An efficient preparation of porous polymeric microspheres by solvent evaporation in foam phase
Yang Yu,Guiying Li,Wanqing Han,Linhua Zhu,Tian Si,Hong Wang,Yanlin Sun,,Yanping He,
1 Faculty of Chemical Engineering,Kunming University of Science and Technology,Chenggong Campus,Kunming 650504,China
2 Faculty of Science,Kunming University of Science and Technology,Chenggong Campus,Kunming 650504,China
Keywords:Continuous process Foam phase High-yield Polymer microsphere Solvent evaporation Time-saving
ABSTRACT This paper reports an efficient method of preparing porous polymeric microspheres by solvent evaporation in foam phase,in which phase separation between polymer and porogen occurs in foam phase instead of that in water phase by using the traditional solvent evaporation method.The method provides outstanding features,including being time-saving,of high-yield and able for continuous production,in which formation of porous polymeric microspheres finished within 3 min with a high production yield up to approximate 95 wt% and the process was able to be developed into a continuous process for production of porous polymeric microspheres.It was also universal to non-crosslinked polymers since the method is a development on the traditional emulsion solvent evaporation method.The new method is efficient and can be used potentially on the industrial scale for continuous production of porous polymeric microspheres.
1.Introduction
Porous polymeric microspheres have drawn intensive interest because of their relatively low density,high specific surface area,light weight and permeability[1–3].These remarkable properties enable porous polymeric microspheres have potential applications in various areas,such as catalysis carriers[3–6],absorption materials[7–9],medical applications[10,11],gas storage[12]and chromatography[13].
So far,porous polymeric microspheres have been reported either to be prepared from monomeric species[14–20]or to be prepared from preformed polymers [21–31].Formation of porous polymeric microspheres from monomeric species always involve heterogeneous polymerizations including several approaches such as suspension,dispersion,emulsion,precipitation polymerization and so on[1,2].In these polymerization approaches,chemical reactions occurs since monomers and initiators are introduced to produce polymer,which leads phase separation between the introduced porogen and the formed polymer to produce porous structures.Although heterogeneous polymerizations have been widely used,batch-processes instead of continuous processes have been used,which is inefficient for industrial production[3].
Another ways to prepare porous polymeric microspheres are from the preformed polymers [21–31].It always start by dissolving the preformed polymer into a volatile organic solvent(a good solvent to polymer);and then mix the polymer solution with porogen(a poor solvent to polymer)to form an oil solution.The oil solution was then dispersed into a continuous phase to produce oil droplets suspension;and after the volatile organic solvent evaporates,diffuses or displaces,polymer solidifies and separate phase with porogen to produce porous structures.Since the method is only a physical process,the preparation process is simplified compared with heterogeneous polymerizations.Currently,methods of making porous polymeric microspheres from the preformed polymer are either by using batch processes in liquid continuous phase[21,22,24–27,29,31]or by using microfluidic devices[23,28,30].For batch operations in liquid continuous phase,several hours were needed since gentle temperature increasing was used to allow the diffusion,displacement or evaporation of volatile organic solvents[22,24–27].Additionally,since the batch process involved dispersion of oil phase in aqueous phase,polymeric microspheres were hardly obtained at high oil/water ratios,because of particle aggregation.Compared with batch operations,the microfluidic device to assistant producing porous polymeric microspheres is different,since it enable a continuous process to produce polymeric microspheres.However,the device is delicate and the yield is relatively low[1,2].Therefore,the reported methods via solvent evaporation to produce porous polymeric microspheres are either of time-consuming,of low-yield,or unsuitable for continuous industrial scale production.Therefore,there exists a need to develop a practical solution to overcome these limitations.
In our previous study,we reported an efficient method of producing porous polymeric microspheres in foam phase through the combination of solvent evaporation and phase separation[29],in which an easy control on the internal structures of polymeric microspheres via introducing a water-soluble organic solvent was obtained[21].In this work,a further research was carrying out to explore the method's outstanding features;in particular,its advantages on time-saving,of being high yield and continuous production.In addition,the universality of the method to produce porous polymeric microspheres was studied.The method may provide an efficient practical industrial solution to make versatile porous polymeric microspheres.
2.Experimental
2.1.Materials
Styrene,methyl methacrylate and butly acrylate were used as monomers to prepare polymers and they were purchased from Sinopharm Chemical Reagent Co.,Ltd.,China (CP).Polylactide (PLA,Mw~160,000)was supplied by NatureWorks(USA)to prepare PLA microspheres.Dichloromethane (DCM),trichloromethane (TCM) and ethyl acetate(EA)were used as organic volatile solvents,which were supplied by Sinopharm Chemical Reagent Co.,Ltd.,China (AR).nheptane(HT),n-dodecane and n-hexadecane were used as porogens and were supplied by Xi Long Chemical Co.,Ltd.,China(AR).Emulsion stabilizer polyvinyl alcohol 1788 (PVA) was supplied by Chengdu Kelon Chemical Regent Co.,Ltd.,China (AR).Azodiisobutyronitrile(AIBN)was supplied by Tianjing Guanfu Fine Chemical Research Center,China(AR)as the initiator.
2.2.Preparation of porous polymeric microspheres
2.2.1.Preparation of polymers
Polystyrene (PS),polymethyl methacrylate (PMMA),polymethyl methacrylate co styrene (PMMA-ST),poly methyl methacrylate co butly acrylate(PMMA-BA)were synthesized by bulk polymerization.In a typical bulk polymerization,styrene and AIBN (2 wt%) were added to a three-neck round-bottom flask,followed by keep stirring at 250 r·min?1and reaction at 60°C for 1 h to obtain pre-polymer solution;and then pour the pre-polymer solution into a beaker to allow reaction at room temperature without agitation and heating.During the process,a large number of bubbles were observed in the viscous polymer solution because of implosion.After 30 min,the temperature of the solution decreased to room temperature and bulk PS was obtained.The recipes and procedures to prepare P(MMA-ST),PMMA and P(MMA-BA) were the same.For P(MMA-ST) and P(MMA-BA),the molar ratio of MMA/ST and MMA/BA were 5:1.
2.2.2.Preparation of porous polymeric microspheres
In a typical experiment,a mixture of PS(10 g),HT(6 g)and DCM(60 g)was decanted into a 250 ml three-neck round-bottom flask,where the mixture was admixed with 100 ml aqueous solution containing PVA at a concentration of 1.0 wt%.The resulting dispersion was immersed in a water bath at the room temperature and thoroughly vortexed at 500 r?min?1for 15 min.After that,the temperature of water bath was ramped up at 1°C·min?1,and is kept at 40°C for 30 min.The bubble phase thus generated,along with porous PS microspheres,was transferred to a beaker wherein a magnetic bar was kept stirring at 500 r?min?1.At the same time,hot water at 90°C was continuously flown into the beaker and interacted thoroughly with the bubbles.The heated water allowed DCM to evaporate and removed HT.Porous polymer microspheres were obtained after defoaming,washing,filtration and vacuum drying.In order to validate the universality of the method,PMMA,P(MMA-ST),P(MMA-BA)and PLA were used to replace PS;TCM and 80 wt%DCM+20 wt%EA were used to replace the volatile organic solvent DCM;n-dodecane and n-hexadecane were used to replace HT to prepare porous polymeric microspheres.
In order to investigate the advantages of being time-saving of preparing polymeric microspheres in foam phase compared with that in water phase.PS polymer microspheres were prepared in water phase.The recipes and procedures to prepare PS microspheres in water phase were the same to that in foam phase.The only difference is that the temperature of the water bath to allow the evaporation of DCM was keeping at 20°C for 4 h.In order to investigate the advantages of being high-yield especially at high oil/water ratios,the oil water ratio was varied to 3:1,2:1,1:1,1:2 by keeping the oil phase at the same and changing the amount of aqueous phase.In foam phase,at the oil/water ratio of 1:1 and 1:2,an airflow at 100 ml·min?1was continuously pumped into the reactor to increase oil droplets'entrainment after all bubbles gone.
2.2.3.Phase diagram for a ternary system
In order to investigate the phase separation behavior of polymer solution and porogen,phase diagram for the ternary system(PS,DCM,nheptane)was studied.A series of polymer solutions(PS+DCM)with different mass ratios was prepared in sealed conical flasks,in which the mass ratio of PS:DCM was less than 0.31,since PS solution was so viscos when the mass ratio of PS to DCM was greater than 0.31.The prepared PS solution was placed into a water bath at 25°C with magnetic stirring at first and then n-heptane was added to PS solution dropwise until the solution became cloudy.Stopped agitation and kept the solution static for 12 h to reach the thermodynamic equilibrium and to see the delamination between PS solution and n-heptane.If delamination was observed,the cloud point was marked as the phase separation point.Otherwise,n-heptane was continually added until seeing delamination.The mass concentrations of PS,DCM and n-heptane at each phase separation point were used to draw phase diagram.
2.2.4.Continuous production of porous polymeric microspheres in foam phase
The continuous production of PS polymer microspheres was carried out in a customized continuous reaction device as showing in Fig.1.Two reservoirs(Fig.1-(1,2))were used to store the oil phase(the mass ratio of PS∶DCM:HT=10:60:6)and the aqueous phase(1 wt%PVA aqueous phase)respectively.A 1000 ml three-neck round-bottom flask was immersed into a water bath as a reaction device(Fig.1-(5,6)),in which 500 ml 1 wt%PVA aqueous phase was warmed up to 45°C with a agitation speed of 500 r?min?1at first.Oil phase was pumped into the flask through a solvent-resistant pump at a feeding rate of 30 g·min?1.Foam phase started to flow out within 1 min after the feeding of oil phase,and then water phase started to feed at a feeding rate of 30 g·min?1by using a peristaltic pump,to keep that there was enough water phase in the reactor.Simultaneously,hot water (90 °C,700 g·min?1)was pump to the outlet of the reactor to wash the foam.The continuous production was running for 10 min and foam collection was extended for another 5 min.All the foam was collected into the foam collector followed by defoaming,washing,filtration and vacuum drying to obtain PS microspheres.
2.3.Characterization
The yield of porous polymeric microspheres was calculated by weight.An optical microscope(LEICA DM4000 M,Leica Microsystems,Shanghai,China)and a scanning electron microscope(SEM,JEOL JSM-6500,Peabody,USA)were used to detect and display the surface morphology and the internal structures of polymeric microspheres.When investigate the time efficiency of the method to prepare polymeric microspheres in foam phase and water phase,the sample was added into a petri dish immediately with 1 wt%PVA solution and then used the optical microscope to detect if phase separation occurs in the oil droplets.The particle size of PS microspheres was measured by using a laser particle diameter analyzer(Microtrac S3500,York,USA),and use matlab to characterize the particle size distribution of the microspheres.
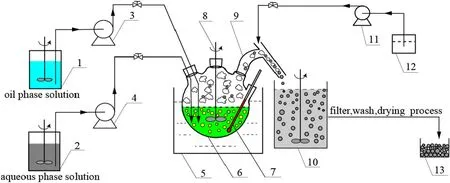
Fig.1.Schematic diagram of the continuous process to prepare porous polymeric microspheres(1,2-revisers;3-a solvent-resistant pump;4,11-a peristaltic pump;5-a water bath;6-a reactor;7-a thermometer;8-stir;9-the foam outlet;10-the foam collector;12-hot water;13-filter).
3.Results and Discussion
3.1.The method of preparation of polymeric microspheres in foam phase
Fig.1 and Video-1 present the preparation of porous polymeric microspheres by solvent evaporation in foam phase and the evolution of oil droplets to porous polymeric microspheres.In this system,PS,DCM and HT were used representatively as the polymer,the organic volatile solvent and the porogen respectively.They were thoroughly mixed at the beginning,enabling the discrete phases to disperse into an aqueous phase that contained an emulsion stabilizer(PVA),leading to oil/water(o/w)dispersions.The organic volatile solvent(DCM)was evaporated at the elevated temperature.The evaporating organic solvent can separate the polymer and the porogen into different phases[3,22].The evaporation of organic volatile solvent is a key step in this study.The primary purpose of increasing temperature is to generate air bubbles to form a foam phase,which,in turn,creates a pull-up force to entrain the oil droplets through the evaporation of the volatile solvent.Importantly,these steps-generating air bubbles,entraining the oil droplets and separating phases-are closely interrelated.The temperature increment needs to be moderate and the maximum of the temperature should be maintained slightly above the boiling point of the volatile solvent.If the temperature increment is too slow,air bubbling would be weak;if it is too fast,air bubbles may burst.Moderate air bubbling is critical in this method and may be achieved at a low temperature in an aqueous phase.The magnetic stirring contributes to the formation a foam phase.Oil droplets are generated and stabilized by an emulsion stabilizer in the foam phase.Phase separation is accelerated to create porous structure.Since the place for phase separation shifts from water phase to foam phase,the method of preparation of polymeric microspheres in foam phase may have several advantages compared with the preparation process in water phase.Specifically,the evaporation rate of volatile solvent in foam phase is significantly higher than that in aqueous phase,as phase resistance at the gas–gas interface is lower than that at the gas–liquid interface [32],and thus oil droplets into the foam phase drove to separate the phases quickly,so it should be timesaving.Secondly,bubbles entrains oil droplets followed by acting as barriers to separate them in foam phase so that less coalescence is going to happen,which may lead a high production yield,especially at high oil/water ratios.Thirdly,if air bubble generation is continuing,it may enable a continuous production process for polymeric microspheres[29].
3.2.Time-saving
As oil droplets were just entrained into foam phase,they were transferred into a petri dish instantaneously.Numbers of white microparticles was observed under the transmission light(Fig.2(a)which indicates that the micro-particles were in solid state instead of oil droplets.The white micro-particles was magnified and polymer was observed to separate phase with porogen at the outer surface since the organic volatile solvent evaporated quickly(Fig.2(a-0min)).When oil droplets were kept in foam phase for 3 min,the resulted polymer microspheres were in porous structures(Fig.2(a-3min)).It indicates a complete phase separation between polymer and porogen.The results revealed that the formation of polymeric microspheres by solvent evaporation in foam phase was finished within 3 min.In water phase,it may take a long time to form polymeric microspheres.In our experiment,when oil phase which was just suspended in water phase was transferred into a petri dish instantaneously,most of them were in oil state;only few ones had phase separation since they were at the interface and contacted with air to drive phase separation(Fig.2(b-0min)).The oil droplets maintained the same status even after 2 h'agitation in water phase at 20°C(Fig.2(b,b-2h)),in which they were transparent under the transmission light.Finally,solidified PS microspheres were obtained at 4 h(Fig.2(b-4h)).Lin[22,33]also reported that few hours were needed to finish the preparation of polymeric microspheres in water phase.Therefore,compared with the method to prepare polymeric microspheres in water phase,the method in foam phase was showing an outstanding feature on time-saving.The main reason is that the evaporation rate of the volatile solvent in foam phase is significantly higher than that in water phase,as phase resistance at the gas–gas interface is much lower than that at the gas–liquid interface[32],and thus an advantage of time-saving is showing.The advantage of time-saving in preparation of polymeric microspheres in foam phase is illustrating in Fig.3.
3.3.High-yield
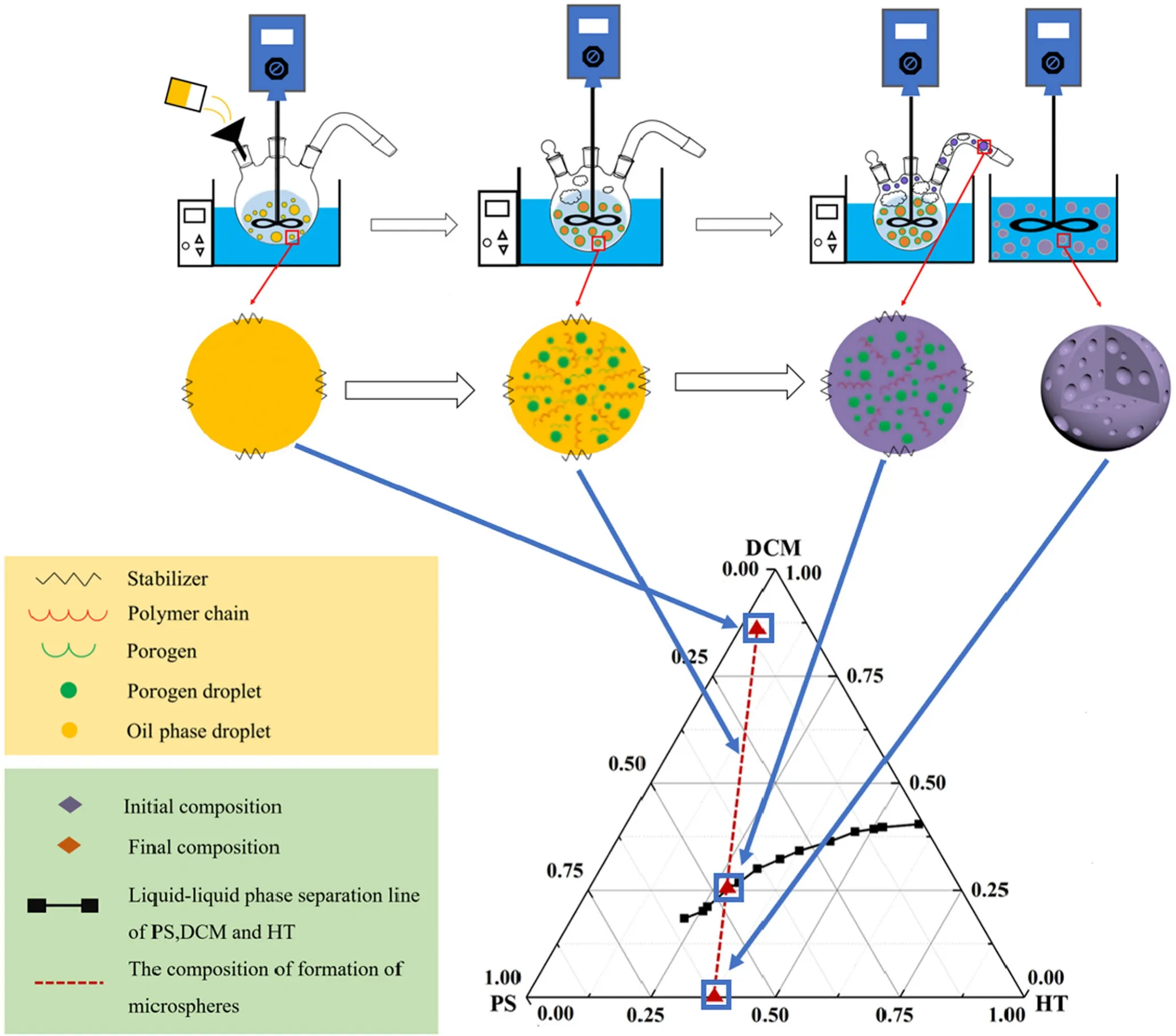
Fig.2.Formation mechanism diagrammatic sketch of porous microspheres prepared by foam phase
The advantage of high-yield in preparation of polymeric microspheres in foam phase is illustrating in Table 1.In foam phase,the obtained polymeric microspheres were in porous structures.At the oil/water ratios of 3:1 to 1:1,the yield of porous polymeric microspheres was approximate 50 wt%.When the oil/water ratio decreased to 1:2,the yield increased to (64 ± 3) wt%.An air flow was continuously pumped into the reactor after no bubble generating in the reactor at the oil/water ratios of 1:1 to 1:2,the left oil droplets was entrained by the new air bubbles and the total yield increased up to approximate 95 wt%.In water phase,it was unable to prepare polymeric microsphere at oil/water ratios of 1:1 to 3:1,because of the coalescences of oil droplets at such high oil/water ratios.At the oil/water ratio of 1:2,(63±2)wt% solid polymeric microspheres were obtained.Therefore,the method to prepare polymeric microsphere in foam phase showed an advantage on production yield of polymeric microspheres compared with that in water phase,especially at high oil/water ratios.The main reason is that along with the bubbles generating oil droplets were gradually entrained into the foam phase and separated by bubbles,leading less oil droplets'collisions and less particle aggregations.
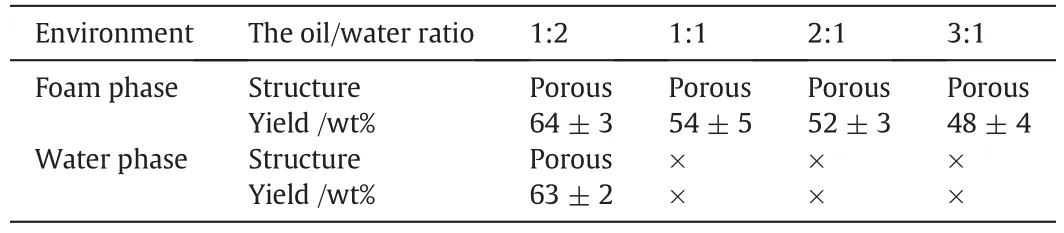
Table 1 The properties of polymeric microspheres prepared in foam phase and water phase
3.4.The continuous production of polymeric microspheres in foam phase
The continuous production of polymeric microspheres was carried out in a customized reaction device by continuous pumping oil phase and water phase into the reactor.Foam phase gradually generated and flowed out from the outlet of the reactor(Fig.3(a)and Video-2).Porous polymer microspheres were obtained after collecting the bubble phase followed by defoaming,washing,filtration and drying.As showing in Fig.3(b),the prepared PS microspheres are porous.The yield of the continuous production of porous PS microspheres is up to(91±4)%in 10 min'continuous running.This work validates that the method to prepare porous polymeric microspheres in foam phase is capable to develop into a continuous process.Although polymeric microspheres can be prepared continuously by using micro-fluidic techniques,delicate micro-fluidic devices are required and the production yield is relatively low.Therefore,this new method is capable to prepare polymeric microspheres in foam phase in large-scale,can be used potentially in industrial continuous production.
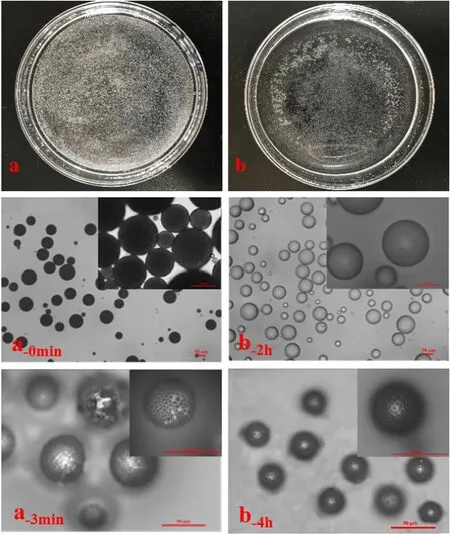
Fig.3.The images of samples in foam phase(a)and water phase(b);the optical microscope images at 0 min for foam phase sample(a-0min )and at 2h for water phase sample(b-2h )(leica transmission);the optical microscope images at 3 min for foam phase sample(a-3min )and at 4 h for water phase sample(b-4h )(leica transmission).
3.5.The universality of preparation of polymeric microspheres in foam phase
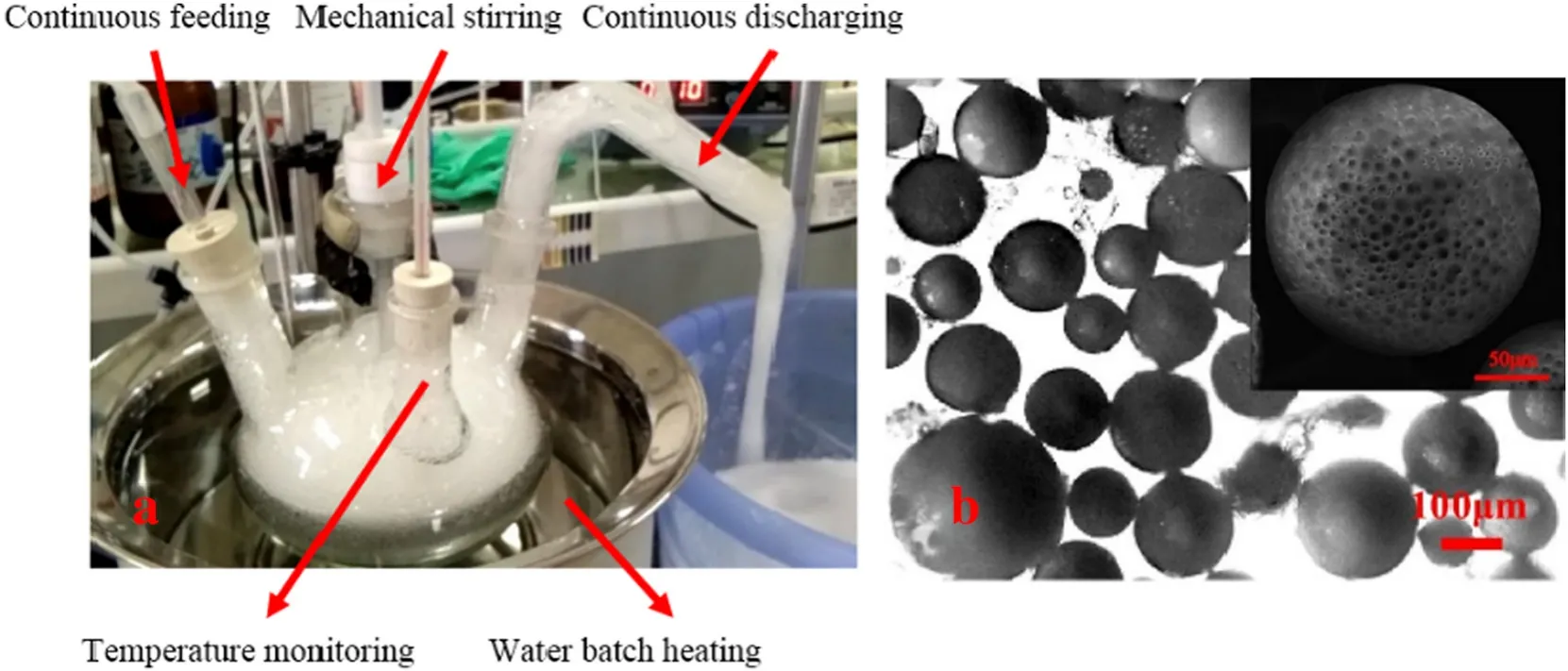
Fig.4.The continuous production of polymeric microspheres(a)and the obtained porous PS microspheres(b).
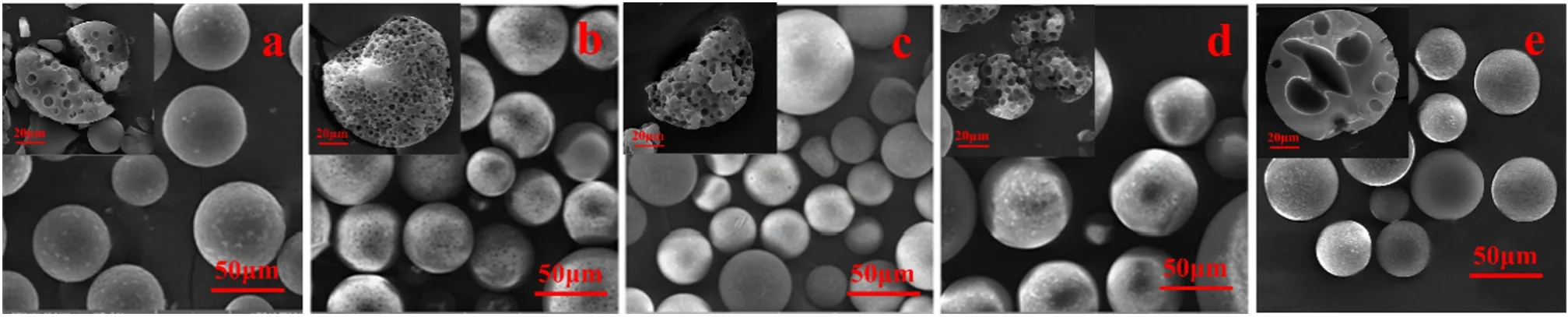
Fig.5.Porous PS(a),P(St-MMA)(b),PMMA(c),P(MMA-BA)(d)and PLA(e)microspheres prepared in foam phase.
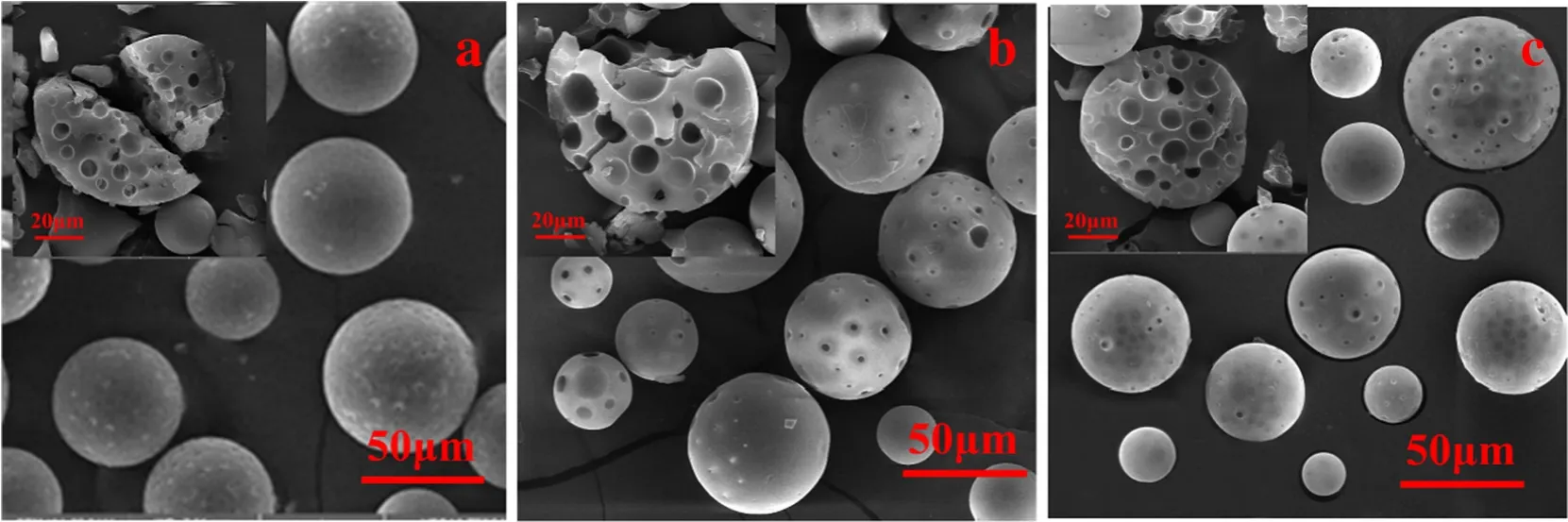
Fig.6.Porous PS microspheres prepared by using DCM(a),80%DCM+20%EA(b)and TCM(c)as the volatile organic solvent in foam phase.
Although the method to prepare porous polymeric microspheres in foam phase has several outstanding features compared with that in water phase,it is a development on the traditional solvent evaporation method[3,22,33].Therefore,it should be universal to non-crosslinked polymer,in which the volatile organic solvent and the porogen should be a good solvent and a non-solvent to the polymer respectively and they should be miscible with each other.In order to validate the universality of the method,various non-crosslinked polymers,volatile organic solvents and porogens were used to prepare porous polymer microspheres,as showing in Figs.4–8.Fig.5(a–e)presents PS,P(St-MMA),PMMA,P(MMA-BA) and PLA microspheres prepared by using the method.Fig.6(a–c)shows PS microspheres prepared by varying the volatile organic solvents including DCM,80%DCM+20%EA and TCM.Fig.7 (a–c) illustrates PS microspheres prepared by varying the porogens including n-heptane,n-dodecane and n-hexadecane.As can be seen,all the prepared microspheres were in porous structures.Since porogen was introduced in this work,macropores were formed predominantly through χ-induced syneresis [1,19].In the previous work,using non-solvents as porogens was reported to have drawback of forming a dense and impermeable polymer layer on the surface[3,34].However,macropores without a dense layer were observed in this study (Figs.5–7).Although highly nonpolar porogen such as nheptane,n-dodecane and n-hexadecane is hardly present on the o/w interface,it is miscible with the volatile organic solvent and can be easily migrated to the outer shell during the volatile organic solvent evaporation.Therefore,macropores were observed on the surface of polymeric microspheres.Fig.8 is the particle size and distribution diagram of the product microspheres.From this diagram,it can be seen that changing the types of polymers,solvents and porogens has little effect on the average particle size and distribution of the microspheres.The results indicate that the method has the universality as the traditional solvent evaporation method(Fig.4).
Specifically,obvious differences have been seen on the pore number and the pore diameter by varying the porogen(Fig.8).The pore number increased and the pore diameter decreased by increasing the hydrocarbon chain length of the porogen.The solubility parameters (δ) of n-heptane,n-dodecane,n-hexadecane and PS are 7.45(cal·cm3)1/2(1 cal=4.1868 J),7.82(cal·cm3)1/2,7.99(cal·cm3)1/2and 9.00(cal·cm3)1/2respectively[35].The difference on solubility parameter(Δδ(Δδ=δpolymer-δporogen))indicates the compatibility between polymer and porogen.Large corresponds to the poor compatibility,causing early phase separation.The earlier phase separation occurred,the large aggregation of the porogen droplets would be obtained [36].Thus,less pore number and large pore size were observed when the difference on solubility parameters is large.
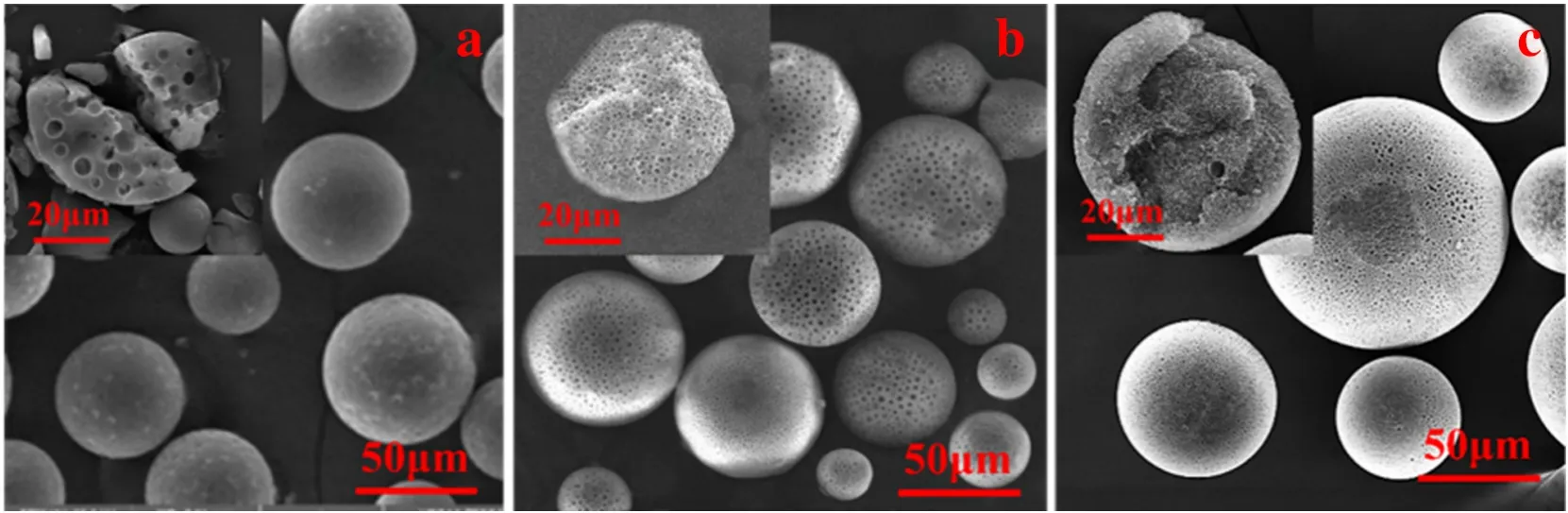
Fig.7.Porous PS microspheres prepared by using n-heptane(a),n-dodecane(b)and n-hexadecane(c)as porogens in foam phase.

Fig.8.The average size and distribution of microspheres were prepared by different porogen(a),polymers(b),solvent(c).
4.Conclusions
In this study,we reported a method of preparing porous polymeric microspheres in foam phase,which is a development on the traditional emulsion solvent evaporation method.The new method to prepare porous polymeric microspheres in foam phase has several outstanding features,including being time-saving,of high production yield and able for continuous production.It validated that formation of microspheres can be finished within 3 min in foam phase with a high production yield up to approximate 95 wt%especially at high oil/water ratios.The process was also developed to a continuous process and PS porous polymer microspheres were obtained with a production yield of(91±4)wt%.The method is universal to various non-crosslinked polymer,volatile organic solvents and porogens,which can be potentially used continuously on an industrial scale.
Acknowledgements
The study was financially supported by National Natural Science Foundation of China(22068018,21466016 and 51863011),Natural Science Foundation of Yunnan Province (2016FB024) and Yunnan Ten Thousand Talents Plan Young&Elite Talents Project.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2020.09.002.
 Chinese Journal of Chemical Engineering2021年1期
Chinese Journal of Chemical Engineering2021年1期
- Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Preparation and properties of a low-cost porous ceramic support from low-grade palygorskite clay and silicon-carbide with vanadium pentoxide additives
- Engineering practice and economic analysis of ozone oxidation wet denitrification technology
- Application of an immobilized microbial consortium for the treatment of pharmaceutical wastewater:Batch-wise and continuous studies
- A polypropylene melt-blown strategy for the facile and efficient membrane separation of oil–water mixtures
- Pyrolysis of single large biomass particle:Simulation and experiments
