Engineering practice and economic analysis of ozone oxidation wet denitrification technology
Yang Li,Defu Che,Chenglong Yang,Mingyu Yao,Tingwen Zhao,Kangli Fu,Hanchen Zhao
1 Xi’an Thermal Power Research Institute Co,Ltd,Xi’an 710032,China
2 State Key Laboratory of Multiphase Flow for Power Engineering,Xi’an Jiaotong University,Xi’an 710049,China
Keywords:Ozone Denitrification Wet process Engineering practice
ABSTRACT SO2 and NO emitted from coal-fired power plants have caused serious air pollution in China.In this study,a test system for NO oxidation using O3 is established.The basic characteristics of NO oxidation and products forms are studied.A separate test system for the combined removal of SO2 and NOx is also established,and the absorption characteristics of NOx are studied.The characteristics of NO oxidation and NOx absorption were verified in a 35 t·h-1 industrial boiler wet combined desulfurization and denitrification project.The operating economy of ozone oxidation wet denitrification technology is analyzed.The results show that O3 has a high rate and strong selectivity for NO oxidation.When O3 is insufficient,the primary oxidation product is NO2 .When O3 is present in excess,NO2 continues to get oxidized to N2 O5 or NO3 .The removal efficiency of NO2 in alkaline absorption system is low (only about 15%).NOx removal efficiency can be improved by oxidizing NOx to N2 O5 or NO3 by increasing ozone ratio.When the molar ratio of O3 /NO is 1.77,the NOx removal efficiency reaches 90.3%,while the operating cost of removing NOx per kilogram is 6.06 USD (NO2 ).
1.Introduction
Coal-fired power generation continues to hold dominant status in China’s energy supply.However,it is an environmentally harmful process that yields anthropogenic emissions,such as SO2,NOx,CO2,and PM2.5 [1,2].During the past several years (from 2014 to 2018) [3],coal-fired power plants in China have experienced ultra-low emission reconstruction,due to which stricter emission standards are implemented for the main pollutants in terms of SO2<35 mg·m-3,NOx<50 mg·m-3and particulate matter(PM) <10 mg·m-3[3–5].Traditional wet flue gas desulfurization(WFGD)technology that uses Ca-based absorbents for SO2absorption can achieve high SO2removal efficiency and holds potential for the synergistic removal of NOx.SO2and NOxare acidic gases with similar chemical properties.The combined removal of NOxin the existing wet desulphurization system can not only reduce the investment and operating costs of the existing pollution control technology,but also solve the corrosion and blockage problems caused by selective catalytic reduction (SCR),which is of great significance to advance the progress of pollution control technologies [6,7].
Since NO is water insoluble,it cannot be directly captured by the WFGD system.To this end,an efficient strategy could be the pre-oxidation of NO to higher oxidation states of NOx,such as NO2,N2O3,N2O4,N2O5,and NO3,after which,Ca-based sorbents can simultaneously remove SO2and these higher oxidation state NOxin the WFGD system.In this regard,many oxidants,such as H2O2[8–11]or additives,KMnO4[12],Fe compound [13–15],NaClO[16],NaClO2[17]and other metal compounds[18–20]have been studied to improve the removal performance of NO.However,the effect is not satisfactory as these oxidants have many drawbacks,such as high cost,poor selectivity,and secondary pollution problems [21,22].
O3is an oxidant used for flue gas denitrification.It has the advantages of strong selectivity and fast oxidation speed [23–25].At the same time,it can oxidize Hg and volatile organic compounds(VOCs) in flue gas to achieve integrated denitrification [26,27].Wang et al.studied the characteristics of NO oxidation by O3using simulated flue gas.The results showed that the oxidation efficiency was more than 90% when the reaction temperature was 150 °C[28].The reaction kinetics of NO oxidation by O3was simulated using Chemkin software.The results showed that the reaction time of O3and NO was only 0.01 s.The decomposition rate of ozone accelerated with the increase of temperature,and it loses its oxidation capability when the temperature exceeds the value of 400 °C[29].There are different products forms originating from the reaction,and include NO2,N2O5and NO3[30,31].Different forms of NOxhave different absorption efficiencies and ozone consumptions[32].At present,O3is generally generated by dielectric barrier discharge.In this process,only a small number of O2molecules are ionized to produce O3,which results in high energy consumption,high consumption of O2and high cost [33–35].These factors limit the popularization and application of O3oxidation denitrification technology.
Oxidation and absorption are two steps of ozone oxidation wet denitrification technology.Both the oxidation and absorption directly affect the efficiency of NOxremoval and operating economy.In this paper,the characteristics of NO oxidation and NOxabsorption using O3were studied in detail using laboratory experiments,and the results were validated in a 35 t·h-1boiler ozone oxidation wet combined desulfurization and denitrification process.The operating economy of ozone oxidation wet denitrification was further analyzed,which provided a reliable theoretical basis for the popularization and application of ozone oxidation denitrification technology.
2.Experimental
NO oxidation test system and NOxabsorption test system were constructed in the laboratory.The NO oxidation test system included a gas distribution system,a steam generation system,an ozone generation system,a reaction system and a detection system,as shown in Fig.1.The simulated flue gas was supplied using four cylinders,which contained 1.0 vol%NO balanced by N2,2.0 vol%SO2balanced by N2,99.999 vol%O2,and 99.999 vol%N2.The flow rate of each gas was controlled using an individual mass flow controller (MFC).Steam was produced using rapid evaporation.Feed water flow rate was controlled using a peristaltic pump and entered the heating tube (200 °C) having a diameter of 1 mm.Steam was mixed with other gases and entered the reactor.Ozone was generated using an ozone generator (3S-5A,Beijing Tonglin Technology Co.,Ltd.,China) using 99.999% O2as the gas source.Ozone flow rate was adjusted by controlling the discharge intensity.The reactor was made of quartz glass tube and placed in a tubular furnace.Porous plates were arranged in the middle of the reactor for uniform airflow.Mixed gases and ozone entered the reactor separately and reacted near the porous plate after preheating.The reaction time was controlled by adjusting the gas flow.The concentration of O3was measured by ozone analyzer at the outlet of the reactor.The inlet concentration is calibrated at room temperature and constant flow,and then the outlet concentration is tested under reaction conditions.The concentrations of NO,NO2,SO2were measured by flue gas analyzer (Rosemount NGA2000)at the outlet of the reactor,when the O3generator is turned off,the test result is the inlet concentration,when the O3generator is turned on,the test result is the outlet concentration.Typical experimental conditions were as follows:flue gas flow rate of 1.5 L·min-1;O2concentration of 6%;NO concentration of 200 μl·L-1;SO2concentration of 1000 μl·L-1;reaction temperature of 150 °C.The O3decomposition rate was calculated using Eq.(1).
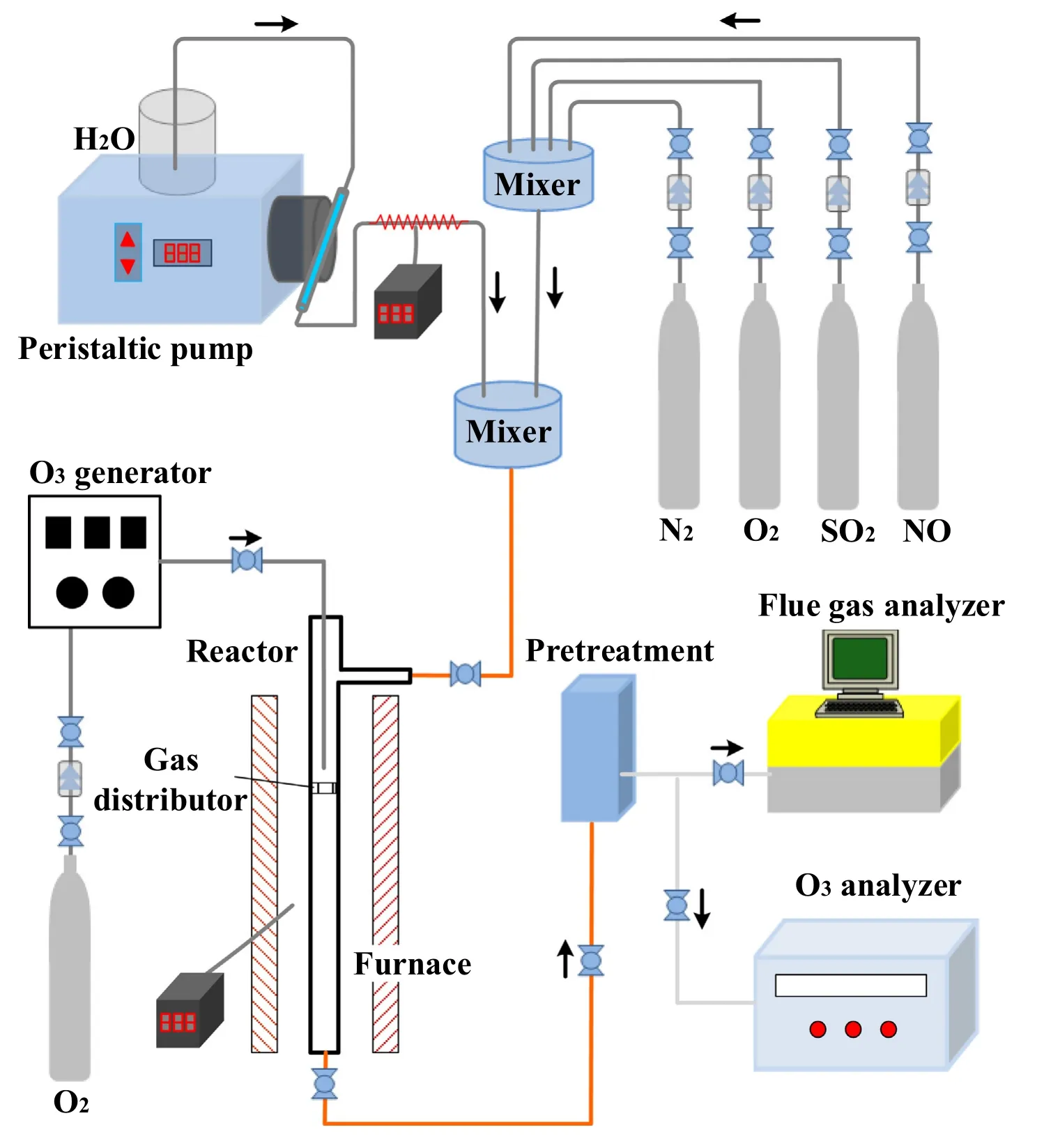
Fig.1.Experimental system for ozone oxidation of NO.

where α represents the O3decomposition efficiency,%;Ainis the O3inlet concentration,μl·L-1;Aoutis the O3outlet concentration,μl·L-1.
The NO oxidation efficiency was calculated using Eq.(2).

where β represents the NO Oxidation efficiency,%;Binis the NO inlet concentration,μl·L-1;Boutis the NO outlet concentration,μl·L-1.
The NOxabsorption test was constructed on a small-scale for multi-pollutant removal system,and included a gas distribution system,a bubbling reactor,an analysis testing system and an exhaust gas recovery system,as shown in Fig.2.The simulated flue gas mixture was made up of gases coming from the four cylinders that contained 1.0 vol%NO2balanced by N2,2.0 vol%SO2balanced by N2,99.999 vol%O2,and 99.999 vol%N2.The flow rate of each gas stream was controlled using an individual mass flow controller(MFC).Water vapor generation system was the same as that used for NO oxidation test system.The gas inlet pipe inside the reactor was made of self-made gas distributor to enhance the gas–liquid reaction.The absorption solution adopted a mixture of liquid catalyst and water,and the pH value was adjusted to 6.5–7.0 by using 0.1 mol·L-1NaOH or 5%ammonia water.The gas concentrations at the inlet and outlet of the reactor were detected using a Rosemount NGA2000 gas analyzer.Typical experimental conditions were as follows:flue gas flow rate of 1.5L·min-1;O2concentration of 6%;NO2concentration of 200 μl·L-1.The NO2absorption efficiency was calculated using Eq.(3).

where γ represents the NO2removal efficiency,%;Cinis the NO2inlet concentration,μl·L-1;Coutis the NO2outlet concentration,μl·L-1.
In order to verify the actual effect of ozone oxidation denitrification process,a wet ozone oxidation denitrification project was undertaken for a 35 t·h-1industrial boiler in a factory.The schematic of the process is shown in Fig.3.The original pollutant treatment equipment of industrial boilers includes a bag filter and a wet desulfurization system (WFD).There is no denitrification system.WFD uses Ca(OH)2as the absorbent.Gypsum is the by-product,which is separated using filter press and transported abroad.In this environmental renovation,ozone injection system was added upstream the absorption tower to realize the combined desulfurization and denitrification.Ozone was produced from liquid oxygen using dielectric barrier discharge (DBD).
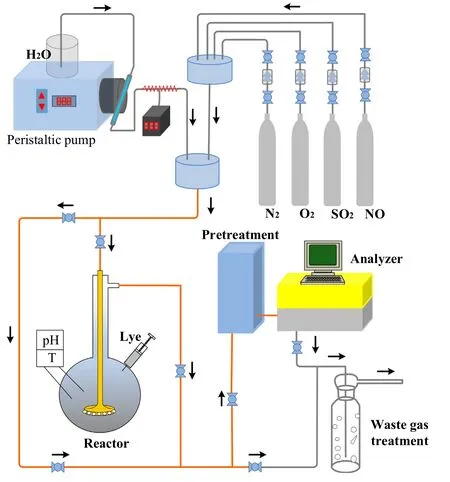
Fig.2.Small-scale experimental system for removing NOx .
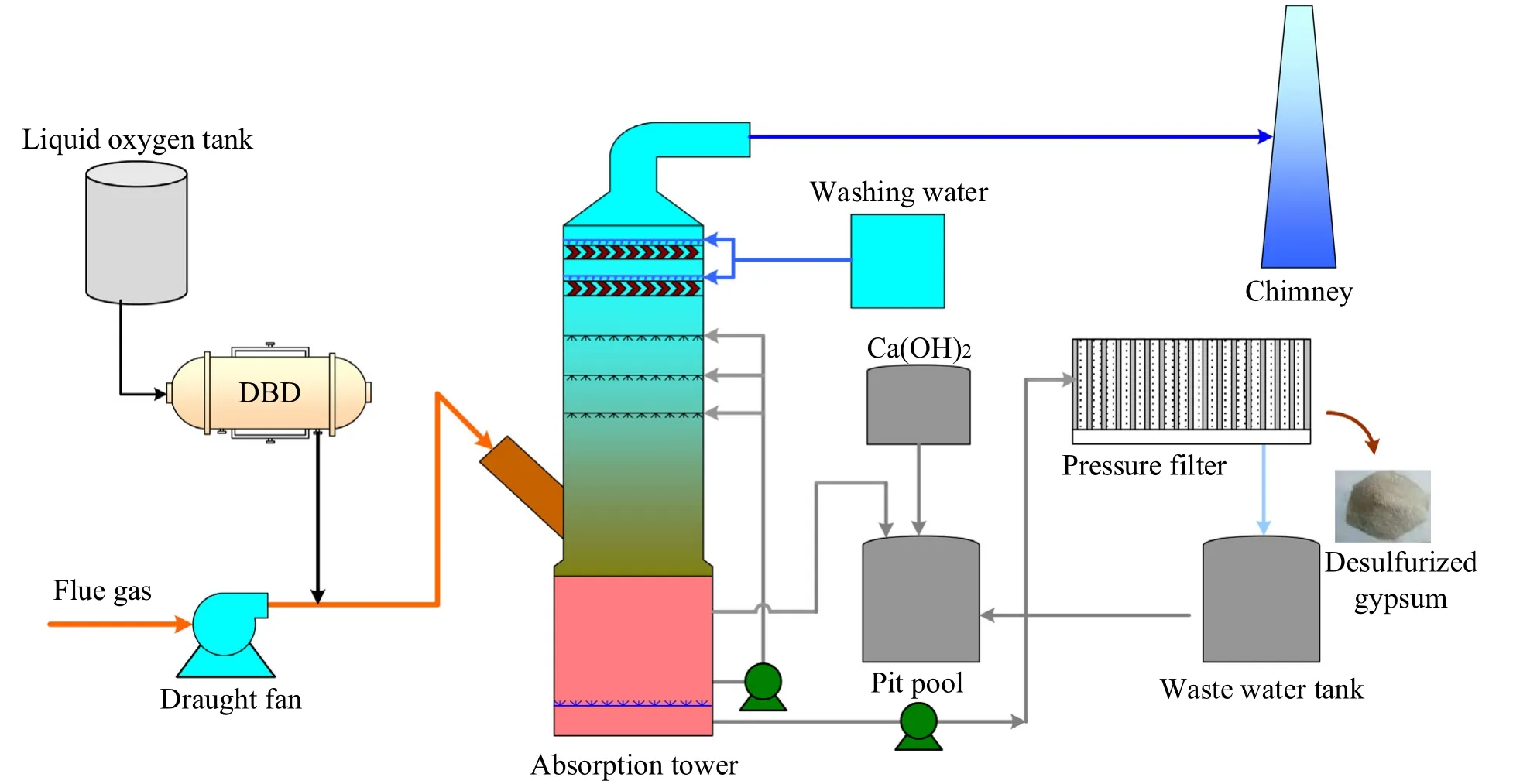
Fig.3.35 t·h-1 boiler ozone oxidation wet combined desulfurization and denitrification process.
During the experiments,the stability of boiler load was ensured.Firstly,the flue gas flow rate and humidity were tested according to the ‘‘Determination of particulates and sampling methods of gaseous pollutants emitted from exhaust gas of stationary source”[36].Then,the O3generator was adjusted to change the O3feed rate.The NO,NO2and O2concentrations at the inlet and outlet of the absorption tower were detected using Rosemount NGA2000 gas analyzer.Finally,the power and oxygen rates of ozone generator were adjusted to record the ozone produced under different operating conditions.The denitrification efficiency was calculated using Eq.(4).
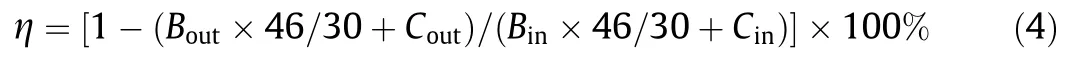
where η represents the denitrification efficiency,%;Binis the NO inlet concentration,μl·L-1;Boutis the NO outlet concentration,μl·L-1;Cinis the NO2inlet concentration,μl·L-1;Coutis the NO2outlet concentration,μl·L-1.
3.Results and Discussion
3.1.Characteristics of O3 oxidation of NO
(1) Thermal stability of O3
O3is a strong oxidant with poor stability and easy decomposition under heating conditions.Therefore,it is important to study the thermal stability of O3for the selection of injection location.The decomposition characteristics of O3at 50–300°C were studied by controlling the reaction time for 1 s and 2 s,respectively,on the experimental system of NO oxidation using O3.The corresponding results are shown in Fig.4.
As shown in Fig.4,the O3decomposition rate increases with the increase in temperature.Within the temperature range of 50–150 °C,the decomposition rate of O3slowly increases.However,the O3decomposition rate is obviously faster when the reaction temperature is higher than 150°C.When the reaction temperature is 250°C,O3completely decomposes within 2 s,whereas when the temperature is 300°C,O3completely decomposes within 1 s.In the process of ozone oxidation wet denitrification,the O3decomposition will lead to a decrease in the NO oxidation efficiency and an increase in ozone consumption.Therefore,it is suggested that the flue gas temperature at the ozone injection site should be controlled to a value of less than 150 °C.
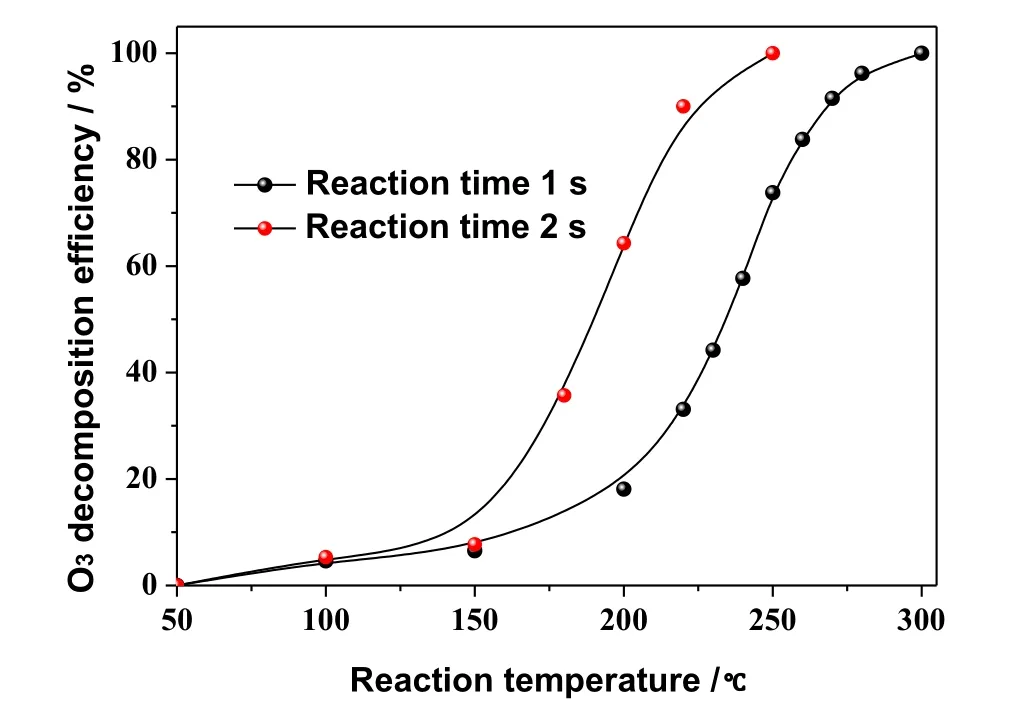
Fig.4.Effect of temperature on thermal stability of O3 .
(2) Priciple of product formation
On the experimental system of O3oxidation of NO,the formation and transformation of NOxwere studied by adjusting the ozone concentration at the reactor’s inlet.The corresponding results are shown in Fig.5.
As shown in Fig.5,with the increase of ozone’s addition,NO is first oxidized to NO2.When the ratio of [O3]/[NO]is 1.2:1,NO is completely converted to NO2,and the total amounts of NO and NO2are basically balanced,which indicates that no other valence NOx(N2O3,N2O4,N2O5,etc.) is generated.With the increase in the value of [O3]/[NO]ratio,NO2decreases obviously.When the ratio of [O3]/[NO]is 2:1,the concentration of NO2is close to zero.The analysis shows that all the NOxis converted to N2O5.The reaction process is shown in Eqs.(5) and (6).Relevant studies show that under the same experimental conditions,the reaction rate of Eq.(5) is about 500 times that of Eq.(6) [37].
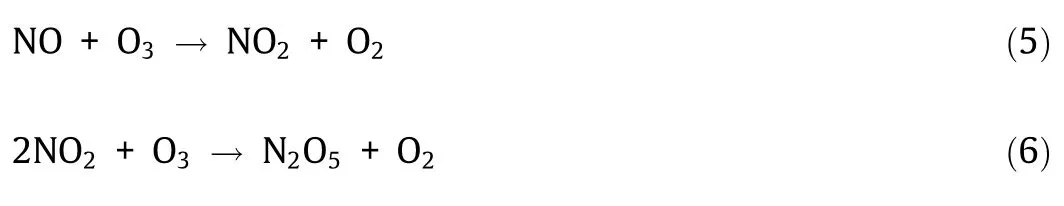
(3) Study on the selectivity of NO oxidation by ozone
SO2and NO are the two main pollutants in coal-fired flue gas.SO2is more active and easier to be oxidized than NO.Moreover,SO2concentration in flue gas is generally higher than NO.If SO2is oxidized in large quantities in the process of NO oxidation using O3,it will lead to a waste of oxidant,causing aerosol emissions and equipment corrosion.Therefore,it is important to study the selectivity of O3for NO oxidation.In order to do so,the concentration of SO2was successively varied through values of 500,1000 and 2000 μl·L-1,and the effects of SO2concentration on NO oxidation were studied at 50 °C and 140 °C.The corresponding results are shown in Fig.6.
As shown in Fig.6,with the increase in SO2concentration,the oxidation efficiency of NO by O3decreases slightly,though it (the oxidation efficiency) is maintained at about 90%.This shows that O3has a strong selectivity for NO oxidation,and is not affected by SO2concentration.The reaction process is shown in Eq.(7).Relevant studies show that under the same experimental conditions,the reaction rate ratio of Eqs.(5),(6) and (7) is 10,000:20:1 [37].
(4) Reaction time
Reaction time is a key parameter for NO oxidation using O3,determines the location of O3injection and affects the construction and operating costs of the technology.On the experimental system of ozonation of NO,the molar ratio of [O3]/[NO]is adjusted to 1:1.The reaction of ozone and NO was studied by controlling the flue gas flow rate and reactor size by within the reaction time range of 0.2–2.5 sec.The corresponding results are shown in Fig.7.
As shown in Fig.7,with the increase of reaction time,the oxidation efficiency of NO basically remained unchanged.When the reaction time changed from 0.2 s to 2.5 s,the oxidation efficiency of NO was about 90%.This indicates that the time required for the ozonation of NO is very short and almost instantaneous.Wang et al.[29]used Chemkin software to simulate the reaction kinetics of ozone and NO.The results showed that the reaction time of ozone and NO was only 0.01 s,which was in agreement with the above experimental results.In actual process design,the uniform injection and diffusion of O3is the key controlling step,and the influence of oxidation time could be neglected.
3.2.NOx absorption characteristics
(1) NO2absorption characteristics
There are many forms of NOx,such as NO,N2O,N2O3,NO2,N2O4,N2O5,and NO3.In the process of NO oxidation using O3,the primary oxidation product is NO2.When ozone is present in excess,NO2will continue to be oxidized to N2O5or NO3.NO is difficult to be absorbed by alkaline solutions,while N2O5is easily absorbed.However,this happens at the cost of higher oxidation expense.There are some differences in the absorption of NO2in the existing desulfurization system.It is important to study the absorption of NO2for improving the technical economy of wet ozonation denitrification.On the small-scale experimental system for removing NOx,the absorption process of NO2was studied in three systems of pure water,alkaline NaOH solution and aqueous ammonia solution.The corresponding results are shown in Fig.8.
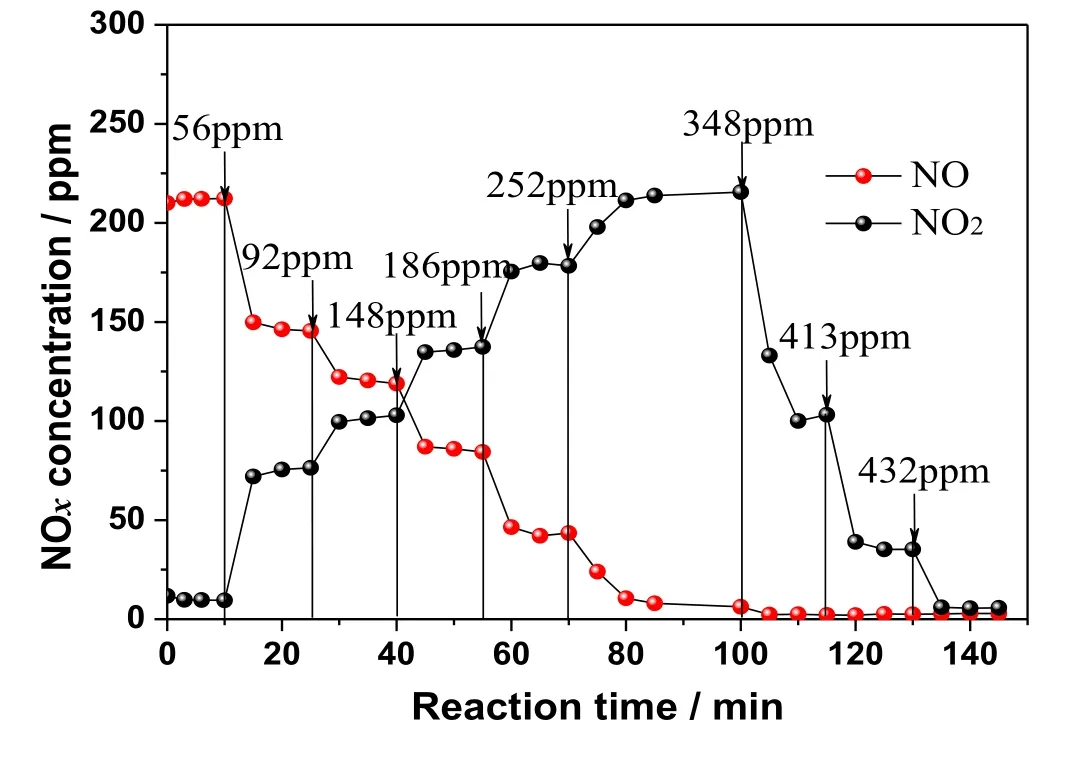
Fig.5.O3 oxidation NO product formation and change law (1 ppm=1 μl·L-1).
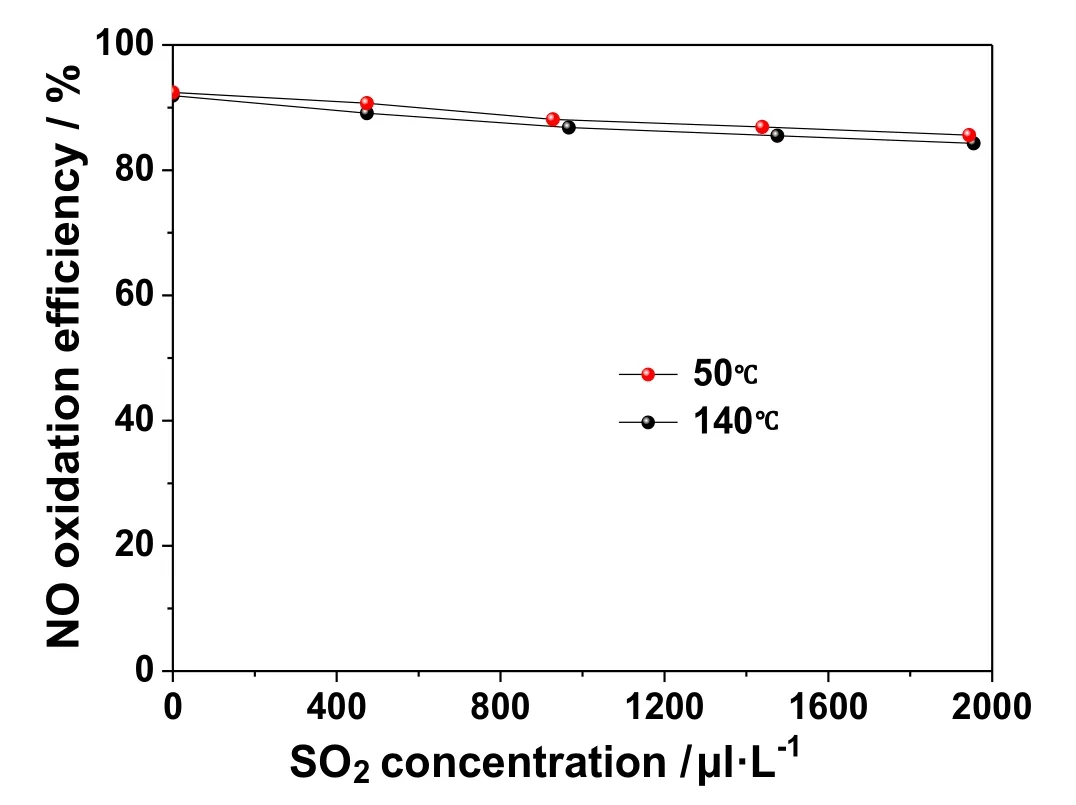
Fig.6.Effect of SO2 Concentration on NO Oxidation by O3 .
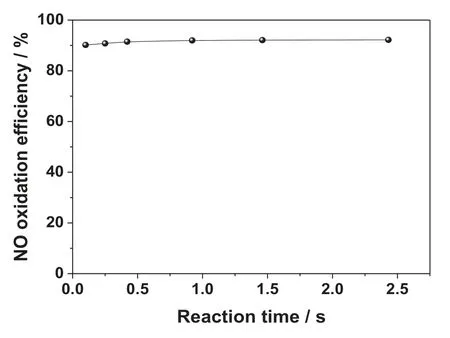
Fig.7.Effect of Reaction Time on NO Oxidation by O3 .
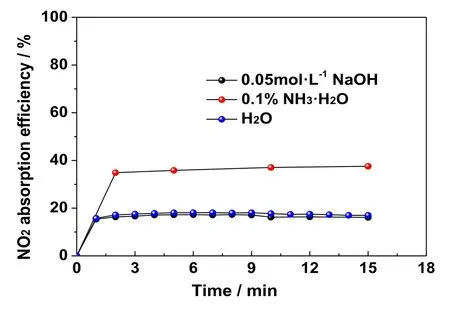
Fig.8.NO2 adsorption performance under different systems.
The results shown in Fig.8 indicate that the absorption performance of NO2in pure water is similar to that in NaOH solution,and the efficiency is around 20%.The NO2removal efficiency in ammonia solution is slightly higher,which is around 40%.All the three systems have poor absorption performance for NO2.The three types of solutions are neutral,strongly alkaline,and weakly alkaline.The absorption efficiency of NO2is similar under neutral and strongly alkaline conditions,however it is higher under weakly alkaline conditions.Therefore,it could be concluded that the absorption performance of NO2is independent of solution’s basicity (pH).Ammonia system has a higher NO2adsorption efficiency than the other two systems due to its higher reducibility.
(2) Effect of oxidation efficiency on NOxremoval
The effect of different amounts of ozone on the NOxremoval performance was studied in industrial boiler wet denitrification project.Firstly,it is ensured that the boiler load remains stable at 80% load.The flue gas flow was tested in the circular flue duct at the outlet of absorption tower.Then,the ozone injection flow was adjusted,and the NO and NO2concentrations at the inlet and outlet of the absorption tower were measured.The results are presented in Table1.The denitrification efficiency and NO2absorption efficiency changed with the ozone’s injection flow under different operating conditions (as shown in Fig.9).
The results showed that with the increase in the amount of O3,the denitrification efficiency increases,while the NO2removal efficiency does not change significantly.NO2adsorption efficiency remained stable at about 13%,which corresponded to the experimental results of NO2absorption performance (Fig.8).The calculated results showed that,when the amount of injected ozone was 11.87 kg,the corresponding molar ratio of O3/NO was 1:1.When the molar ratio of O3/NO was less than 1:1,with the increase in the amount of O3,the inlet concentration of NO2obviously increases,while the denitrification efficiency increases rather slowly.This is because the product of NO oxidation was NO2and the absorption efficiency of NO2was poor.When the molar ratio of O3/NO was greater than 1:1,the denitrification efficiency increased significantly.This is due to the reason that NO2was oxidized to higher valency and easily absorbed as N2O5or NO3.When the molar ratio of O3/NO was 1.77,the denitrification efficiency reached 90.3%.Therefore,in O3oxidation wet denitrification,excessive O3should be sprayed so that NO can be oxidized to valence N2O5or NO3,which ensures high denitrification efficiency.
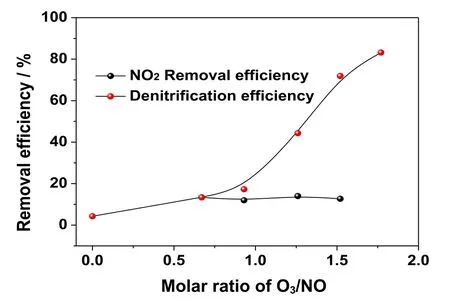
Fig.9.Effect of oxidation efficiency on NOx removal.
3.3.Economic analysis of the O3 oxidation wet denitrification
(1) Boundary conditions
The research results of industrial boiler ozone wet denitrification project showed that,when the amount of injected ozone was 21.0 kg,the denitrification efficiency reached 90.3%,which could meet the demand of ultra-low emissions of NOx.Based on the operating conditions,the operating cost of ozone denitrification was analyzed,whereas the boundary conditions are presented in Table 2.
(2) Analysis of the operating cost
In the wet O3denitrification process of industrial boilers,ozone is produced by dielectric barrier discharge (DBD),and Ca(OH)2is used as the absorbent.O3is produced by liquid O2using a 25 kg·h-1O3generator (CF-G-3-25,Shandong Zhiwei Environmental Technology Co.,Ltd.,China).When the discharge power of O3generator is less than 75%,ozone cannot be produced.The relationship between the amount of O3produced and the amount of input oxygen was studied when the discharge power was between 75% and 100%.The cost of ozone generation under different operating conditions was analyzed.The corresponding results are presented in Table 3.
The results showed that the cost of ozone generation gradually decreases with the increase in ozone generator power,which indicates that the operation of ozone generator under low power conditions was not economical.The optimum operating cost of O3generator was about 2.61 USD·(kg O3)-1.The corresponding costof liquid oxygen was 1.22 USD·(kg O3)-1,whereas the corresponding cost of power consumption was 1.38 USD·(kg O3)-1.For the O2consumption of 181 kg·h-1,the power consumption was 160 kW.Converted to ozone per kilogram,oxygen and electricity consumption are 11.0 (kg O2)·(kg O3)-1and 9.7 kW·(kg O3)-1,respectively.
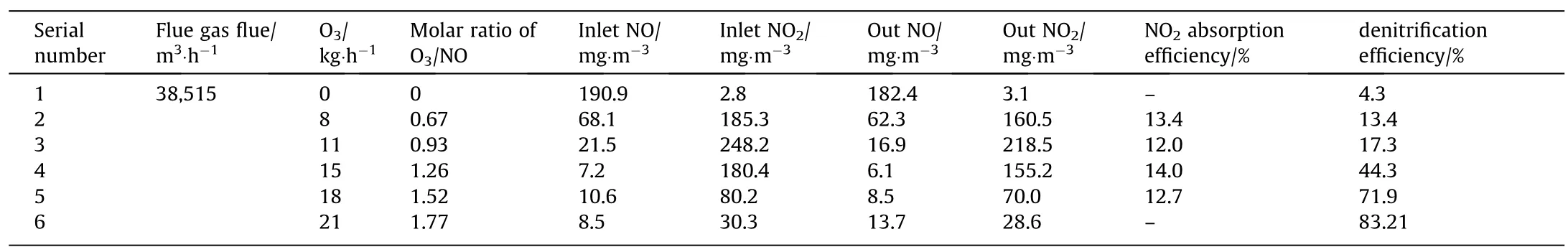
Table 1 Effect of oxidation efficiency on NOx removal
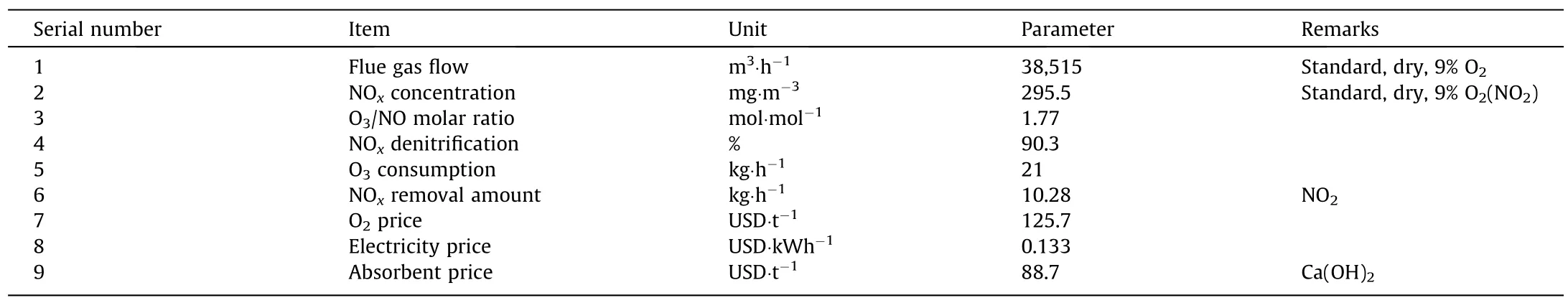
Table 2 Calculation basis for the economic analysis
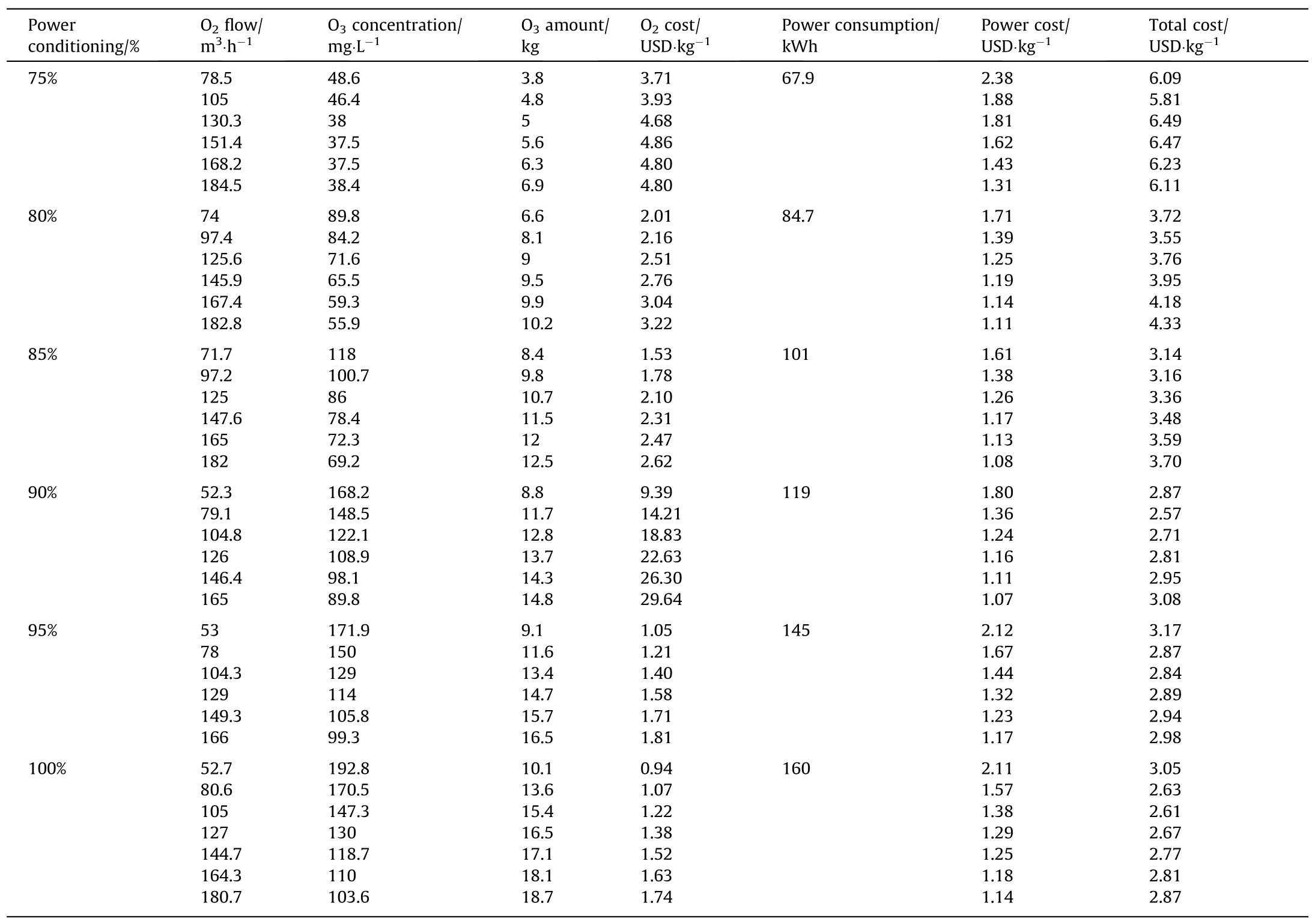
Table 3 Cost analysis of O3 generation
The cost of O3generation is calculated to be 2.61 USD·kg-1,while the cost of O3is 5.33 USD.Moreover,the cost of absorbent is 0.73 USD for removing one kilogram of NO2.The total cost of the process is 6.06 USD·kg-1.
4.Conclusions
In this paper,the mechanism of NO oxidation using O3and the absorption performance of NOxare experimentally studied.In the O3oxidation wet denitrification project of 35 t·h-1industrial boiler,the mechanism of NO oxidation using O3and the absorption performance of NOxare verified.The operating cost of O3oxidation wet denitrification is analyzed.Based upon the results,following conclusions are drawn.
(1) Under the flue gas environment of wet desulfurization inlet,O3can exist stably,and has strong selectivity for NO oxidation.The oxidation time is less than 0.2 s.The primary oxidation product is NO2,while the advanced oxidation product is N2O5or NO3.
(2) It is difficult for NO2to be absorbed efficiently by existing wet desulfurization systems.The NO2adsorption efficiency is less than 20%in the conventional wet desulfurization process,while it is about 40% in the ammonia desulfurization process.NO must be oxidized to high valence N2O5or NO3to achieve high efficiency of denitrification.
(3) The research results of industrial boiler O3oxidation wet denitrification process show that,when the molar ratio of O3/NO reaches 1.77,the denitrification efficiency reaches 90.3%.The corresponding operating cost of denitrification is 6.06 USD·kg-1(calculated as NO2).
(4) Ozone oxidation wet denitrification has the advantages of being simple,having low investment cost,flexible load regulation,no specific temperature window and low temperature denitrification.However,the operating cost is much higher than that of traditional selective catalytic reduction(SCR) and nonselective catalytic reduction (SNCR) denitrification processes.This technology is suitable for low flue gas volumes,low NOxconcentrations and low temperature denitrification.The proposed technology could be applied in industrial boilers,industrial kilns,sintering machine,coke ovens,etc.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Huaneng Group Science and Technology Project (HNKJ17-H14) and the Project of National Science and Technology Supporting Plan(2014BAA07B00)for their financial support.
 Chinese Journal of Chemical Engineering2021年1期
Chinese Journal of Chemical Engineering2021年1期
- Chinese Journal of Chemical Engineering的其它文章
- Highly interconnected macroporous MBG/PLGA scaffolds with enhanced mechanical and biological properties via green foaming strategy
- Preparation and properties of a low-cost porous ceramic support from low-grade palygorskite clay and silicon-carbide with vanadium pentoxide additives
- An efficient preparation of porous polymeric microspheres by solvent evaporation in foam phase
- Application of an immobilized microbial consortium for the treatment of pharmaceutical wastewater:Batch-wise and continuous studies
- A polypropylene melt-blown strategy for the facile and efficient membrane separation of oil–water mixtures
- Pyrolysis of single large biomass particle:Simulation and experiments
