approach;second,focusing on the forest dynamics variables.In the first part,we assessed the effects of altitude (m),soil and inventory year on the following response variables:number of trees,aboveground biomass (AGB),AGB/tree,species richness and the standardized effects sizes (ses) of phylogenetic diversity (sesPD),mean phylogenetic pairwise distance(sesMPD) and mean nearest taxon distance (sesMNTD)(Webb et al.2002;Kembel et al.2010).For this,we fitted generalized linear mixed models (GLMM) considering sample units as a random factor to control for possible temporal dependence on the observations.First,we fitted the following global model to each response variable:y~ altitude × soil+year+(1|sample unit).Second,using a variable selection criterion (Pearson correlation <| 0.6 |),we obtained all possible variable combinations in models containing only uncorrelated variables through the functiondredge(package MuMIn;Bartón,2009).Third,we ranked the best model fits based on second-order Akaike information criterion (AICc)and selected the models with ?AICc ≤ 2 relative to the best model fit (?AICc=0).Fourth,we applied a multi-model inference approach (Burnham et al.2011) to calculate average model coefficients and their significance through themodel.avgfunction from the MuMIn package (Bartón 2009).We used Poisson residuals distribution in the absence of overdispersion for the count variables (i.e.,number of individuals and species richness),and for all others,we used Gaussian residuals distribution ensuring residual normality and homoscedasticity.Lastly,we assessed the role of altitude and soil (PCA 1 axis) on floristic similarity (Bray—Curtis) and phylogenetic composition (UNIFRAC;Lozupone et al.2006) among the plots in each inventory year (2010 and 2015),using theadonisfunction of theveganpackage(Oksanen et al.2019),also including the interaction between the two variables in the model.
In the second part,we assessed the role of altitude and soil on tree community dynamics between inventory years(2010 and 2015).We calculated tree mortality rate (M) and tree recruitment (R);biomass gain (G) and biomass loss (L)(Sheil et al.1995);within-plot temporal species substitution(betatemp)(Baselga et al.2013);and within-plot variation of phylogenetic diversity metrics,which we called ? sesPD,? sesMPD and ? sesMNTD.We fitted generalized linear models (GLM) with each dynamics variable as a response variable and altitude in interaction with soil as explanatory variables.We obtained all possible model combinations without collinear variables (Pearson’s correlation | 0.6 | criterion) and repeated the first part’s approach and criteria for variable selection and multi-model inference to obtain average coefficients and their significance values.We used Gaussian family of residuals distribution for all variables,ensuring homoscedasticity and residual normality.
We calculated tree aboveground biomass (AGB,in tons(t)) with the Pantropical equation of Chave et al (2014),using thebiomasspackage (Rejou-Mechain et al.2017).We calculated the phylogenetic diversity metrics (sesPD,sesMPD,sesMNTD) and the phylogenetic similarity index(UNIFRAC) based on an ultrametric phylogenetic tree of the site’s species pool,using the mega-tree available in theV.PhyloMakerpackage (Jin and Qian 2019).When genera and species were absent from the mega-tree (GBOTB.extended),we adopted the approach recommended by Qian and Jin (2016),in which the tips of these genera and species were bound to the midpoint of the genus or family branch.All analyses were performed in the R version 3.6.1 environment (R Core Team 2020),adopting a significance level of 5%.
Results
We sampled a total of 2072 individuals from 194 species,113 genera and 51 families.Total tree density was 1726.7 ind.ha?1and total aboveground biomass (AGB) was 255.4 t ha?1.Most of the soil variation was explained by the first PCA axis (54.21%,while the second axis explained 15.41%),so we used it as a proxy for soil variation.PCA1 described a gradient of soil fertility from lower altitudes(which displayed high Ca,Mg and pH) to higher altitudes (which displayed high Al,P and Sand) Fig.2.In other words,plots with high PCA1 scores displayed more restrictive soil conditions (i.e.,low soil fertility and high acidity,sand and aluminum content) than plots with low PCA1 scores (Fig.2).
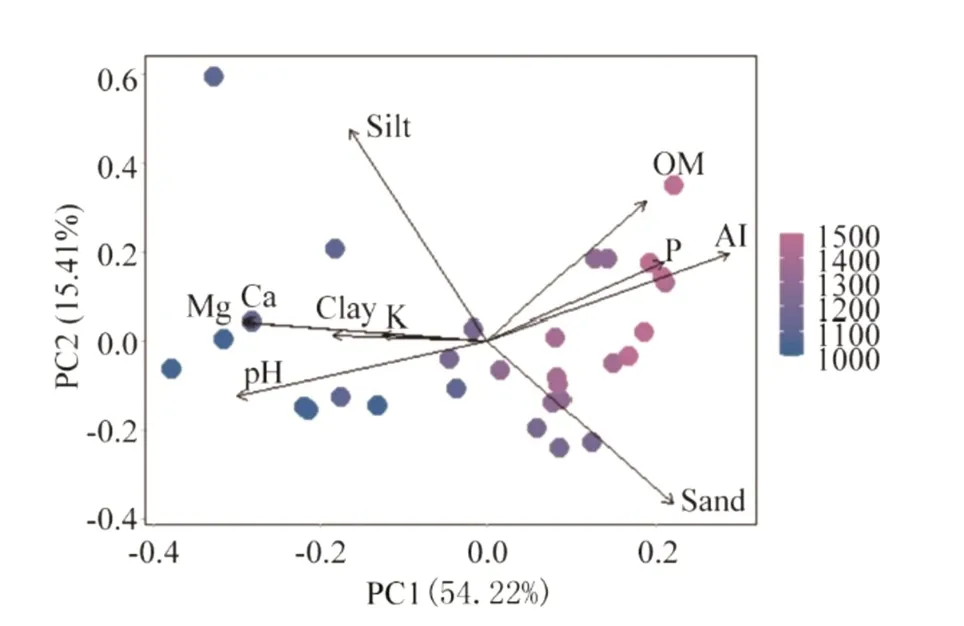
Fig.2 Principal component analysis (PCA) of 10 soil variables collected in 30 tropical forest plots along an altitudinal gradient in southeastern Brazil.Point colors indicate plot altitude.Note:pH:pH in water,P:phosphorus;K:potassium;Ca:calcium;Mg:magnesium,Al:aluminum;OM:organic matter of soil;Sand:sand percentage;Silt:silt percentage;Clay:clay percentage
Spatial patterns of phylogenetic structure and diversity were explained by altitude and soil variation.Altitude and soil explained spatial variations in number of individuals and phylogenetic diversity metrics (sesPD,sesMPD,sesMNTD):higher altitudes and low fertility soils displayed a greater number of individuals;lower altitudes and high fertility soils displayed greater phylogenetic diversity Table 1;Fig.3;Fig.4.Punctual patterns of community temporal dynamics were marked by a significant increase in total biomass (AGB) in 2015 and by altitude’s influence on individual tree biomass Table 1;Fig.5.Average community-level AGB (t ha?1) increased from 240.9 to 255.4 t ha?1,while AGB per tree increased from 0.15 to 0.17 t Fig.5.
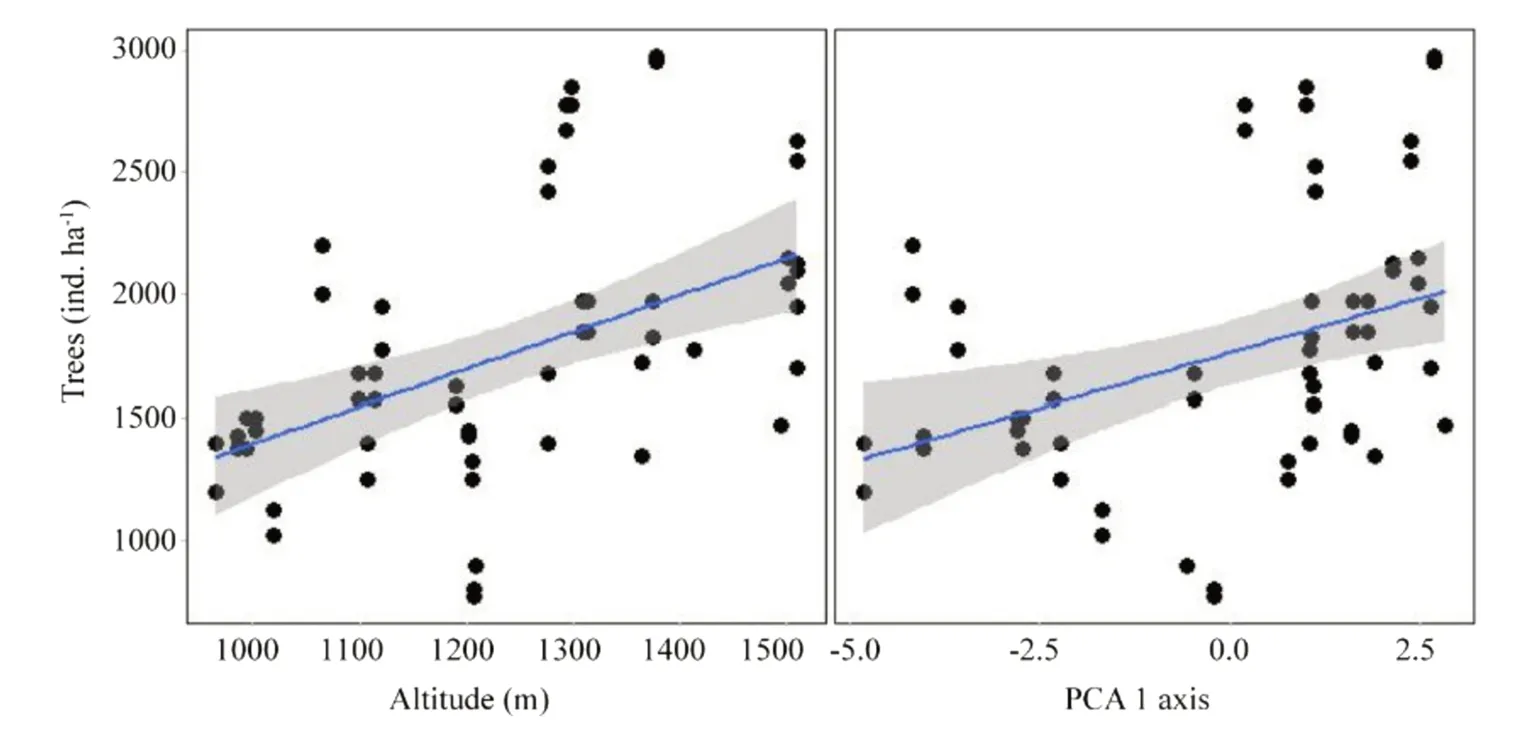
Fig.3 Tree density variation(ind.ha?1) along altitude (m)and soil change (PCA 1 axis)in a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil.Blue lines represent the model estimates,and shaded areas,the standard error
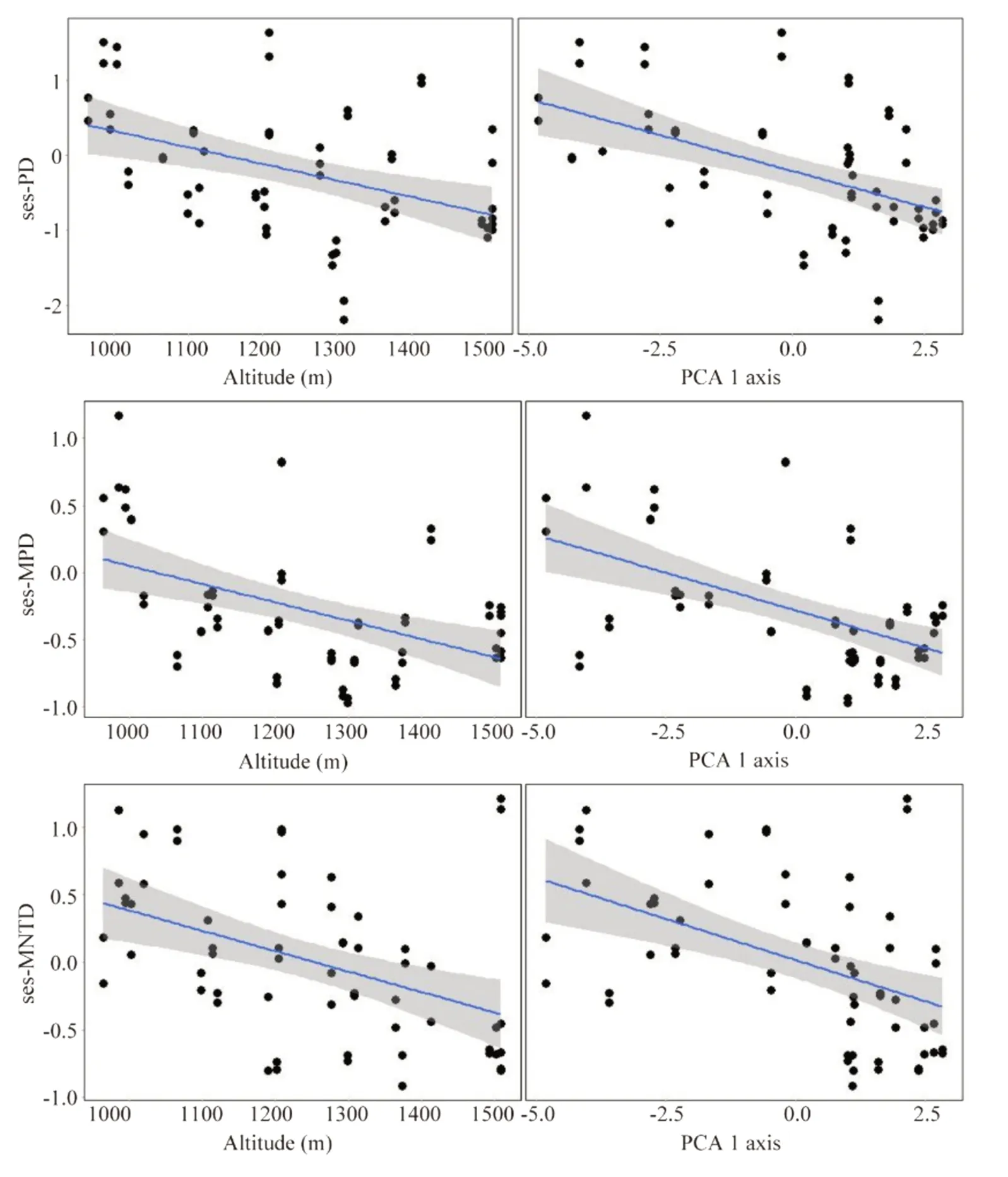
Fig.4 Phylogenetic diversity variation with altitude (m)and soil change (PCA 1 axis)in a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil.Blue lines represent the model estimates,and shaded areas,the standard error.Note:PD:phylogenetic diversity;MPD:mean phylogenetic pairwise distance;MNTD:mean nearest taxon distance;ses:standardized effect size
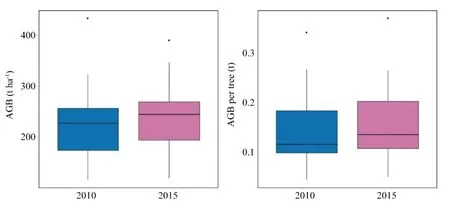
Fig.5 Boxplots for the aboveground biomass (AGB) (t ha?1)and AGB per tree (t) in each inventory year in a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil.The two years are significantly different (values higher in 2015,see Table 1)
Unlike the spatial variables,community dynamics (or temporal variables) was not explained by altitude and soil Table 2.In other words,although altitude and soil may explain spatial patterns of phylogenetic structure and composition,the community’s temporal patterns do not vary with altitude and soil.This suggests that community dynamics are somewhat similar across the broad scale of the forest.For the forest as a whole,average mortality was of 1.96%;average recruitment was 1.02%;AGB loss was 1.61%;and AGB gain was 2.81% Fig.6.We found an average of 0.1 for species temporal substitution (betatemp),and averages of ? 0.004,? 0.008 and 0.007 for sesPD,sesMPD and sesMNTD,respectively.
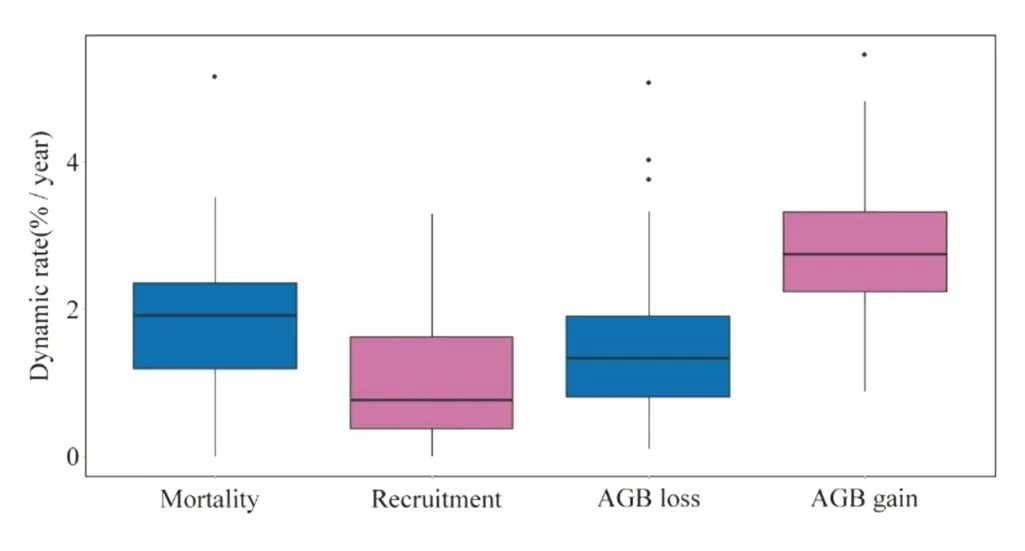
Fig.6 Boxplot for the dynamics rates (%/year) obtained in 2010 ? 2015 for the tree community sampled in a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil.Note that the values of mortality and AGB loss are presented as positive values,but they represent losses in the community
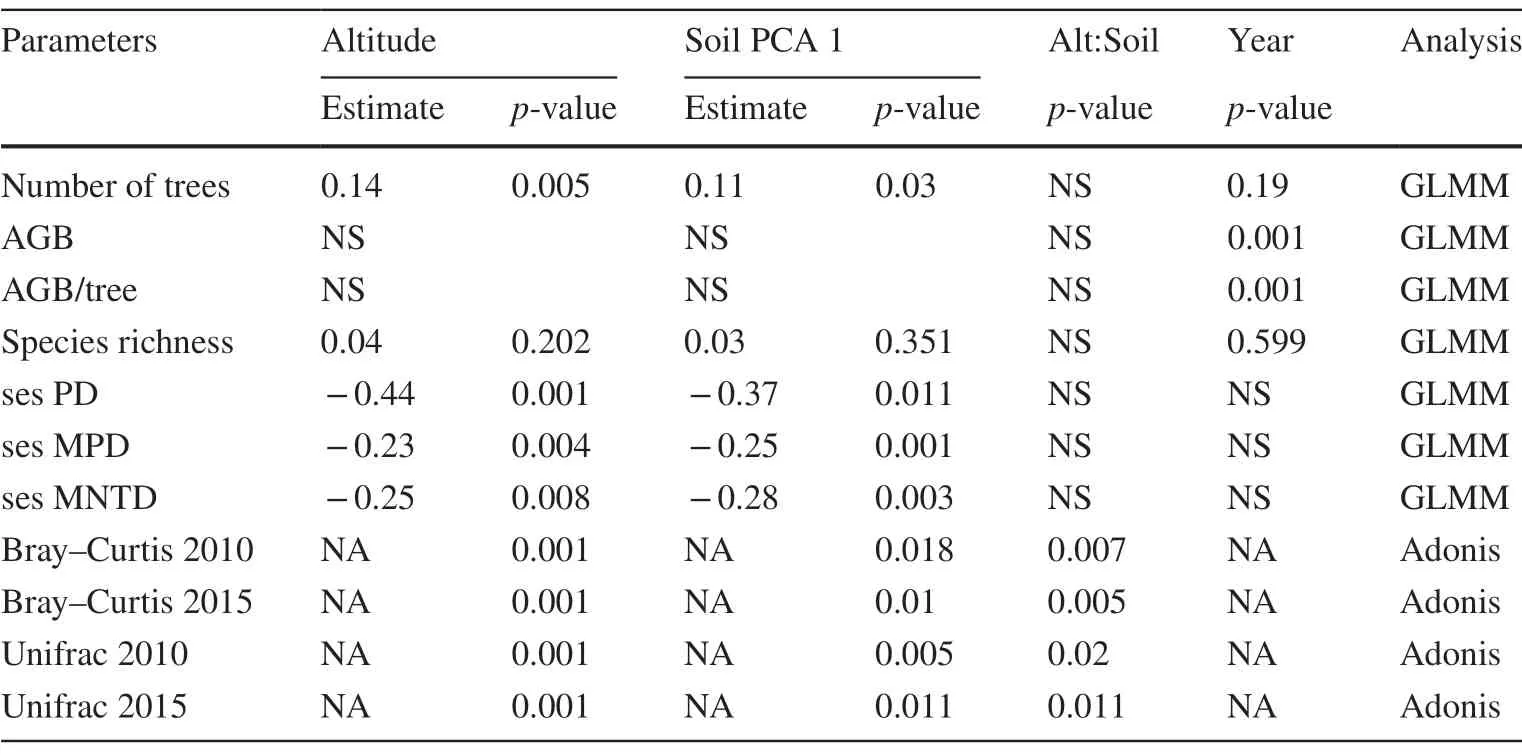
Table 1 Summary of analysis results on the influence of predictor variables on spatial response variables in a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil
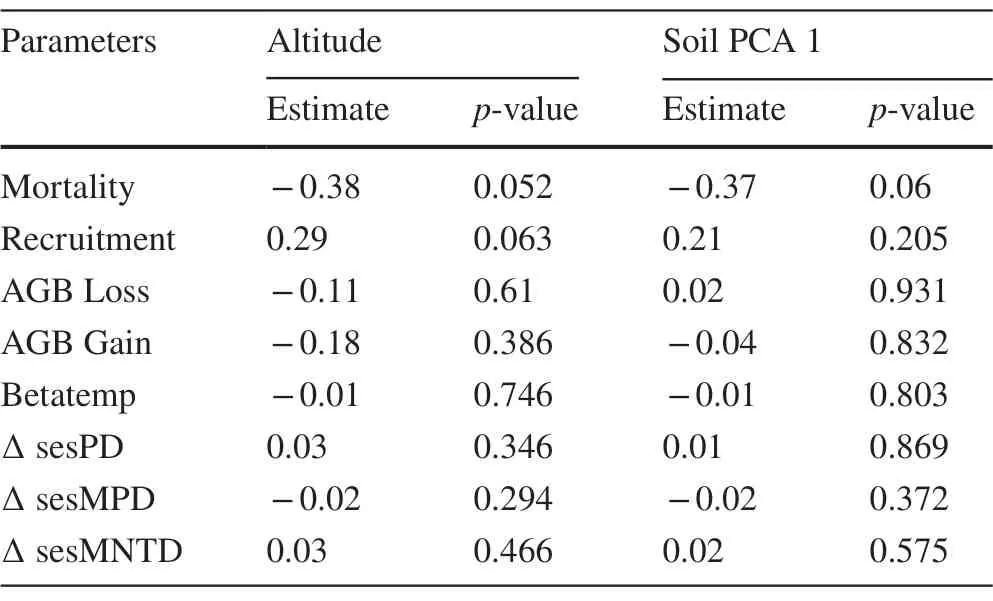
Table 2 Summary of GLM results on the influence of predictor variables on dynamics response variables (2010 ? 2015) of a tropical forest along a small-scale altitudinal gradient in southern Minas Gerais,southeastern Brazil
Discussion
We found that,in the study’s timeframe,altitude-related environmental heterogeneity drove spatial differences (in community structure,f loristic composition and phylogenetic diversity) but not temporal dynamics of the tree community of our study site.This result conf irms that local-scale community assembly and structure are driven by habitat heterogeneity in the site,since abiotic factors control the amount and availability of resources that have a direct influence on plant growth and survival (Quesada et al.2012;Baldeck et al.2016).Similar results have been found in previous studies conducted in other regions (Girardin et al.2010,2014;Gonmadje et al.2017).
Our results imply that a gradient of altitude and soil variation drives community structure across space in our study site.For instance,we found that higher altitudes tend to harbor high individual density and low individual biomass,while the opposite is true for the lower altitudes.In general,montane forests have few large trees,likely because of growth-limiting factors such as lower temperatures,fog,low incidence of light and high relative humidity.Altogether,these abiotic factors decrease productivity and limit tree biomass gain (Girardin et al.2014;Cuni-Sanchez et al.2017).But at the community level,there was no significant influence of altitude and soil in total aboveground biomass(AGB),and altitude only affected tree-level AGB.Ecological drivers of temporal dynamics vary depending on scale(e.g.,at the individual,species or community level);therefore,different ecological processes may have affected these parameters (van der Sande et al.2016).
Altitude and soil are determining factors for species abundances and distribution at local scales (Bohlman et al.2008;Pan et al.2013;Kipkoech et al.2019).We found that altitude and soil also played a significant role in local variation of phylogenetic diversity (Table 1).Floristic diversity and phylogenetic diversity are related,and the former usually determines the extent to which phylogenetic diversity can vary (Hardy et al.2012).Besides,greater phylogenetic diversity is expected to increase functional diversity,leading to niche complementarity,greater resource acquisition,more efficient resource use and higher productivity (Poorter et al.2 015).The lower values of sesPD,sesMPD and sesMNTD found at higher altitudes and low fertility soils,suggest that phylogenetic structure is clustered and that plant diversity is lower in this part of the gradient.This result may be due to the stressful environment found at high altitudes.Based on the assumption of phylogenetic niche conservatism,environmental filtering in altitudinal gradients leads to coexisting species with similar physiological tolerances that may result in phylogenetic clustering (Qian et al.2014).
Although the imprint of evolutionary history is more evident on flora composition at broader scales (e.g.,regional,continental and global;Nagalingum et al.2015;Thornhill et al.2016;Vellend et al.2017),our results revealed the importance of evolutionary processes at local-scale community assembly in tropical forests.Phylogenetic diversity informs about the influence of adaptations inherited over evolutionary time and also about the current environmental restrictions that lead to variation in ecological strategies along environmental gradients (Fine 2015;Gerhold et al.2015).
Because species composition is one of the main factors that govern forest dynamics and productivity (Toledo et al.2017),we expected the influence of environmental variation on structural and phylogenetic differences to extend to community’s temporal dynamics in our study site.However,the dynamics parameters did not differ significantly along the altitudinal gradient,leading us to reject our hypothesis about the influence of altitude and soil on the forest’s temporal dynamics.Other studies have also failed to detect a significant influence of environmental heterogeneity on shortterm forest dynamics variation in local tree communities(Higuchi et al.2008a,b;Santos et al.2019).In this case,these environmental factors are expected to act as a filter selecting the most adapted species to exploit the resources available in each habitat (Weiher and Keddy 1995;Pulliam 2000;Gonmadje et al.2019).However,since the established species are already adapted to local conditions,the environment should have little influence on community temporal dynamics,and similar temporal patterns should emerge.Altogether,the significance of altitude and soil’s influence on community structure,composition and phylogenetic diversity,and absence of such significance on community temporal dynamics,suggest that structural changes are slow in this tropical forest.
Furthermore,disturbances (either natural or anthropogenic) are usually the main cause of spatial variation in temporal dynamics along environmental gradients,with environmental factors usually playing a secondary role(Machado and Oliveira-Filho 2010;Miguel et al.2011).In fact,disturbances are recognized as drivers of forest dynamics because they destabilize the patterns of the affected community (Swanson et al.2011;Pugh et al.2019),promoting a decrease in tree individual density and an increase in biomass loss that directly impact community dynamics rates(Lieberman et al.1985;Swaine et al.1987).The studied area has no reports of major disturbances,except for selective logging near the forest edges.For removing a specific group of interest,this type of disturbance usually has more effect on species richness and single population dynamics than it has on overall community dynamics rates.Based on these results,we could expect long-term stability in the temporal dynamics of this forest,as also observed in other undisturbed Atlantic Forest sites in the region (southern Minas Gerais)(Chagas et al.2001;Appolinario et al.2005;Higuchi et al.2008a;Higuchi et al.2008b;Meyer et al.2015).Lastly,we suggest that future studies encompassing a longer monitoring timeframe test whether our expectations (community stability and similar dynamics along the gradient) hold in longer periods of time.
Conclusion
Our study concluded that the altitude and soil gradients led to the structural and phylogenetic patterns observed in the tree community.The small-scale variations in altitude and soil determine the conditions and resources that directly influence the selection of species adapted to each habitat.This has led to spatial differences in floristic composition,community structure and phylogenetic diversity across the environmental gradient.However,we have also found that forest dynamics are spatially similar and temporally stable across the same gradient.Altogether,these results suggest that the species present in each habitat are adapted to exploit locally available resources,which could explain the spatially similar dynamics.At the same time,the temporally stable dynamics could be explained by an absence of major disturbances.
AcknowledgementsThe authors wish to thank the Federal University of Lavras (UFLA),Minas Gerais State Research Foundation(FAPEMIG),the Brazilian National Council for Scientific and Technological Development (CNPq),and the Coordination for the Improvement of Higher Education Personnel (CAPES) for all the support.
 Journal of Forestry Research2021年5期
Journal of Forestry Research2021年5期
- Journal of Forestry Research的其它文章
- Performance and genetic diversity of 23 provenances of northern red oak (Quercus rubra L.) after 25 years of growth in South Korea
- Morphological and genetic differentiation in isolated populations of Mexican beech Fagus grandifolia subsp.mexicana
- Tree species classification using deep learning and RGB optical images obtained by an unmanned aerial vehicle
- The composition and diversity of natural regeneration of tree species in gaps under different intensities of forest disturbance
- Shade and sapling size influence restoration of Araucaria angustifolia
- Genetic diversity and differentiation among populations of the pedunculate oak (Quercus robur) at the eastern margin of its range based on a new set of 95 SNP loci
