Pyrolysis of single large biomass particle:Simulation and experiments
Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education,School of Energy and Environment,Southeast University,Nanjing 210096,China
Keywords:Pyrolysis Large biomass particle Simulation Heat/mass transfer
ABSTRACT Pyrolysis and heat transfer characteristics of single large biomass particle were investigated using threedimensional unsteady heat transfer model coupled with chemical reactions.The consumption of biomass and the production of products were simulated.Some experiments were designed to provide model parameters for simulation calculations.The simulation was verified by pyrolysis experiments of large biomass particle in a vertical tube furnace.The simulation results show the internal heat and mass transfer law during the pyrolysis of large biomass particle.When the biomass particle diameter is between 10 and 30 mm,for every 5 mm increase in particle diameter,the time required for complete pyrolysis will increase on average by about 50 s.When the pyrolysis temperature is between 673 K and 873 K,a slight decrease in the pyrolysis temperature will cause the time required for the biomass to fully pyrolyze to rise significantly.And the phenomenon is more obvious in the low temperature range.The results indicate that the numerical simulation agrees well with the experimental results.
1.Introduction
Biomass pyrolysis is an important method of biomass utilization and a significant step in the process of biomass combustion and gasification [1].As a common thermal process that converts raw biomass into secondary fuel in an anaerobic or anoxic environment,biomass pyrolysis is an extremely complex process,including a series of homogeneous and heterogeneous reactions,accompanied by mass,momentum and energy transfer.In addition,due to the high porosity,biomass is a poor conductor of heat.Especially in a large-scale biomass utilization device,the biomass particles usually have a large size,and the temperature rises slowly during pyrolysis.It seriously restricts the effective utilization of biomass.Therefore,it is important to thoroughly understand the pyrolysis behavior of large biomass particle.
Despite great efforts have been made on the studies on biomass pyrolysis,most of works have focused on strategies for fluidized bed and the study of small particles or straw,including zerodimensional and one-dimensional research methods [2–9].Some models have achieved higher accuracy for the combustion stage using two-dimensional and three-dimensional models [10–13].For example,Gomez et al.[14]considered the internal temperature gradient of the fuel particles during the thermal conversion of solid biomass,and obtained more accurate results.Mlonka et al.[15]used TG GC/MS to conduct a laboratory study on the effect of biomass particle size on pyrolysis and combustion,found that it had a great influence on the activation energy of combustion.Medrano et al.[16]established a mathematical model for the pyrolysis process of walnut shells under thermogravimetric balance and proposed a kinetic progressive conversion model (PCM) of major biomass components.Pham et al.[17]used analytical methods to study the unsteady temperature field of the rapid pyrolysis of bagasse and wood in a fluidized bed reactor and first studied the relationship between residence time and particle size.Reschmeier et al.[18]studied the pyrolysis process of wooden balls with a diameter of 6 mm in a fluidized bed,and obtained the thermal degradation kinetic information.
However,there are limited studies on pyrolysis of large particles of biomass.Particle size of biomass in practical industrial applications is relatively large,usually in the order of centimeters.Besides,pyrolysis of large particles is a complex process in which heat and mass transfer and chemical reactions within and between phases interact.Complex reaction process and numerous chemical changes have made it main barrier to systematically reproduce the physical phenomena of the pyrolysis process.In these few studies on pyrolysis of large biomass particle,some researchers have explored the process of pyrolysis of large biomass particle and developed mathematical models of biomass particle pyrolysis in a certain way.These models include the continuity of gas in the particles,the conservation of energy in the particles,and onestep and two-step kinetic reactions model.Pyle et al.[19]experimented on the pyrolysis process of single large particles,distinguished different particles controlled by kinetic reaction or diffusion according to Biot number (Bi) and pyrolysis number (Py),and established corresponding simplified models.Benkoussas et al.[20]studied the pyrolysis process of three types of flat,spherical and cylindrical thermally thin particles,and found that for a given heat flow,the size of the thermally thin particles and the thick hot particles were independent of the moisture content.Bryden and Shen [21,22]combined experiments and models to study the pyrolysis process of water-containing particles,which showed that the pyrolysis rate of the particles was mainly affected by the particle surface temperature and particle diameter,followed by moisture.Sadhukhan et al.[23]employed an implicit Finite Volume Method (FVM) with Tridiagonal Matrix Algorithm (TDMA)to solve the energy conservation equation and established a transient model for pyrolysis of spherical and cylindrical particles to predict the temperature rise and weightlessness process within the particles.Biswas et al.[24]established models for thermallythick particles,modeled cylindrical and spherical particles according to different characteristics,which was obtained through parametric studies.
Related research shows that in the pyrolysis reaction of single biomass particle,heat transfer and reaction kinetics are the most important rate control mechanisms.Current simulations mainly have focused on the influence of a certain physical property on pyrolysis or the development of a more accurate algorithm to reduce the simulation error.However,it rarely involves detailed processes inside a particle,such as the temperature distribution and composition distribution inside the particle.Our research has focused on biomass consumption,volatility analysis,bio-char generation,temperature transfer and material transfer inside a particle.It can provide us a further understanding of the internal heat transfer mechanism of biomass pyrolysis process,and a clearer understanding of how external conditions affect pyrolysis.Naturally,it can also offer experience for the development of more accurate numerical models,and shed light on the design of biomass pyrolysis reaction devices in practical applications.
In this work,a three-dimensional unsteady state model that simulates the pyrolysis of large biomass particles is built.The model uses a one-step reaction kinetic model to describe the pyrolysis reaction,and defines a set of Euler variables to simulate the main parameters of the gas phase and solid phase.The dynamic convective heat transfer coefficient including other influencing factors is used to consider the convective heat transfer between the particle surface and the outside environment.The experiments on the pyrolysis of single large biomass particle were used to verify the model.Importantly,this study details heat transfer,interaction,and internal mass and energy diffusion through the solid and gas phase.The model established can predict the rate of pyrolysis and gasification,and predict the temperature distribution and product formation.And the model was used to study the influence of the pyrolysis temperature and particle size on the biomass pyrolysis process.
2.Material and Methods
2.1.Wood model
The biomass used in the experiment was mainly Gugertree ball particles,which were purchased from Zhejiang Province,China.The raw wood balls were processed to obtain three kinds of experimental raw materials.Gugertree powder was ground for ultimate analysis and thermogravimetric experiment.And the results provide data for subsequent simulations.Wood fibers was obtained with hammers and scissors which have a diameter of 2–3 mm for proximate analysis.The main type used in the experiment is spherical particles,10–30 mm in diameter,used for pyrolysis experiments.
The instrument used for ultimate composition analysis was the Vario MICRO elemental analyzer of Nanjing University,and the measurement results according to the general rules of the JY/T017-1996 elemental analyzer method are shown in Table 1.The instrument used for proximate composition analysis was the KSL-1200X-J Muffle furnace of Southeast University.The measured results according to international measurement standards are shown in Table 1.
2.2.Experimental setup
The experimental device can realize the pyrolysis of particles at 673–873 K and monitor and collect the product in real time.A schematic of the experimental setup is shown in Fig.1.In order to achieve anaerobic pyrolysis of biomass,high-purity nitrogen is used as a protective gas to flow through the reactor through a control valve,the actual flow rate is 350 ml·min-1.The reactor is made of quartz and controls the temperature through an electric heating device.A telescopic device is used to control the dropping and lifting of the biomass particles.Two thermocouples are installed in the center and 1/2 radius of the pellet to capture temperature information.
After the particles were dried overnight in the drying box,two holes with a diameter of 0.5 mm were drilled in the wooden balls for the installation of thermocouples and fixed with a small amount of glue,shown in Fig.1.The biomass particle is suspended on the upper part of the reactor when the furnace body heats up.After the reactor warms up to the set temperature,the particle is quickly dropped and heated.At the same time,the temperature collection device starts to collect temperature information.The products produced by pyrolysis are passed into an ice-bath condenser for condensation.After that,the bio-oil is left in the conden-sation tube,and the remaining material is collected into the air bag after passing through the absorbent cotton silicone tube.The gas in the air bag is passed into a gas analyzer connected to a computer for gas analysis to determine the type and content.To ensure complete pyrolysis of the particles,the experiment was continued for 20 min.In fact,the particles pyrolysis were finished at about 5 min for particle which has a diameter of 20 mm.
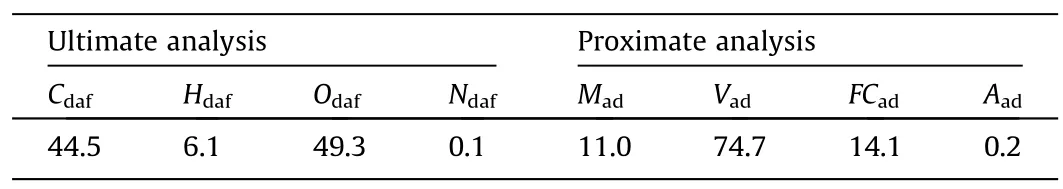
Table 1 Proximate and ultimate analysis of the biomass (wt%)
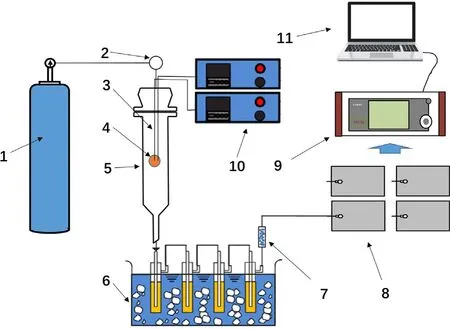
Fig.1.Schematic diagram of a single biomass pyrolysis system.(1) nitrogen;(2)flowmeter;(3) thermocouple;(4) biomass pellet;(5) reactor;(6) ice bath condenser;(7) absorbent cotton silicone tube;(8) air bag;(9) gas analyzer;(10)temperature acquisition device;(11) computers.
In the experiment,the Gugertree particles with diameter of 20 mm were first selected for pyrolysis at a temperature of 773 K and a nitrogen flowrate of 350 ml·min-1as the reference experiment.And then,16 identical wood particles were chosen to pyrolysis for the study of weight loss during heated.After the particle was put in and pyrolyzed after the reactor was heated to 773 K.After 30 s,the first particle was quickly taken out and cooled quickly,meanwhile,insulated from oxygen to avoid subsequent reactions.The second particle was also laid down after the reactor was heated to 773 K,and after 60 s the particle was quickly taken out and cooled quickly;16 identical particles were sequentially pyrolyzed for 30,60,90,120,150,180,210,240,270,300 330,360,390,420,450 and 480 s.The mass loss and pyrolysis residue characteristics of each particle were studied and compared with subsequent numerical simulation data.
2.3.Numerical model
In this study,the commercial software FLUENT was used for research and analysis,and the continuity equation,incompressible momentum equation,energy equation and composition equation were taken as the governing equations.The standard k-ε model of the momentum equation is used as the turbulence model.The discrete ordinates (DO) model is used to simulate heat transfer and treat biomass particles as gray body.Eddy Dissipation Model is used to simulate the overall chemical reaction.A simple algorithm to deal with pressure–velocity coupling is proposed.The density is calculated by the ideal gas relationship,and the physical properties such as thermal conductivity and heat capacity of particles and products are calculated by piecewise linear interpolation.
An important step in the establishment of particle model is to simplify the actual process.The main purpose of this is to capture the main influences and ignore the secondary ones.In the present study,the pyrolysis reaction loss is expressed as an overall onestep reaction.Volatile matter and coke are generated by particle pyrolysis.The model used to simulate the biomass pyrolysis is based on the following main assumptions:
a.Particle is considered not to shrink during pyrolysis.
b.Thermophysical properties of wood particles vary linearly with the local temperature such as thermal conductivity and heat capacity.
c.Heat transfers from the hot gas to the surface of particles by a combination of heat convection and radiation.
d.Structure of wood particles is assumed homogenous despite the fact that the particles are anisotropic.
e.The biomass particle is considered as a porous medium.
f.Char heterogeneous reactions occur from the outer layer of the particle to the core,resulting in a shrinking core regime.
Under the above assumptions the equation for conservation of mass,or continuity equation,can be written as follows:

Eq.(1) is the general form of the mass conservation equation and is valid for incompressible as well as compressible flows.The source Smis the mass added to the continuous phase from the dispersed second phase(for example,due to volatilization of biomass)and any user-defined sources.
Two-equation turbulence models allow the determination of both,a turbulent length and time scale by solving two separate transport equations.The turbulence kinetic energy,k,and its rate of dissipation,ε,are obtained from the following transport equations:
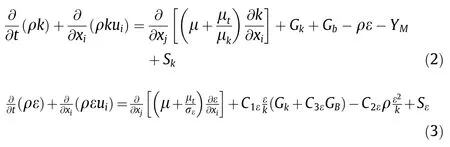
where Gkrepresents the generation of turbulence kinetic energy due to the mean velocity gradients,Gbis the generation of turbulence kinetic energy due to buoyancy,YMrepresents the contribution of the fluctuating dilatation in compressible turbulence to the overall dissipation rate.are constants.σkand σεare the turbulent Prandtl numbers for k and ε,respectively.Skandare user-defined source terms.
The main heat transfer path in the simulation is radiation,in our simulation,the discrete ordinates (DO) model is used to simulate radiant heat transfer.The radiative transfer equation (RTE) for an absorbing,emitting,and scattering medium at positionin the directionis
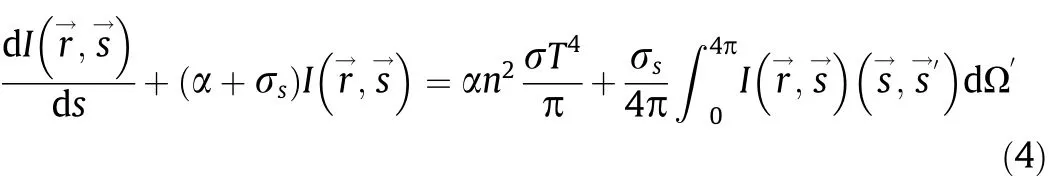
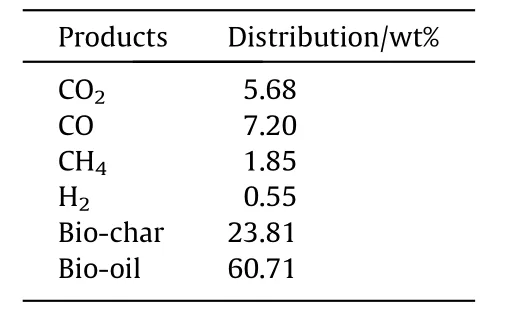
Table 2 Distribution of pyrolysis products
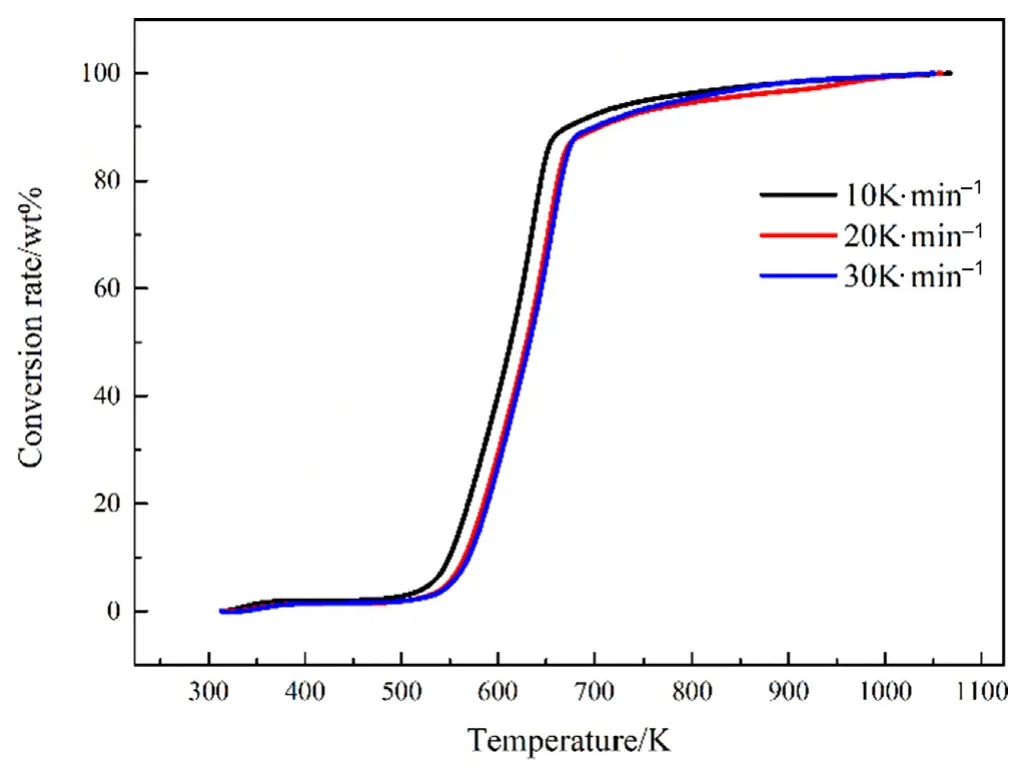
Fig.2.Conversion rates at different heating rates.
The discrete ordinates radiation model solves the radiative transfer equation for a finite number of discrete solid angles,each associated with a vector direction fixed in the global Cartesian system.The DO model considers the radiative transfer equation in the directionas a field equation:

CFD techniques have been extensively used in combustion and pyrolysis simulations for many different systems.Gas dynamics are typically modelled using conservation equations of variables such as pressure,momentum,energy,turbulence,radiation and chemical species.Gas flow inside the biomass pores is modelled using a porous media approach with a momentum source based on Darcy’s law,Since the Reynolds number inside the pore structure is low and the permeability of biomass and carbon is low,the inertia term is ignored.As the temperature increases and the reaction progresses,the density of solid phase biomass decreases gradually.At the same time,the density of carbon in the solid matrix gradually increased,and CO2,CO,CH4,H2,H2O,tar and a small amount of O2separate out from the particle surface.
3.Results and Discussion
3.1.Model validation
Firstly,some experiment was designed to obtain some parameters needed for simulation,including TG/DTG experiment and pro-duct analysis experiment.The products obtained by multiple pyrolysis were analyzed and tested.With the experiment results,the obtained product was analyzed and listed in Table 2.The results in the table are the average results obtained from multiple experiments.According to previous proximate analysis experiments,in addition to the substances listed in the table,there is also an ash content of 0.2%.
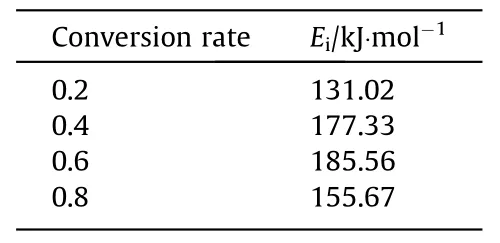
Table 3 Kinetic data used in the model
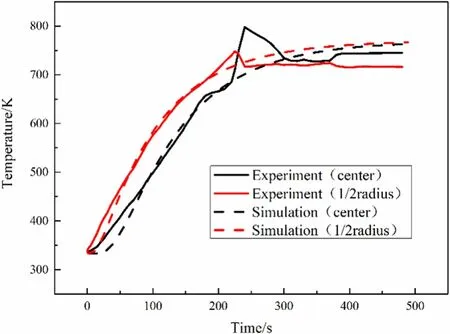
Fig.3.Particle internal temperature of experiment and simulation.
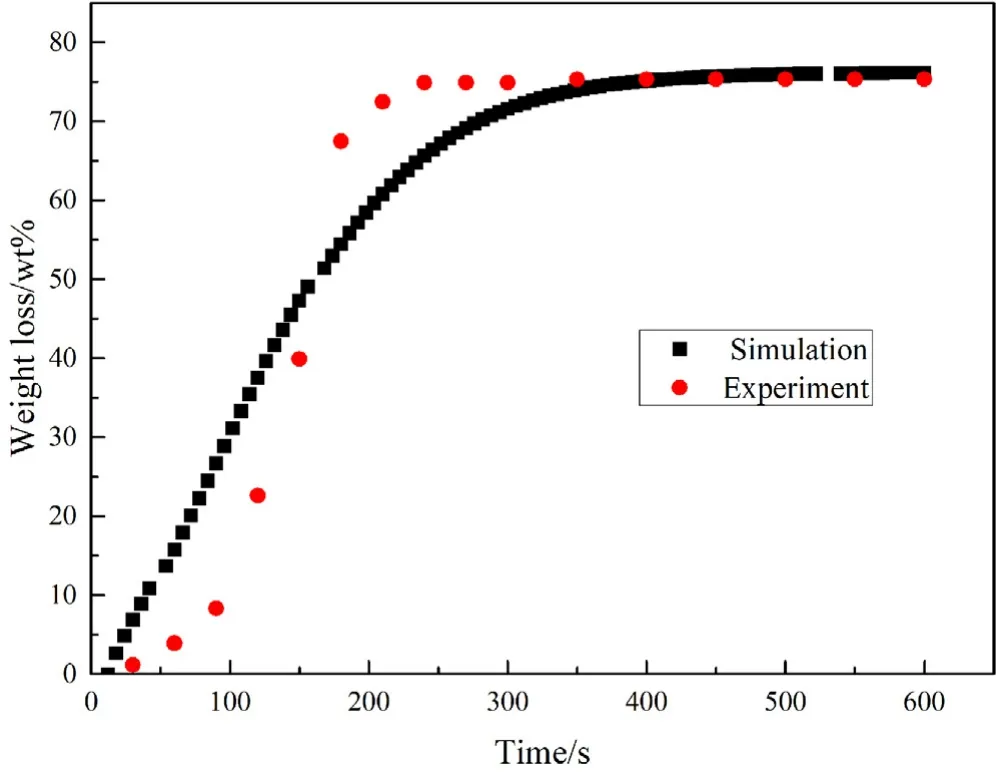
Fig.4.Particle weight loss ratio of experiment and simulation.
From the above results combined with the results of ultimate analysis,the chemical equation of one-step turnkey reaction for pyrolysis of biomass can be obtained:
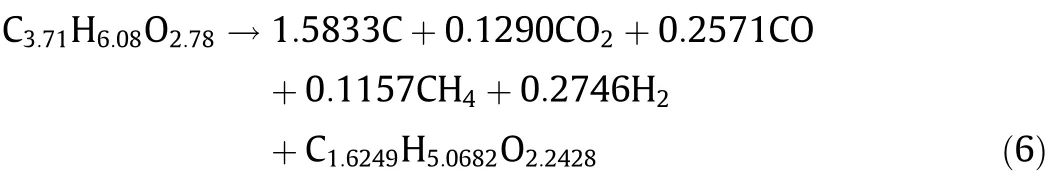
For the reason that the composition of bio-oil is complex,and there is no need to analyze the content of each component,so bio-oilcanbedirectlyregardedasasubstance C1.6249H5.0682O2.2428.Such treatment is more conducive to simplify the difficulty of simulation calculation.
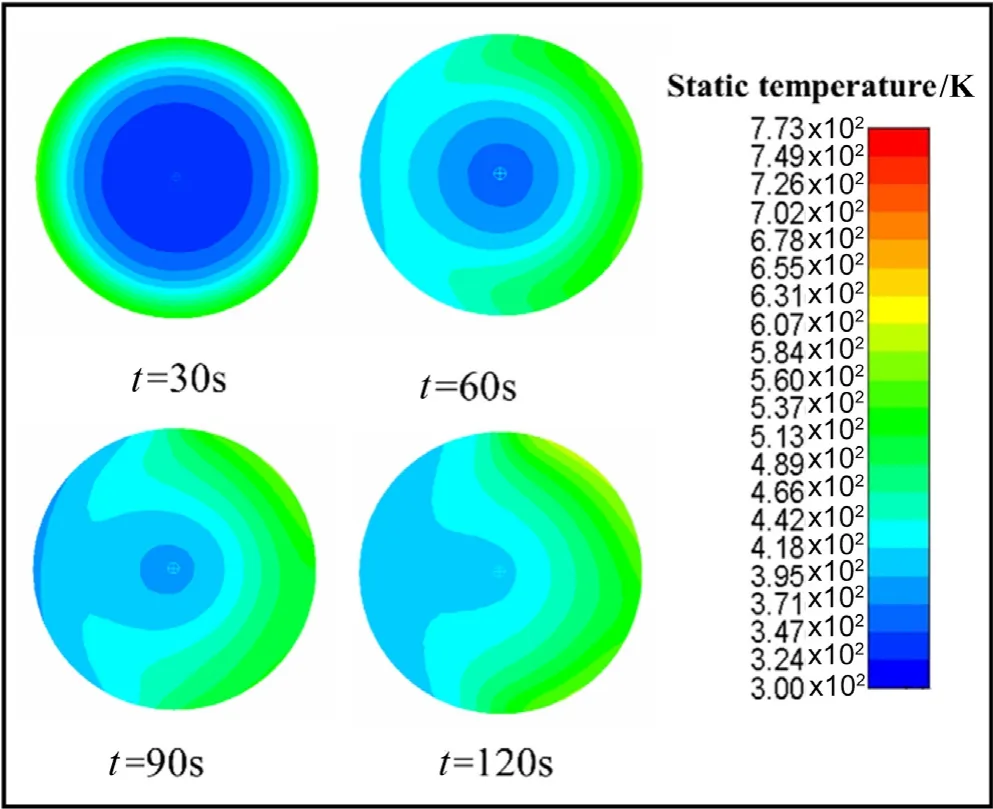
Fig.5.Static temperature in the particle.
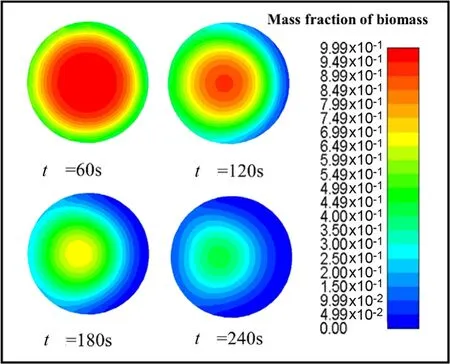
Fig.6.Mass fraction of biomass in the particle.
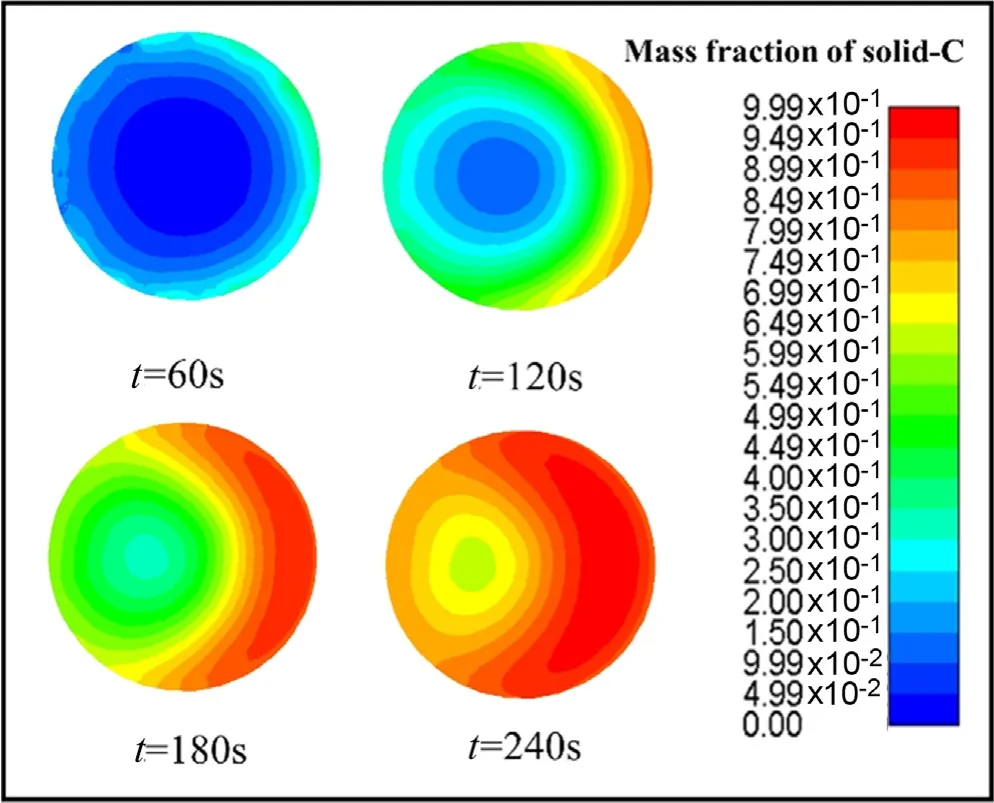
Fig.7.Mass fraction of solid-C in the particle.
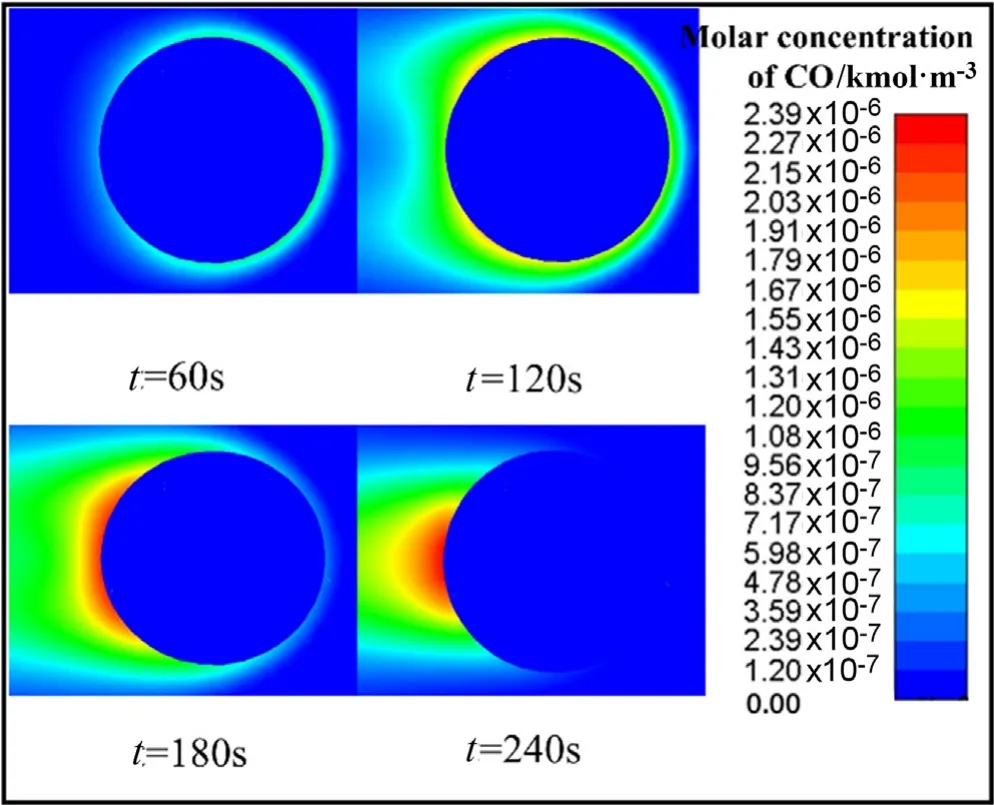
Fig.8.Molar concentration of CO in the particle.
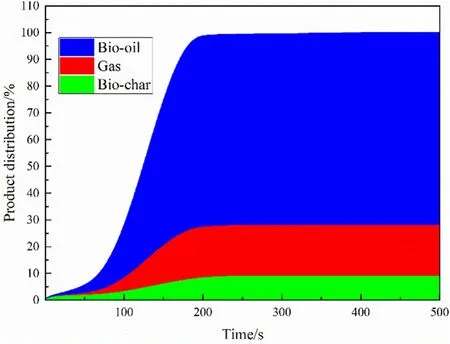
Fig.9.Proportion distribution of pyrolysis products of particle.
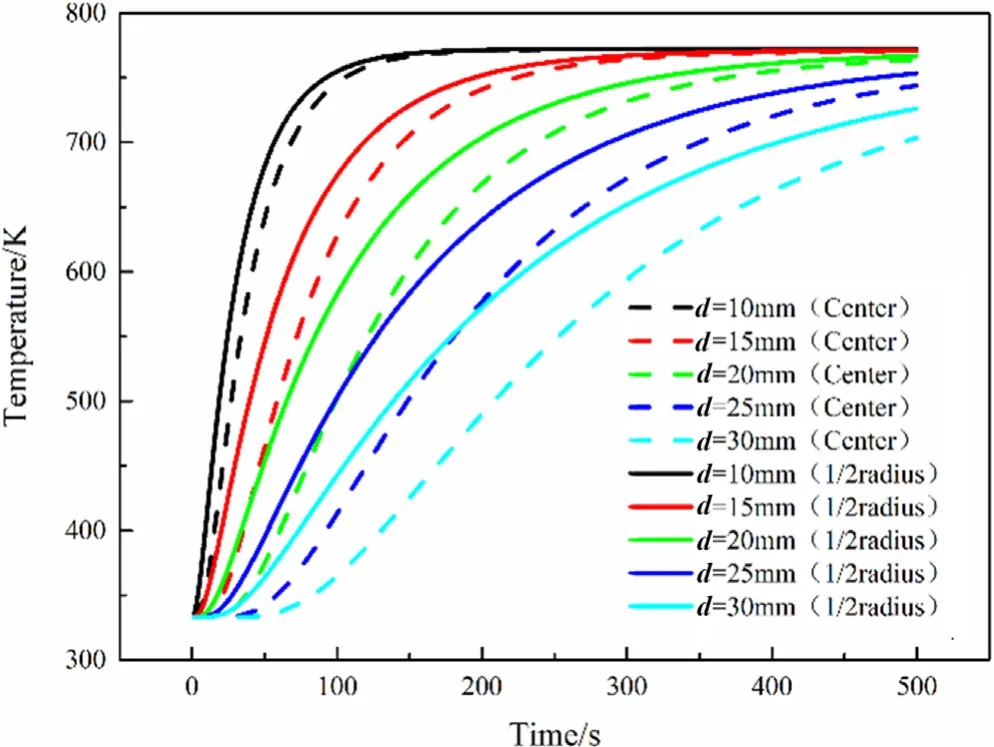
Fig.10.Temperature of particles with different diameters.
The process of biomass pyrolysis is approximately regarded as a first-order kinetic reaction.The activation energy can also be obtained without assuming a reaction mechanism.In the Fig.2,three thermogravimetric curves with a heating rate of 10 K·min-1,20 K·min-1,and 30 K·min-1are selected.When the conversion rate α=0.2,the calculated activation energy E=131.02 kJ·mol-1can be obtained.Similarly,activation energy and other kinetic factors of each stage can be obtained,as shown in Table 3.
In order to verify the accuracy and rationality of the model,the experimental results of the simulation and the experimental results were compared and verified.Figs.3 and 4 compare the time-varying temperature and weight loss rate of the particles predicted in this simulations with the author’s experimental results.It can be seen from the figures that under the conditions of a diameter of 20 mm and setting temperature of 773 K the simulation results show that the particle center temperature rises to 600 K at 150 s,and the particle center temperature slowly rises after 200 s of pyrolysis.At t=300 s,the volatiles of the particle are basically volatile completed and the weight loss rate basically does not change.In the final stage of the pyrolysis experiment,there will be a jump increase in temperature,which is particularly evident at the center of the particle.The reason for this phenomenon is that pyrolysis is a process that first absorbs heat and then releases heat,and is releasing heat at this time.Although this phenomenon is not well reflected in the simulation.The focus of this paper is on the temperature change and material evolution inside the particles,so the resolution and simulation of the reaction phase in this paper are not very accurate.And this is enough for some qualitative research.Of course,the different stages of this reaction are indeed worthy of further study.We will carry out relevant research next on this point.
In the comparison of weightlessness graphs,the experimental results show that pyrolysis occurs in a relatively concentrated period of time.Correspondingly,in the simulation,pyrolysis lasts longer and the rate is slower.All in all,the experimental and simulation results are in good agreement,and numerical simulation accurately reproduces the main process of pyrolysis of large particles.
3.2.Simulation results
In the high temperature environment,heat is transferred to the surface of solid particles through convection and radiation.When inside the particle,the main way of heat transfer is the heat conduction between solids and the radiation in the porous area.As the heat is continuously transferred from the surface of the particles to the interior of the particles,the temperature inside the particles gradually increases,and the increase in temperature causes a chemical reaction to occur,generating volatiles,solid carbon and small amount of ash.
The numerical results shown in Figs.5–10 exhibit that the changes of the main parameters of the particles during the simulated pyrolysis process,which helps us to visually understand the heat and mass transfer processes inside the particles.Fig.5 shows the process of heat conduction layer by layer after the particles are heated.At t=30 s,the particle center is only 360 K,and when t=90 s,the particle center temperature has risen to about 450 K.
As mentioned earlier in the paper,because the solid particle temperature transfer process is a gradual process that heat transfer from the outer surface to the inner layer,the internal area of the biomass particles during pyrolysis can be divided into three areas:the first area is the outer layer of the particle where the pyrolysis reaction is completed,and the bio-char generated after the reaction occupies this position;the second area is the middle layer of the particles that undergoing the pyrolysis reaction,and this position is the area where the reactants transition to the product;the third area is the center of biomass where no pyrolysis reaction occurred,which is also basically occupied by raw biomass temporarily.
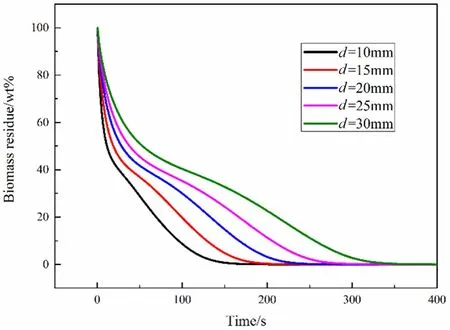
Fig.11.Biomass residue with different diameters.
The results reproduce these three areas very well in our simulation.Fig.6,Figs.7 and 8 show the consumption of primary biomass and the generation of two representative products when the 20 mm particles were pyrolyzed at a temperature of 773 K.At 60 s,some parts of the outer layer of the particle have not yet been pyrolyzed,but bio-char has generated,and the inner layer of the particle has not yet begun to pyrolysis,and only a small amount of non-condensable gas has precipitated around the particle.With the progress of time,the pyrolysis gradually transitions to the interior.At 120 s,most of the particles have been converted into products.There is almost no biomass remaining in the outer layer of the particles,and some of the biomass in the core of the particles has been transformed at high temperature.Volatile matter fills around the particles.When it reaches 180 s,most of the primary biomass in the particle has been pyrolyzed,and only a small amount of biomass is reacting at the center.
From Fig.7,it can be found that the solid components in the outer layer of the particle have been completely converted into bio-char which represented by solid-C in the simulation at 180 s.It is more obviously in the side near the air inlet,which at the right of the figure.The possible reason is that the convective heat transfer is more intense.And at this time,since there is almost no biomass residue on the right side,there is almost no volatile detected on the outside,this can be further confirmed from Fig.8.When the time advances to 240 s,the particle have basically been pyrolyzed,but there is still a small amount of raw biomass being pyrolyzed at the center of the particles.At the same time,only a small amount of volatiles are precipitated from the rear of the particles.
Fig.9 gives the relationship between the product distribution and the time of pyrolysis.It is slightly different from the experiment that the bio-oil production accounted for about 60% of all products.In the simulation,our bio-oil production reached 70%,while the solid product the ratio has dropped slightly,but the overall error is still within the allowable range.
3.3.Effect of particle size on pyrolysis
The size of the particles have great influence on the pyrolysis process of the biomass.Figs.10 and 11 show the changes in the radial temperature and solids residual rate of particles with different particle sizes when the pyrolysis temperature is 773 K.The particle size affects the temperature rise rate and pyrolysis time of the particles for the larger particles have the greater heat transfer resistance,which significantly affects the reaction rate.
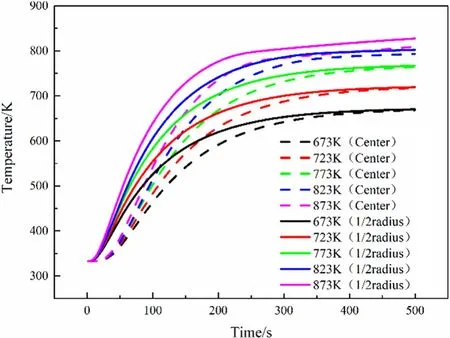
Fig.12.Inner temperature with different pyrolysis temperatures.
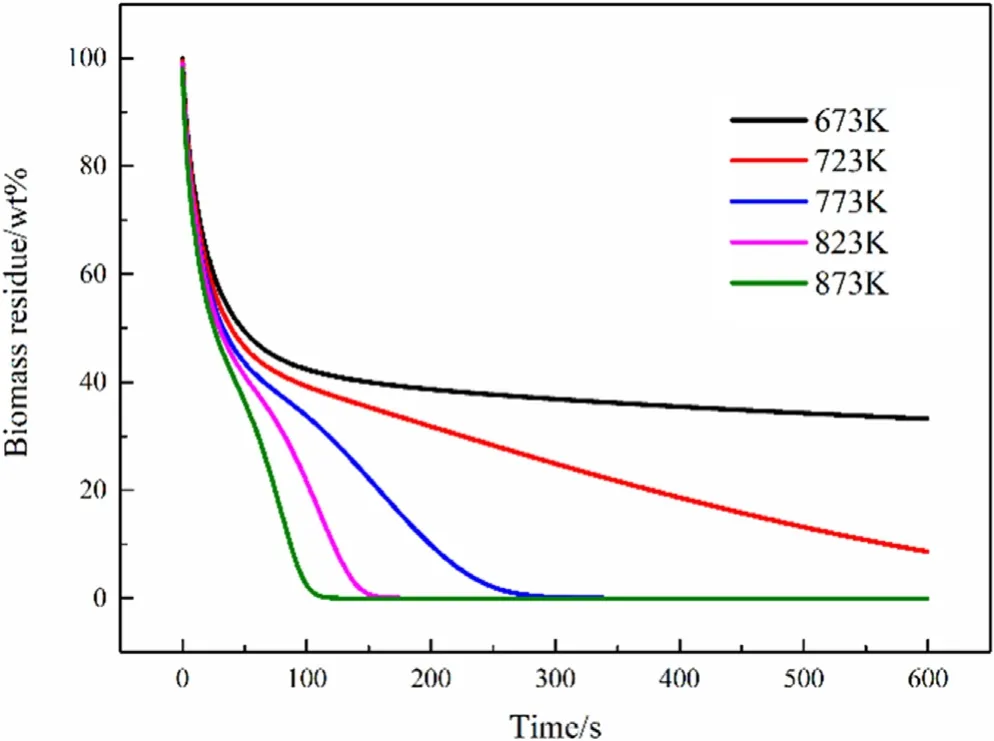
Fig.13.Biomass residue with different pyrolysis temperatures.
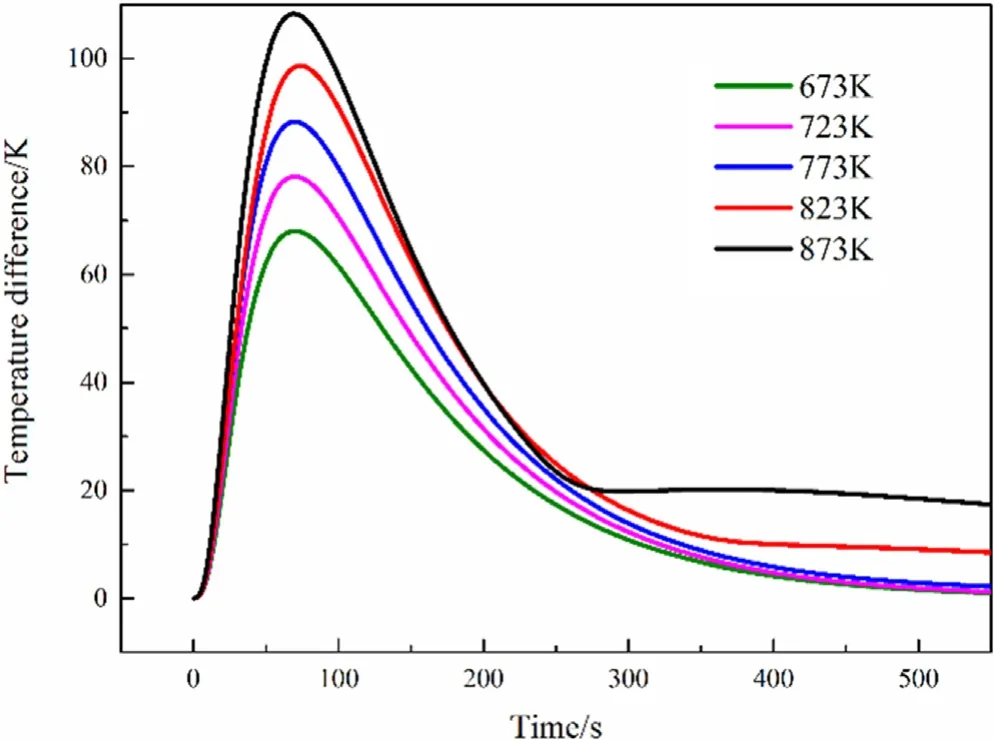
Fig.14.Inner temperature difference between inside and outside of particle.
The curves given in Fig.10 shows that during the pyrolysis of particles of different diameters,the temperature rise in different regions is significantly different.The particle with d=10 mm has been heated to about 770 K at about 100 s.For particle with d=20 mm,the particles only reach about 550 K within 100 s of the particle.And it takes more than 300 s to achieve the set temperature.For particle with d=30 mm even at 500 s,the temperature of 1/2 radius of the particle is just over 700 K,and the center of the particle is only about 550 K.In addition,it takes shorter time for the internal location to reach the same temperature for the smaller particles.
Fig.11 shows the pyrolysis time of the particles of five different sizes is about 140 s,180 s,230 s,280 s and 330 s respectively.For every 5 mm increase in particle diameter,the time required for pyrolysis will increase by about 50 s on average.Moreover,the primary biomass residue is almost zero with d=10 mm at 100 s,and almost all biomass has been converted into products.However,for biomass particles of d=30 mm,we can see that the pyrolysis process proceeds very slowly.Even after 300 s of pyrolysis,some biomass residue can still be found.It will take more time to simulate to the end of pyrolysis.It should be noted that the time required for complete pyrolysis of particle with d=25 mm is twice of particle with d=10 mm.Under the same external conditions,smaller particles reaching higher temperatures results in faster pyrolysis,and the size of the particles has a significant impact on the pyrolysis process.It will provide significant guidelines to help engineers and workers make decisions in reactor design and manufacturing strategy from the aspect of efficient pyrolysis.
In summary,the particle size has a great influence on the pyrolysis time of the particles.Within a certain range,for every 5 mm increase in particle diameter,the time required for pyrolysis will increase by about 50 s on average.
3.4.Effect of pyrolysis temperature on pyrolysis
Pyrolysis temperature is also critical to the biomass pyrolysis process.Figs.12–14 show the pyrolysis features of 20 mm diameter particles at different pyrolysis temperatures.
Although the set pyrolysis temperature is different,the time for the interior of the particles to be heated to the set temperature is not much different.On the contrary,the progress of the pyrolysis reaction shows a more obvious difference.Fig.13 shows that the pyrolysis time of particles with pyrolysis temperatures of 873 K,823 K,and 723 K is about 110 s,150 s,and 280 s,respectively.And after 500 s,the particles with a diameter of 25 mm and 30 mm still have not been completely pyrolyzed.Simulation shows that about 40% of the raw biomass remained after pyrolysis for 500 s at a temperature of 673 K.This result may be slightly different from the actual situation at low temperatures,which may be due to some problems during kinetic analysis.Table 3 shows that the kinetic parameters obtained by our experiment have a temperature of more than 600 K at a conversion rate of 0.2,so simulations at lower temperatures may not be as accurate as that at higher temperatures,but qualitative conclusions are still valid.
Higher pyrolysis temperature will also make the temperature difference between the center of the particle and the 1/2 radius of the particles more obvious.When the particle is pyrolyzed at a pyrolysis temperature of 873 K,the maximum temperature difference between the two locations can exceed 100 K,at 673 K the maximum temperature difference between the two places is about 70 K,which can been see in the Fig.14.But it was unexpected that the physical model is the same,and the peak temperature difference between the two points arrives at almost the same time.
In a word,the pyrolysis temperature has an obvious effect on the pyrolysis process of particles.When the particle size is a certain value,as long as the pyrolysis temperature is slightly increased,the time required for complete pyrolysis can be greatly reduced.
4.Conclusions
This paper presents a comprehensive model that simulates the thermal conversion of single large biomass particle.In this model,several sub-models of heat transfer,reaction and material diffusion represent the physical phenomena in the biomass pyrolysis process,and realize several variables representing biomass materials in the CFD environment.In a vertical tube furnace,pyrolysis experiment of large-grained Gugertree was carried out.The simulation results are compared with the experimental measurement results to evaluate the accuracy of the temperature and reaction progress predicted by the model.The heat transfer characteristics inside the particles during pyrolysis,as well as the product formation and biomass consumption rules were obtained.Considering the uncertainty of some physical properties of biomass,certain errors have occurred in the simulation,but overall the simulation reproduces the pyrolysis behavior well and shows behaviors that are difficult to be observed in experiments.Moreover,this paper has studied that the effects of particle size and temperature on the pyrolysis.It was shown that the biomass particle size has a significant effect on the pyrolysis process.Within a certain range,if the biomass size increases by 5 mm,the time required for pyrolysis will increase by about 50 s.It is worth noting that the effect of different pyrolysis temperatures on the heating time of the particles is not as obvious as expected.But different pyrolysis temperatures have a significant effect on the time required for complete pyrolysis.A slight increase in pyrolysis temperature can significantly reduce the time of pyrolysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grand No.2019YFD1100602);the National Natural Science Fund for Excellent Young Scholar of China (Grant No.51822604);the National Natural Foundation of China (Grand No.51676045);and the Natural Science Fund of Jiangsu Province for Distinguished Young Scholar (Grand No.BK20180014).
 Chinese Journal of Chemical Engineering2021年1期
Chinese Journal of Chemical Engineering2021年1期
- Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Preparation and properties of a low-cost porous ceramic support from low-grade palygorskite clay and silicon-carbide with vanadium pentoxide additives
- An efficient preparation of porous polymeric microspheres by solvent evaporation in foam phase
- Engineering practice and economic analysis of ozone oxidation wet denitrification technology
- Application of an immobilized microbial consortium for the treatment of pharmaceutical wastewater:Batch-wise and continuous studies
- A polypropylene melt-blown strategy for the facile and efficient membrane separation of oil–water mixtures
