Shade and sapling size influence restoration of Araucaria angustifolia
Simone Aparecida Zolet Sasso·José Abramo Marchese·Amanda Pacheco Cardoso Moura·Bruna Valéria Gil·Anelise Tessari Perboni·Joel Donazzolo·Fabrícia Lorrane Rodrigues Oliveira·Bruno Francisco Sant’Anna-Santos·Angela Rohr·Moeses Andrigo Danner
Abstract Toward improving reforestation of Brazilian pine(Araucaria angustifolia),two contrasting sapling sizes in either full sun or in the shade of a mixed plantation and the effect of opening the canopy were evaluated for survival,growth,gas exchange,photosynthetic pigments,and leaf anatomy 18 months after being planted.At 23 months after planting,a partial opening was made in the canopy in the mixed plantation,then the saplings were evaluated again after 2 months for the same morphophysiological traits.After 18 months,saplings planted in the full sun had higher survival,growth,pigments,and photosynthesis compared to the shaded saplings.Large saplings had higher survival and growth compared to the small ones.Shaded leaves were thinner and little differentiation of palisade parenchyma and hypodermis.After opening of the canopy,photosynthetically active radiation was 10 times higher,and the saplings quickly grew in height due to increased photosynthesis.Thus,although the species can tolerate shade,growth in the shade is limited.We recommend that for reforestation purposes of Brazilian pine,large saplings should be selected and planted in the open for better development.
Keywords Brazilian pine·Luminosity·Gas exchanges ·Photosynthetic pigments·Leaf anatomy
Introduction
The Brazilian pine [Araucaria angustifolia(Bert.) O.Kuntze] dominates the Araucaria forest canopy in southern Brazil (Eisenlohr and Oliveira-Filho 2014).However,because of intense deforestation for wood gathering,urban expansion,and agriculture,especially between 1930 and 1970,the Araucaria forests were reduced to less than 3%of the original area (Guerra et al.2002) andA.angustifoliareached endangered species status (Thomas 2013).
The high fragmentation and low regeneration of the species in natural forests indicate that,for its effective conservation,reforestation must be stimulated by encouraging the production and sale of the large,delicious araucaria pine nut(pinh?o),instead of the wood (Danner et al.2012).Furthermore,populations ofA.angustifoliahave sufficient genetic diversity to help preserve the regional genetic diversity of the species of the native forests when planted in reforestation areas (Stefenon et al.2008;Ferreira et al.2012).However,the high mortality of transplanted saplings and rodent damage during direct seeding (Maran et al.2016) hampers the success of these plantations.Thus,more studies are needed to understand the survivability of transplanted saplings during reforestation and verify whether sapling size affects the regeneration and development of this species.
Shade could also be detrimental for reforestation of Brazilian pine,which develops different morphological and physiological traits depending on the light conditions(Duarte and Dillenburg 2000;Duarte et al.2002;Franco and Dillenburg 2007).Most of the research on these aspects,however,were done in natural forests;the lack of information on many important ecological and physiological factors that affect Brazilian pine in restoration forests further limits the success of this species in reforestation programs (Duarte et al.2002).Considering that the Araucaria forest in southern Brazil is a highly reduced and fragmented ecosystem of the Atlantic Forest Biome (Ribeiro et al.2009),restoration is recommended for the conservation of theA.angustifolia,an endangered conifer of this ecosystem.
The aim of this study was to evaluate growth,gas exchange and leaf anatomy of large and small saplings sizes ofA.angustifoliagrown in a plantation in partial shade or full sun and how opening the canopy affects growth and morphophysiological traits.
Materials and methods
The experiment was performed in Dois Vizinhos,Paraná,Brazil (25°42′01″ S,53°05′54″ W,529 m a.s.l.) in a demonstration plantation managed according to agroforestry principles.The climate is classified as Cfa (Humid subtropical,Oceanic climate,without dry season,with hot summer) by K?ppen (álvares et al.2013),and the soil was classified as a Dystric Nitisols (IUSS Working Group WRB 2015).The mixed plantation was established on 1350 m2in August 2013;a base of 56 banana saplings were planted 7 m apart in eight rows that separated by 4 m.Each row were completed by plantation of 33 saplings,1.40 m apart of each other,with heterogeneous composition of 40 tree and shrubs species,organizing to obtain 11× the small,medium,and large canopy size sequence.
Saplings ofA.angustifoliaoriginated from seeds and cultivated for 18 months in nurseries with 50% shade were selected by two size:large (39.3 ± 3.6 cm height and 5.7 ± 0.4 mm diameter,with two verticils) and small(18.9 ± 2.9 cm height and 3.6 ± 0.3 mm diameter,with one verticil).In October 2014,31 saplings of each size were planted at the edge of the plantation (in full sun) and within the mixed plantation (in the shade of banana canopy),1.5 m apart each other in the same schemes of mixed plantation rows.
The saplings ofA.angustifoliawere checked for survival 18 and 25 months after planting to calculate the percentage of live saplings among the total planted.Height and stem diameter growth of saplings of each size in each light condition were measured at the time of planting and after 18 and 25 months to calculate change in growth from planting to 18 months and from 18 months to 25 months.After 23 months from planting,50% of the banana pseudostems were removed to provide more sunlight for the shaded araucaria saplings.
At 18 and 25 months after planting,between 10:00 and 12:00 on sunny days,an infrared gas analyzer (model LICOR 6400-05)wasusedtomeasureCO2assimilation rate(μmolCO2m?2s?1),stomatalconductance(mol H2O m?2s?1),intercellular CO2concentration(μmol CO2mol?1),transpiration rate (mmol H2Om?2s?1) andphotosyntheticallyactiveradiation(PAR,μmol m?2s?1)inthree leaves of each survived saplings.
Photosynthetic pigments were extracted and quantified from three disks (0.3 cm diameter,0.21 cm2) from each of three leaves.The disks were immersed in 5.0 mL of dimethyl sulfoxide for 18 h in the dark,at 65 °C in water bath.Absorbance at 470 nm (carotenoids),649.1 nm (chlorophylla),and 665.1 nm (chlorophyllb) was measured with a spectrophotometer (Shimadzu UV-1800;Kyoto,Japan).The respective pigment concentrations were calculated as proposed by Wellburn (1994).
Leaf anatomy was examined for three leaves in each sapling and treatment combination at 18 and 25 months after planting.The leaves were fixed in FAA solution (formaldehyde—acetic acid—50% ethanol,1:1:18 v/v) for 24 h(Johansen 1940),washed in 50% ethanol and stored in 70%ethanol.For microscopic analyses,fragments of 0.5 cm2of the middle region of the leaf blade were dehydrated in an ethanolic series and included in methacrylate (Historesin,Leica Instruments,Heidelberg,Germany).Cross-sections 10μm thick were stained with aqueous 0.12% w/v toluidine blue O in 5% w/v borax,then mounted in distilled water for observation and imaging.In addition to qualitative analysis,the thickness of the leaf blades was measured using Image-Pro Plus 4.1 version.Images were captured using a Zeiss Axiolab photomicroscope and Sony Cybershot 7.2MP digital camera.
All data separately at 18 and 25 months from planting were analyzed by Shapiro—Wilk normality test,two-way ANOVA and Tukey multiple range comparison when significant differences were found among treatment combination.The analysis were performed using the MASS,ExpDes,and Agricolae packages in the R platform v.4.0.2 (R Core Team 2020).
Results
The sapling size × light level interaction was not significant for any analyzed variables.When each factor was evaluated separately,for sapling size,the large saplings had grown more in height and diameter than the small saplings by 18 months after planting.After 25 months,there were no statistically significant differences in growth between the two sapling sizes.Saplings in full sunlight had grown more in height and diameter compared to shaded ones both at 18 and 25 months after planting (Fig.1).
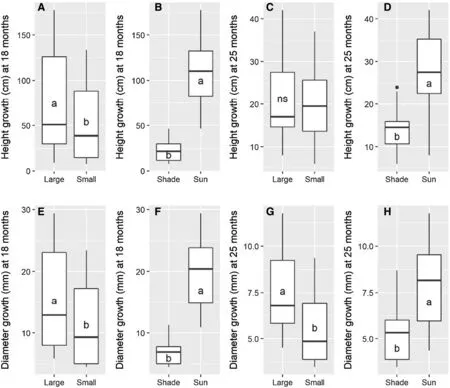
Fig.1 Height and diameter growth of large and small A.angustifolia in shade and sun at 18 and 25 months after planting.Diff erent letters indicate significant differences among means of treatments and ns indicate not significant by F-test (P ≤ 0.05)
Notably,after opening of the canopy at 23 months,in just 2 months with more light intensity in the mixed plantation,the saplings grew more than half of what they had grown in the first 18 months after planting,regardless of sapling size.Even so,in those 2 months,A.angustifoliasaplings in full sun had increased to almost double the height of the shaded ones.
Survival after 18 months was 69% in full sun and 64%in shade.The large saplings had higher survival rate (80%)when compared to small ones (58%).After canopy opening at 23 months,in the re-evaluation at 25 months,there was no mortality of saplings in full sun,whereas shaded saplings had their survival rate reduced to 61% for large saplings and to 58% for small ones.
At 18 months,PAR was close to 850μmol m?2s?1for leaves in full sun,and PAR for leaves in the shade was only 1% of those in full sun (Fig.2).Saplings in full sun had a higher CO2assimilation rate (A),stomatal conductance (gs),transpiration rate (E),and lower intercellular CO2concentration (Ci) than in the shaded plants.The values for these variables did not differ significantly between the two sapling sizes within the same light condition (Fig.2).
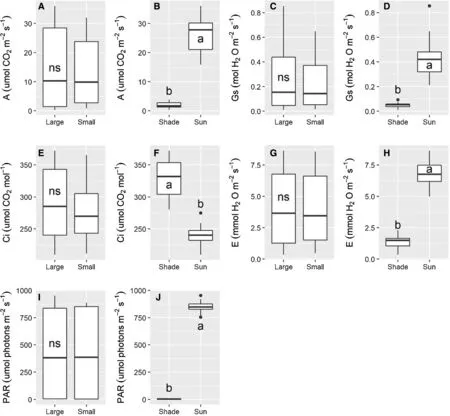
Fig.2 Gas exchange variables of A.angustifolia saplings of two sizes (large and small) and under two light conditions (shade and sun) 18 months after planting.Diff erent letters within a panel indicate significant differences among means,and ns indicates not significant by F-test (P ≤ 0.05).CO2 assimilation rate (A);stomatal conductance(gs) ;intercellular CO2 concentration (Ci);transpiration rate (E);photosynthetically active radiation (PAR)
The PAR for saplings ofA.angustifolia,after 25 months in the shade of the mixed plantation was about four times greater than after 18 months,due to the partial opening of the canopy.The PAR in the shade reached 11.5% compared to the saplings in full sun.At 25 months,as found at 18 months,the saplings in full sun had higherA,Eandgsand lower Ci,when compared to shade.There were no significant differences between the two sapling sizes (Fig.3).
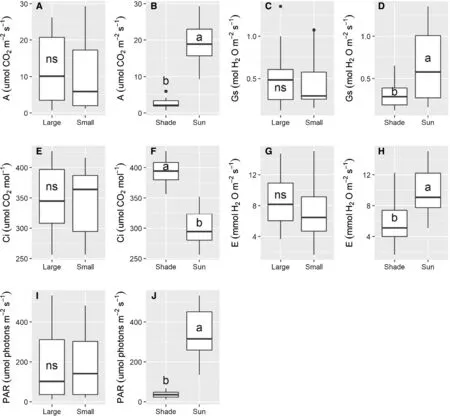
Fig.3 Gas exchange parameters of A.angustifolia saplings of two sizes (large and small) and under two light conditions (shade and sun)25 months after planting.Diff erent letters within a panel indicate a significant difference among means,and ns indicates not significant by F-test (P ≤ 0.05).CO2 assimilation rate (A);stomatal conductance(gs) ;intercellular CO2 concentration (Ci);transpiration rate (E);photosynthetically active radiation (PAR)
Carotenoids and chlorophyllalevels were higher in the saplings exposed to full sun than in the shade plants,chlorophyllbdid not differ.Nor did any pigment levels differ between the large and small saplings within the respective light conditions.These results were the same at 18 and 25 months after planting (Fig.4).
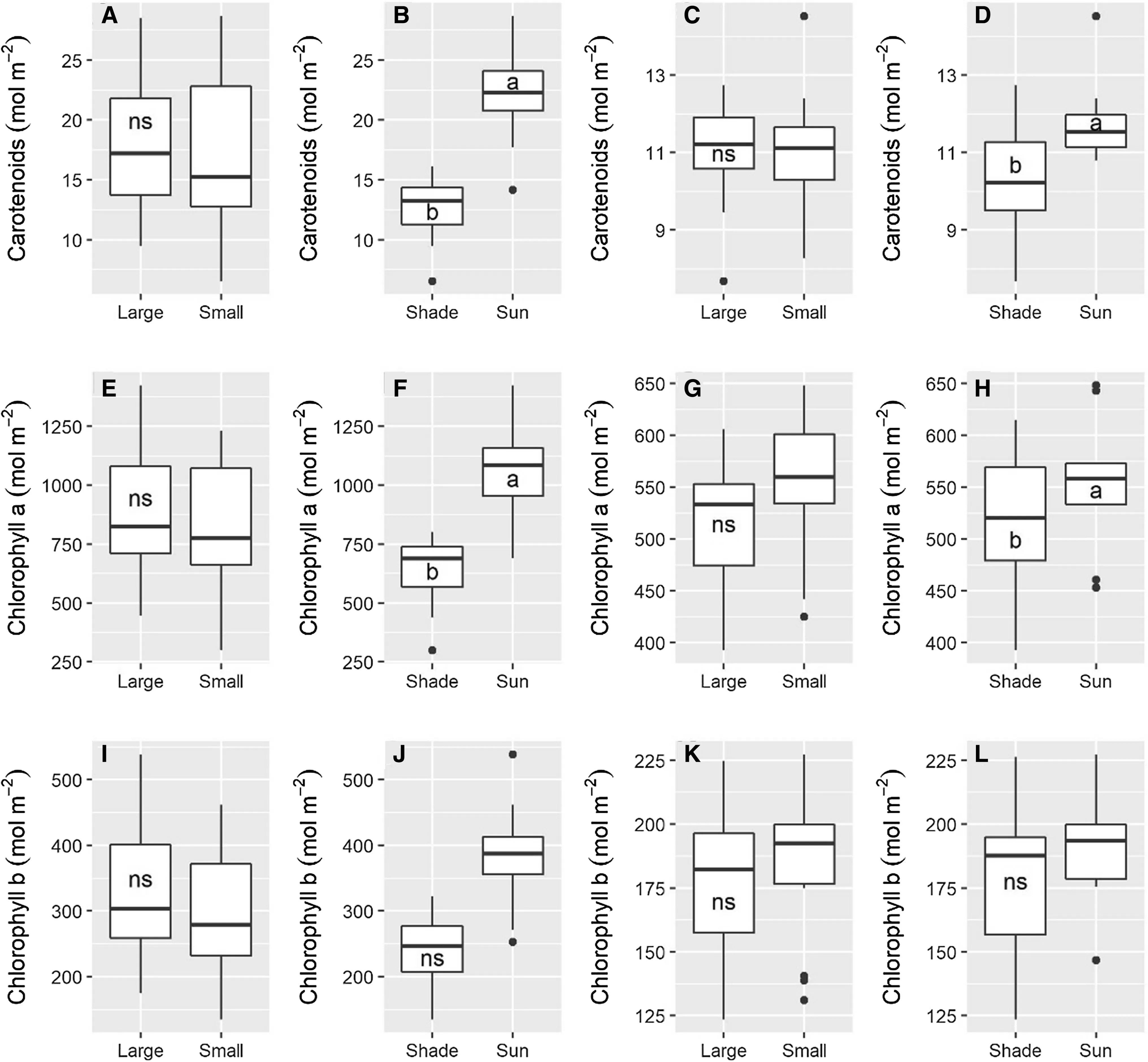
Fig.4 Photosynthetic pigments of A.angustifolia saplings of two sizes (large and small) and under two light conditions (shade and sun)18 months after planting (A,B,E,F,I,J) and 25 months after planting (C,D,G,H,K,L).Diff erent letters within a panel indicate significant differences among means,and ns indicate not signif ciant by F-test (P ≤ 0.05)
For leaf anatomy after 18 months,the blades from saplings in full sun were thicker than those in the shade,but after 25 months,the leaf thickness did not differ between full sun and shade.Nor did the thickness differ among sapling sizes in the respective light conditions after 18 or 25 months(Fig.5).
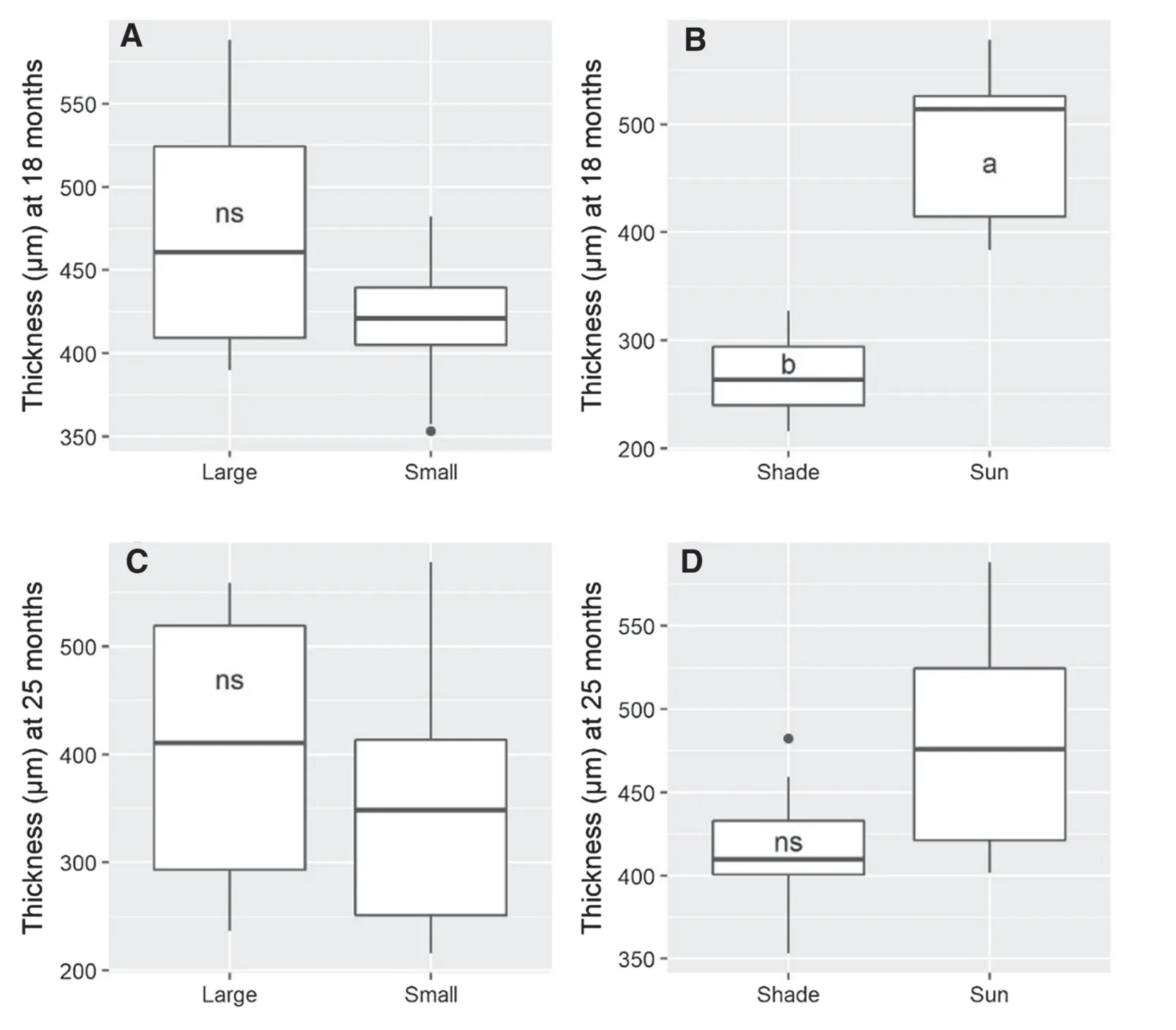
Fig.5 Leaf thickness of A.angustifolia saplings of two sizes (largelarge and small)and under two light conditions(shade and sun) 18 and 25 months after planting.Diff erent letters within a panel indicate significant differences among means,and ns indicates not significant by F-test (P ≤ 0.05)
In most of the leaves of saplings in full sun,the palisade and spongy parenchyma were differentiated,and the mesophyll was isobilateral at 18 and 25 months.In leaves of shaded saplings,the palisade parenchyma was barely differentiated,and the mesophyll was homogenous.The abaxial epidermis comprised up to four layers of cells in sun leaves,but only one in shade leaves.The adaxial epidermis of leaves comprised one cell layer in both light conditions.
Two months after the canopy opening,the anatomy of leaves from saplings that were no longer shaded had changed.The palisade parenchyma was more differentiated on the adaxial surface,and the mesophyll was dorsiventral on the adaxial side.In both full sun and shade,the hypodermis was composed up of four layers of cells on the abaxial surface and one to two cell layers in the adaxial surface.In most of the cuts,the leaf structure was similar in both sapling sizes (Fig.6).
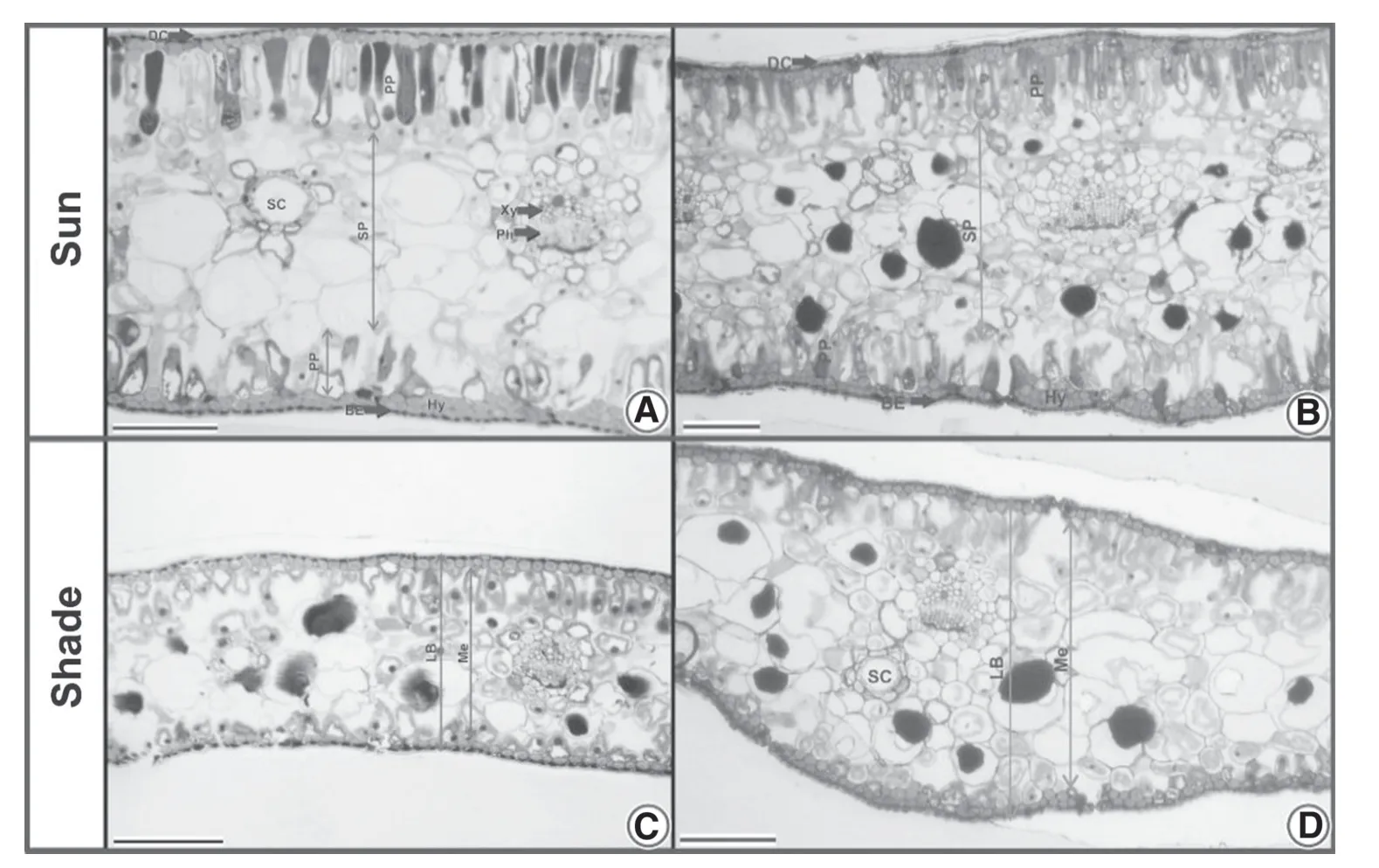
Fig.6 Structural leaf anatomy of A.angustifolia saplings at full sun and under shade 18 months (A and C) and 25 months after planting (B and D).Bars:150 mm.BE (abaxial epidermis);DC (adaxial cuticle);Hy (hypodermis);LB (leaf blade);Me (mesophyll);Ph(phloem);PP (palisade parenchyma);SC (secretory duct);SP(spongy parenchyma)
Discussion
This study showed thatA.angustifoliacan acclimate to low light;saplings survived and grew,although slowly,even when light was restricted by the banana canopy to only 1%of the PAR in full sun,18 months after planting.The survival rate of the saplings was high in both light conditions(~65%),compared to another study where only 17% of the saplings ofA.angustifoliasurvived in full sun (Maran et al.2016).Between 18 and 25 months,no saplings in full sun had died;only saplings in shade or opened canopy had died.Thus,although most saplings survived,PAR restriction in the shade can make the plants weaker,mainly the small saplings.We have no def initive information yet on how many yearsA.angustifoliacan remain alive in deep shade without an opening in the canopy,but when growth rings were counted,regenerating saplings ofA.araucanawere estimated to be quiescent in forest understory and retained the ability to resume growth after the canopy opening for more than 150 years (Grosfeld et al.1999) and those ofAbies albafor more than 90 years (Szymura 2005).
The lower values for gas exchange variables,except for CO2accumulation in intercellular spaces,in saplings in heavy shade compared to those in full sun,shows that the low density of photons trapped by shaded plants limits photosynthesis,thus reducing Nicotinamide and adenine dinucleotide phosphate (NADPH),Adenosine TriPhosphate (ATP) and Rubisco production,consequently reducing the amount of CO2fixed and carbohydrates synthesized (Flexas et al.2004;Dias and Bruggemann 2007).The saplings in shade condition in this experiment,accumulated CO2in intercellular spaces,so this high content in the leaves discourages stomatal opening,which explains the lower stomatal conductance and transpiration rate in the shaded plants (Aasamaa and S?ber 2011;Kinoshita and Hayashi 2011).These results may explain,in part,the slower growth of saplings in the shade compared to saplings in the sun.
When the canopy was partially opened in the mixed plantation was performed after 23 months,PAR increased by 10×,and saplings had a significant increase in stomatal conductance,transpiration and in CO2accumulation in intercellular spaces,but no significant increase in the assimilation rate.Within just two months of the canopy opening,growth in height of the saplings was more than half of their growth in the first 18 months.This results demonstrates the need for higher light intensities for the best growth and the ability ofA.angustifoliasaplings to adapt quickly to absorb and efficiently use the light for photosynthesis,and thus grow quickly.
The better growth ofA.angustifoliasaplings in the sun compared to shaded saplings could be associated to greater gas exchange and pigment levels.The higher content of chlorophyllaenables greater capture and transfer of energy to the photosystem reaction centers,factors that are also associated with shade-tolerant plants (Valladares and Niinemets 2008).In addition,the higher carotenoid content increases the ability to dissipate the energy from the excited state of chlorophyll to avoid damage from photoinhibition,factors associated with plants adapted or acclimated to high light levels (Gratani et al.2006;Tang et al.2015).
The large saplings had also grew more than the small ones,although the assimilation rates and pigment levels were similar,possibly because the large saplings also have greater leaf area for light absorption and photosynthate production.Hence,for mixed plantations with other species,we recommend the planting of large saplings ofA.angustifoliaat the edges of planting rows or with continuing opening of the canopy to ensure exposure to full sun.
Changes in thickness and differentiation of tissues were evident between shaded and full-sun-exposed leaves.The change in blade thickness is closely associated with the regulation of light and CO2within the leaves,maximizing photosynthetic efficiency when in risk of light uptake reduction (Dickison 2000;Santos et al.2015).However,in our study of leaves in deep shade,this compensation was not adequate;photosynthesis was greatly reduced.Probably,the partial differentiation of the palisade parenchyma interfered negatively with this compensation and suggests that,in deep shade,the amount of light in the environment was not enough for total differentiation of photosynthetic tissues.Light is fundamental for this process because it acts on auxin signaling,which is required for cell relaxation and expansion(Gomes et al.2014).It is worth mentioning that the reduction in leaf thickness is a way to compensate for a reduction in the amount of light;the reduction in the number of layers in the hypodermis in a shaded environment,as noted in this work,reduces the barrier of light ref lectance,also maximizing light absorption in low-light environments.In leaves exposed to the sun,tissues external to the chlorophyll parenchyma increase light ref lectance of the leaf and protect the most superf icial cells of the chlorophyll parenchyma against excess radiation (Dickison 2000).
The results of this study indicate that this point of light compensation of saplings in the shade is low for the species,since the saplings maintained their growth when PAR was less than 9.0μmol photons m?2s?1.We assume that PAR must have been variable during the 18 months after planting the saplings due to the closing of the canopy with the growth of the trees,mainly bananas,in the mixed plantation.In another study,at 110 days after germination ofA.angustifoliaseeds,the light compensation points were 35,38 and 75μmol m?2s?1for the leaves in basal,median and upper parts of the saplings,respectively (Einig et al.1999).
Conclusions
Growth ofA.angustifoliasaplings was plastic when light availability was low;the saplings survived but grew little until the canopy opening increased the light exposure.In practice,our f nidings of morphophysiological acclimation to light conditions are important for advancing our biological knowledge of this species and for managing its growth in single or mixed plantations for commercial or reforestation purposes.For reforestation purposes of the Brazilian pine,we recommend the planting of large saplings in full sun and opening the canopy in mixed plantations to avoid growth stagnation that takes place in deep shade.
AcknowledgementsS.A.Z.Sasso,A.P.C.Moura,B.V.Gil and F.L.R.Oliveira wish to thank the Brazilian agency CAPES for master’s and doctoral degree fellowships.A.Rohr thanks CAPES and FUNDA??O ARAUCáRIA for postdoctoral fellowship.M.A.Danner thanks to Brazilian agency CNPq for research fellowship.
 Journal of Forestry Research2021年5期
Journal of Forestry Research2021年5期
- Journal of Forestry Research的其它文章
- Performance and genetic diversity of 23 provenances of northern red oak (Quercus rubra L.) after 25 years of growth in South Korea
- Morphological and genetic differentiation in isolated populations of Mexican beech Fagus grandifolia subsp.mexicana
- Tree species classification using deep learning and RGB optical images obtained by an unmanned aerial vehicle
- Can small-scale altitudinal gradients predict spatial and temporal patterns in tropical forests?
- The composition and diversity of natural regeneration of tree species in gaps under different intensities of forest disturbance
- Genetic diversity and differentiation among populations of the pedunculate oak (Quercus robur) at the eastern margin of its range based on a new set of 95 SNP loci
