Morphological, physiological and biochemical traits of Cordia trichotoma under phosphorous application and a water-retaining polymer
álvaro Luís Pasquetti Berghetti · Maristela Machado Araujo · Luciane Almeri Tabaldi · Felipe Turchetto · Suelen Carpenedo Aimi · Daniele Guarienti Rorato · Carina Marchezan · Adriana Maria Griebeler · Felipe Manzoni Barbosa · Gustavo Brunetto
Abstract The application of phosphorus (P) and a waterretaining polymer to the soil can increase the availability of P for Cordia trichotoma, having a positive effect on the plants. The objective of this study was to evaluate the morphological, physiological and biochemical characteristics of C. trichotoma plants were cultivated in a red argisol and treated with 120, 240 and 360 kg P 2O 5 ha? 1 and no phosphorous addition as a control, in the presence (5 g L ?1 per seedling) and absence of a water-retaining polymer. Twentyfour months after planting, survival, height, stem diameter, shoot and root dry matter, leaf area, photosynthetic pigment concentration, chlorophyll a fluorescence, acid phosphatase enzyme activity (APase) and P in tissues and soil were determined. The polymer had no effect on survival and the other parameters. The addition of P increased growth, dry matter production, photosynthetic pigment concentrations, the use of light energy and maximum quantum yield of photosystem II. Plants cultivated in soil with 240 kg P 2 O 5 ha ?1 application had 4.7 and 5.4 times more shoot and root dry matter, respectively, than control plants. This dosage also showed 52.1% greater photochemical energy use than the control plants. Plants cultivated without the addition of P showed higher activity of the APase enzyme.
Keywords Fluorescence · Louro-Pardo · Morphological traits · Phosphate nutrition · Photosynthetic apparatus
Introduction
The species, louro-pardo, Cordia trichotoma (Vell.) Arrab. ex Steud., belongs to the Boraginaceae family and is widely distributed in tropical and subtropical forests of Argentina, Bolivia, Brazil, Paraguay and Uruguay. This species has great potential for cultivation in commercial stands (Rossi and Sartoretto 2014). Its wood has high economic value and is widely used for the manufacture of luxury furniture and decorative coatings (Coradin et al. 2011; Berghetti et al. 2016). However, C. trichotoma is rarely established in soils with low fertility, and when it occurs, growth is poor without fertilization (Rossi and Sartoretto 2014; Antonelli et al. 2015).
Research indicates that one of the major diffi culties in the development of plantations in Brazil is associated with weathered soils that are low in available phosphorus (P) (Gon?alves et al. 2008a, b). This is because of the adsorption affi nity of the phosphate ion to functional groups of reactive particles such as Fe, Al and Mn oxides and hydroxides, but also to clay minerals which decreases P availability (Fink et al. 2016; Freitas et al. 2017; Ngatia et al. 2017). To overcome this, phosphate fertilizers are applied with the expectation of increasing its availability. However, for C. trichotoma, the optimum dosage is unknown. Because of this, soils to be planted with C. trichotoma are treated at P application rates recommended for species such as Eucalyptus (Antonelli et al. 2015), which may partially explain why slow growth and smaller wood yield does not reflect its genetic potential.
In addition, in plantations of C. trichotoma, the effectiveness of water-retaining polymers of acrylamide copolymer potassium acrylate to reduce mortality soon after transplanting is unknown. These polymers are used as soil conditioners in silviculture (Fonseca et al. 2017; Pontes Filho et al. 2018) because they absorb water during periods of water availability and release it in periods of water scarcity (Leciejewski 2009; Felippe et al. 2016). This is common in summers in the southern regions of Brazil (Wrege et al. 2012). Exploratory studies have shown that the use of water-retaining polymers promotes higher survival rates of seedlings because they increase water availability in the soil (Fonseca et al. 2017; Pontes Filho et al. 2018). However, there may also be an increase in soil pH (Bernardi et al. 2012; Lopes et al. 2017). As a result, the availability of P may be increased, enhancing its uptake by plants (von Tucher et al. 2018), considering the acidic soils of the natural occurrence of C. trichotoma.
Therefore, the association between P application and water-retaining polymers in young plantations could be an effective strategy to reduce mortality and stimulate seedling growth. This response to higher absorption and accumulation of P can be demonstrated by measuring height, stem diameter, leaf area and dry matter production (Stahl et al. 2013; Crous et al. 2015; Piccin et al. 2017b). Seedlings would be expected to have higher concentrations of photosynthetic pigments (Wu et al. 2014) in the leaves, less energy loss by chlorophyll a fluorescence (Tiecher et al. 2016, 2017), enhanced physiological processes such as ATP biosynthesis and the carboxylation/regeneration of ribulose-1,5-bisphosphate and rubisco activity (Warren 2011; Hidaka and Kitayama 2013). However, information on the effectiveness of P applications and of water-retaining polymers in C. trichotoma plantations is unknown.
The objective of this study is to evaluate the morphological, physiological and biochemical characteristics of C. trichotoma seedlings in a field planting treated with dosages of P in the presence and absence of a water-retaining polymer.
Materials and methods
Experimental area
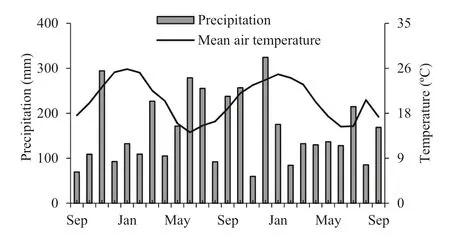
Fig.1 Mean precipitation (mm) and mean air temperature (°C) during the 24 months of the experiment
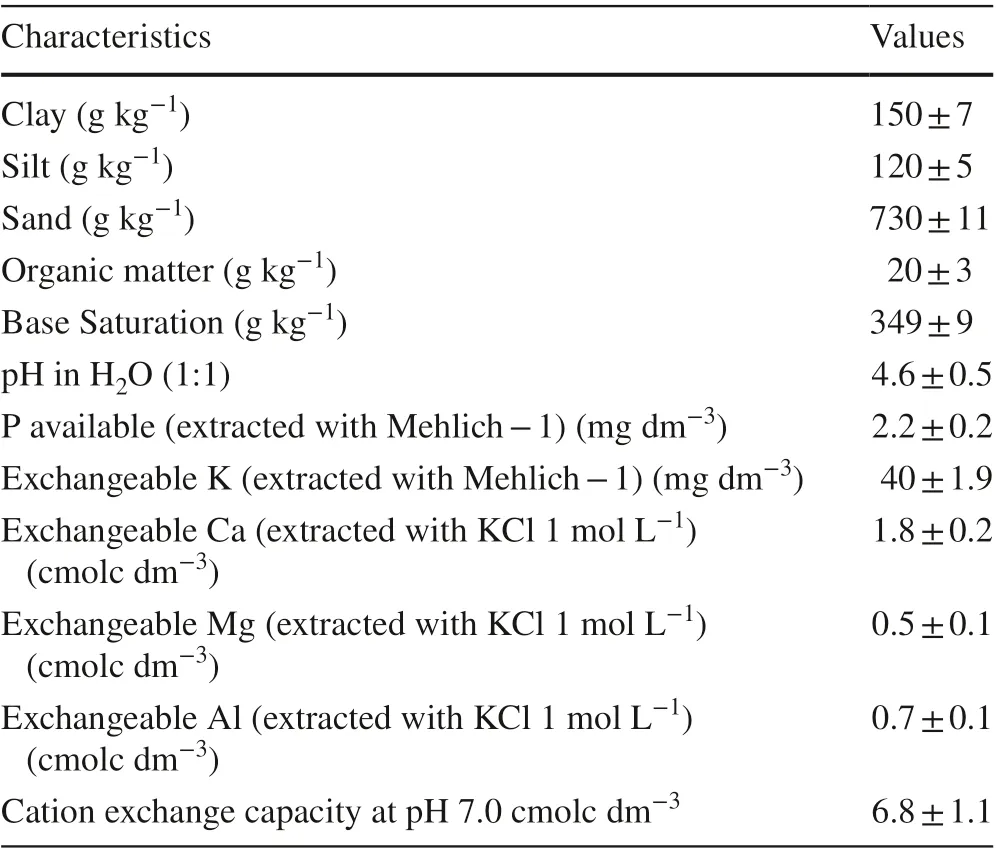
Table 1 Soil physical and chemical characteristics in the upper 20 cm layer before planting C. trichotoma seedlings
The study was carried out from September 2013 to September 2015 in Santa Maria, Rio Grande do Sul in southern Brazil (29° 47? 37″ S; 53° 40? 08″ O). Based on the K?ppen climate classification, the climate is Cfa, humid subtropical characterized by rains well distributed throughout the year (Alvares et al. 2013). The precipitation and mean temperatures throughout the study period were 162.8 mm month?1and 20 °C, respectively (Fig.1) (Bdmet-Inmet 2019). The soil was classified as a dystrophic sandy red argisol (Embrapa 2013). The physical and chemical characteristics of the soils are described in Table 1.
Experimental design and treatments
The experiment was carried out in a randomized block design in a 4 × 2 factorial scheme, considering that the levels of factor “A” referred to the dosages of P2O5(120, 240 and 360 kg of P2O5ha?1)and no phosphorous addition as a control, and the levels of factor “D” to the presence of 5 g L?1of water-retaining polymer per seedling?1or to its absence. There were four replicates, each consisting of 16 plants per treatment. The source of P2O5was triple superphosphate (46% P2O5) and dosages were 100, 200 and 300% of the recommended P2O5for eucalyptus cultivation according to the Committee on Soil Chemistry and Fertility—CQFS-RS/SC ( 2004).
The polymer was hydrated an hour before its application in seedling hole before planting. The water-retaining polymer is a mixed product of acrylamide copolymer (C3H5NO) potassium acrylate (K2S2O8) and is a white powder insoluble in water, with particles ranging in size from 0.3 to 1.0 mm, anionic, with 10% humidity and a density of 0.8 g cm?3. After application, the polymer slowly degrades with carbon dioxide, water and ammonia the end products (Wallace and Wallace 1986; Stahl et al. 2000). According to Wallace et al. ( 1986) there is no issue related to residual toxicity.
Silvicultural practices
For preparation of the area, the vegetation, predominantly composed of Vernonanthura tweedieana (Baker) H. Rob, Sida spp. Senna obtusifolia (L.) Irwin & Barneby and Bidens alba (L.) DC. was removed. Subsequently, 683 kg ha?1of dolomitic limestone [CaMg(CO3)2], with a total relative neutralizing power of 76%, were applied to the surface to raise the pH to 5.5. A non-selective systemic herbicide (N-(phosphonomethyl) glycine) was applied for the control of herbaceous species prior to planting.
Planting was done in 30 × 30 × 30 cm holes with a 3 m × 2 m spacing. The soil was at about 80% of field capacity. The seedlings were produced in 280 cm 3 tubes containing a mixture of soil, a commercial substrate based on Sphagnum peat and carbonated rice husks in a ratio of 2:2:1. At the time of planting, the seedlings had an average height of 40 cm, stem diameters of 5.5 mm, shoot dry matter of 3.1 g plant?1and root dry matter of 3.4 g plant?1. After planting, irrigation (1 L of water per seedling?1) was carried out to eliminate air pockets around the roots. In addition, all the seedlings were fertilized with 50 kg N ha?1and 45 kg K2O ha?1. The source of N used was urea (45% N) and the source of K was potassium chloride (KCl 60% K2O). Fertilizer application was carried out in two lateral holes approximately 15 cm from the seedling.
Evaluation of survival and morphological traits of the plants
The survival was evaluated 24 months after planting. At the same time, height (H) of four plants per plot was measured using a millimeter ruler, and stem diameter (SD) with a digital caliper (accuracy of 0.01 mm). The above ground portions of three plants per plot were cut and separated into leaves, branches and stem. The tissue was dried in a forced circulation oven at 65 °C to constant weight, followed by weighing with an analytical balance (0.001 g) to constant mass to determine the shoot dry matter (SDM).
Leaf area (LA) was determined on 30 leaves randomly collected from the lower, middle and upper third of the plant. The leaves collected were scattered on white paper with a millimeter scale, pressed with transparent glass and photographed with a digital camera (SONY Cyber-shot). The images were adjusted for contrast and brightness and processed for determination of leaf area with Image J. software. The leaves were then dried at 65 °C in an oven to constant weight to determine dry matter. Subsequently, from leaf dry matter and leaf area ratio, the total plant LA was determined (Coelho Filho et al. 2012).
The same plants used to determine shoot dry matter had roots removed and manually separated from the soil and washed with running water. To obtain the root dry matter (RDM), the material was oven-dried at 65 °C to constant weight and determined with an analytical balance (0.01 g).
In addition, an analysis of the absolute growth rate (AGR) for H, SD, SDM and RDM was performed using the following: AGR = (X1? X0)/(T1? T0) where AGR is the value of the absolute growth rate; X1and X0refer to values for each morphological trait (H, SD, SDM and RDM) at time T1(24 months after planting) and T0(at the moment of planting) (Aimi et al. 2017).
Detection of photosynthetic pigments
Twenty-four months after planting, concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids were determined according to Hiscox and Israelstam ( 1979). Fully expanded leaves from the upper third of the plants were frozen in liquid N2and stored at ? 80 °C until the time of quantification. Leaf samples (0.05 g) were incubated with dimethylsulfoxide (DMSO) at 65 °C for 1.5 h. The absorbance readings of the extracts were performed in a spectrophotometer (Celm E-205D—Bel Engineering, Italy), at 663, 645 and 470 nm for Chl a, Chl b and carotenoids, respectively. The photosynthetic pigments levels were calculated according to Lichtenthaler ( 1987).
Evaluation of chlorophyll fluorescence
The emission of chlorophyll a fluorescence was analyzed in 24- month-old leaves using a portable light-modulated fluorometer (Junior-Pam Chlorophyll Fluorometer, Walz, Germany). Measurements were taken in the morning (8:00 am to 11:00 pm) on sunny days using the first fully expanded leaf (Souza et al. 2013) of four plants per treatment. Previously, the leaves were dark-adapted for 30 min for measuring the initial fluorescence (Fo) and subsequently subjected to a pulse of saturating light (10.000 μmol m?2s?1) for 0.6 s, thereby determining maximum fluorescence (Fm) and maximum quantum yield (Fv/Fm) of photosystem II (PSII).
Extraction and quantification of the acid phosphatase enzyme (APase)
Foliar tissue (0.5 g) were macerated with liquid N2 homogenized with Tris/HCl buffer (0.1 M), EDTA (0.001 M) and albumin (0.1 g) (pH 7.4). Samples were filtered and centrifuged at 20,000 × for 30 min and the resulting supernatant used to determine acid phosphatase enzyme activity (Tabaldi et al. 2007).
Inorganic phosphate (Pi) content was measured at 630 nm using malachite green as a colorimetric reagent and KH2PO4standard for the calibration curve using spectrophotometer (Celm E-205D) for sample reading. Specific activities of the enzyme were reported as nmol Pi released min?1mg?1protein. Protein was determined according to Bradford ( 1976) using bovine serum albumin as a standard.
Phosphorous in tissues and soil
To determine leaf nutrient concentrations, dry leaf tissues were ground in a Wiley mill, passed through a 20-mesh sieve and total P contents determined after sulfur digestion (Tedesco et al. 1995). The concentration of phosphorous was determined by colorimetry in a spectrophotometer (SF325NM, Bel Engineering, CITY? Italy) according to Murphy and Riley ( 1962).
For soil nutrient content, four samples of seven subsamples were collected per treatment. Soil sampling was carried out with a sampling probe at depths of up to 20 cm and 20–40 cm. The soil was air dried, sieved with a 2 mm mesh and P was extracted with Mehlich 1:0.05 mol L?1HCl + 0.0125 mol L?1H2SO4) according to Tedesco et al. ( 1995).
Statistical analysis
The data were analyzed for assumptions of normality of residues and homogeneity of variance by the Shapiro–Wilk and Bartlett tests, respectively. Subsequently, an ANOVA was carried out and the data analyzed according to the model:
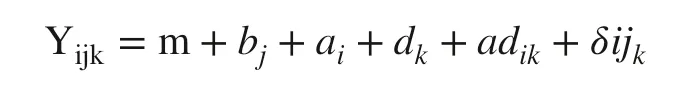
where Yijkis the value observed for each seedling; m a constant; bjthe effect of the blocks; a i the fixed effect of the dosages of P; dkthe fixed effect of the water-retaining polymer; adikthe effect of the interaction between the dosages of P and the polymer; and δijkthe effect of the random error in each experimental unit.
When there was a difference between treatments, a polynomial regression analysis ( p < 0.05) was performed for the quantitative factor, and a t test for the qualitative factor, using the statistical package Sisvar (Ferreira 2014).
Results
Survival and morphological traits
The survival of C. trichotoma seedlings after 24 months was 100% regardless of the P dosage and the presence or absence of the polymer. However, heights (H), stem diameters (SD), leaf areas (LA), shoot dry matter (SDM) and root dry matter (RDM) increased quadratically ( p < 0.0001) with an increase in applied P ( p = 0.0033) (Fig.2). However, the water-retaining polymer ( p > 0.05) did not alter morphological traits (Fig.2).
The largest height values (252.9 cm) were with the maximum technical efficiency dosage (DMET) of 216.0 kg P2O5ha?1. Growth was 83.6% greater than the controls. Seedlings grown in soil with 360 kg P2O5ha?1had 37.8% less growth compared to the DMET (Fig.2 a).
The lowest SD values (46.2 mm) were control plants and the highest (64.9 mm) in the DMET of 214.0 kg P2O5ha?1. Seedlings cultivated with the addition of 360 kg of P2O5ha?1had a 23.7% reduction in stem diameter compared seedlings treated with 214.0 kg P2O5ha?1.
Leaf area increased in a quadratic curve with the increase of P dosage. The highest LA was with the DMET of 236.0 kg P2O5ha?1, 8.3 times higher than control seedlings (0.40 m 2 plant?1) (Fig.2 c).
The highest accumulate of SDM (Fig.2 d) and RDM (Fig.2 e) were in plants with a maximum technical effi ciency dosage of 236.7 and 241.7 kg P2O5ha?1, respectively. Under these conditions the shoot dry matter (SDM, 2.07 g plant?1) and the root dry matter (RDM, 2.18 g plant?1) were 4.7 and 5.4 times higher than the controls. However, seedlings raised in soil with 360 kg P2O5ha?1had SDM and RDM of 37.9% and 60.1%, respectively, lower than DMET values (Fig.2 d, e).
Absolute growth rates (AGR) in height, stem diameter, shoot dry matter and root dry matter increased quadratically with the phosphorous application regardless of the presence or absence of the polymer (Fig.3). The highest AGRs were with seedlings grown with 120 and 240 kg P2O5ha?1(Fig.3). The highest monthly height growth rate (7.9 cm month?1), stem diameter (2.2 mm month?1), shoot dry matter (83.7 g plant?1month?1) and root dry matter (167.5 g plant?1month?1) were in seedlings with DMETs of 193.7; 162.5; 227.7 and 222.9 kg P2O5ha?1, respectively. The lowest monthly growth and dry matter accumulation were with seedlings grown in soil without phosphate (Fig.3).
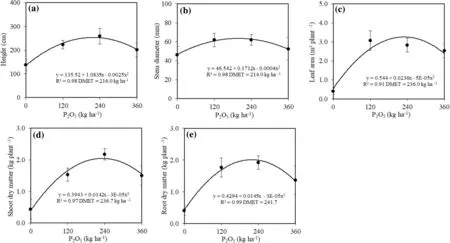
Fig.2 a Height, b stem diameter, c leaf area, d shoot dry matter, and e root dry matter of C. trichotoma seedlings in soil with different P dosages, 24 months after planting
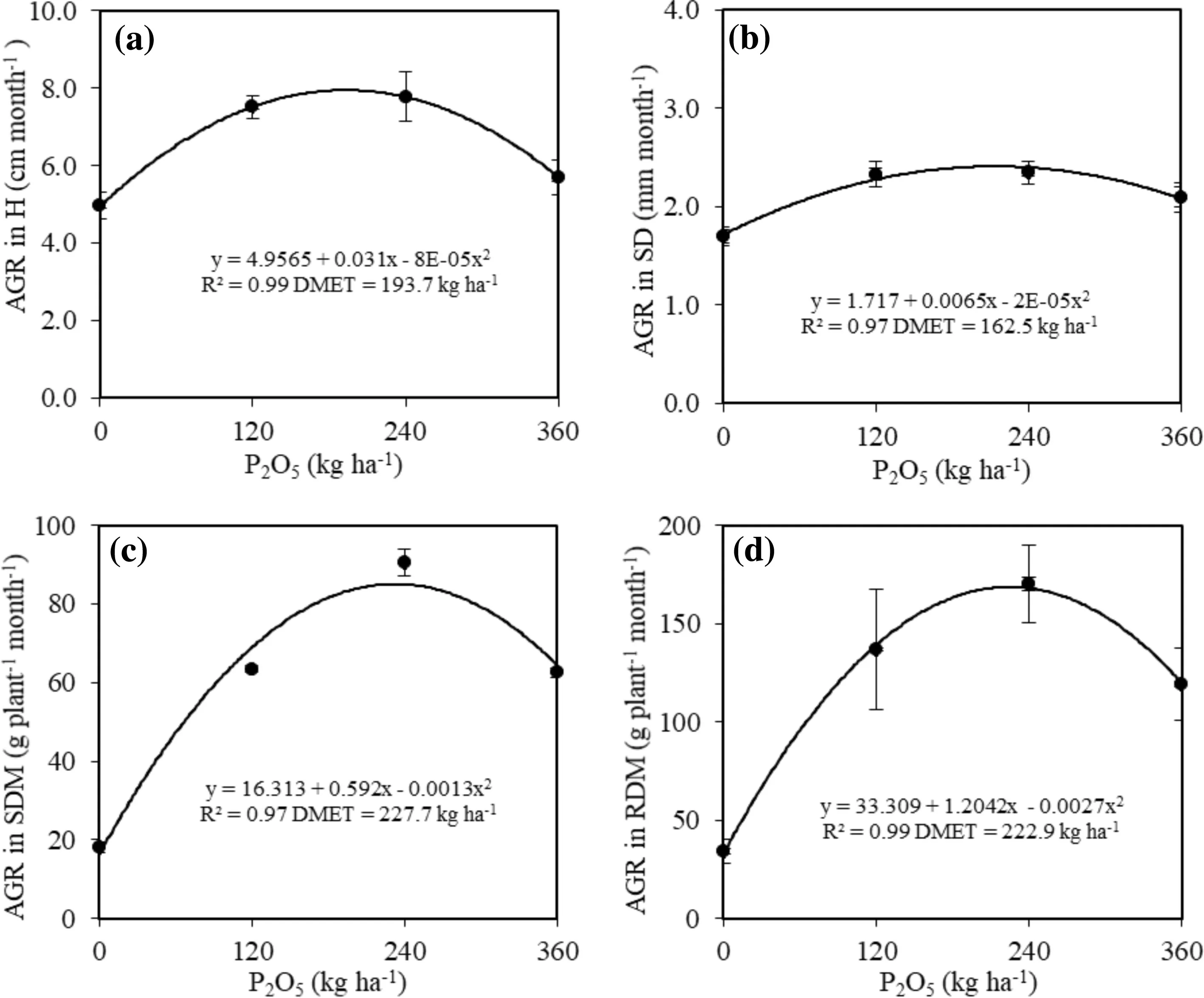
Fig.3 Absolute growth rate (AGR) in a height, b stem diameter, c shoot dry matter, and d root dry matter of C. trichotoma seedlings in soil with different P dosages after 24 months
Photosynthetic pigments
The addition of phosphorous influenced the levels of photosynthetic pigments, increasing chlorophyll a, chlorophyll b, total chlorophyll and carotenoids in a quadratic pattern in seedling leaves. Application of water-retaining polymer did not change the pigment content. The DMET for chlorophyll a, chlorophyll b, total chlorophyll and carotenoids was similar (Fig.4), with a mean value of 194.6 kg P2O5ha?1. Seedlings grown without P supplement had the lowest levels (Fig.4).
Fluorescence of chlorophyll a
The traits of chlorophyll a fluorescence, initial fluorescence (Fo) and maximum quantum yield (Fv/Fm) of photosystem II showed that the increase in P availability alters the photochemical effi ciency of C. trichotoma seedlings regardless of the use of the water-retaining polymer in planting.
The Fodecreased quadratically with the increase of P. The lowest values were in plants treated with 240.5 kg P2O5ha?1(Fig.5 a). In this condition, the use of photochemical energy was 52.1% higher than in the controls (Fig.5 a).
The maximum quantum yield (Fv/Fm) of the seedlings increased quadratically with phosphorous levels (Fig.5 b). Seedlings in soil supplied with 210.0 kg P2O5ha?1had the highest quantum yield of 0.66. The lowest Fv/Fmvalues were in control plants with reductions of 41.3% compared to the DMET of 210.0 kg P2O5ha?1(Fig.5 b).
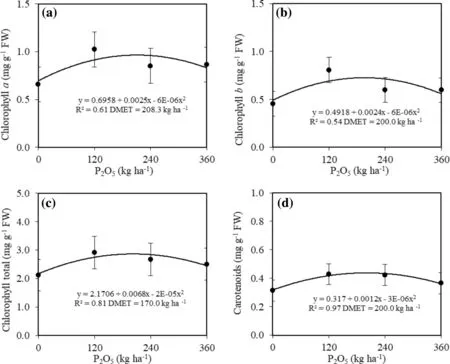
Fig.4 a Values of chlorophyll a, b chlorophyll b, c total chlorophyll, and d carotenoids of C. trichotoma seedlings in soil with different P dosages 24 months after planting
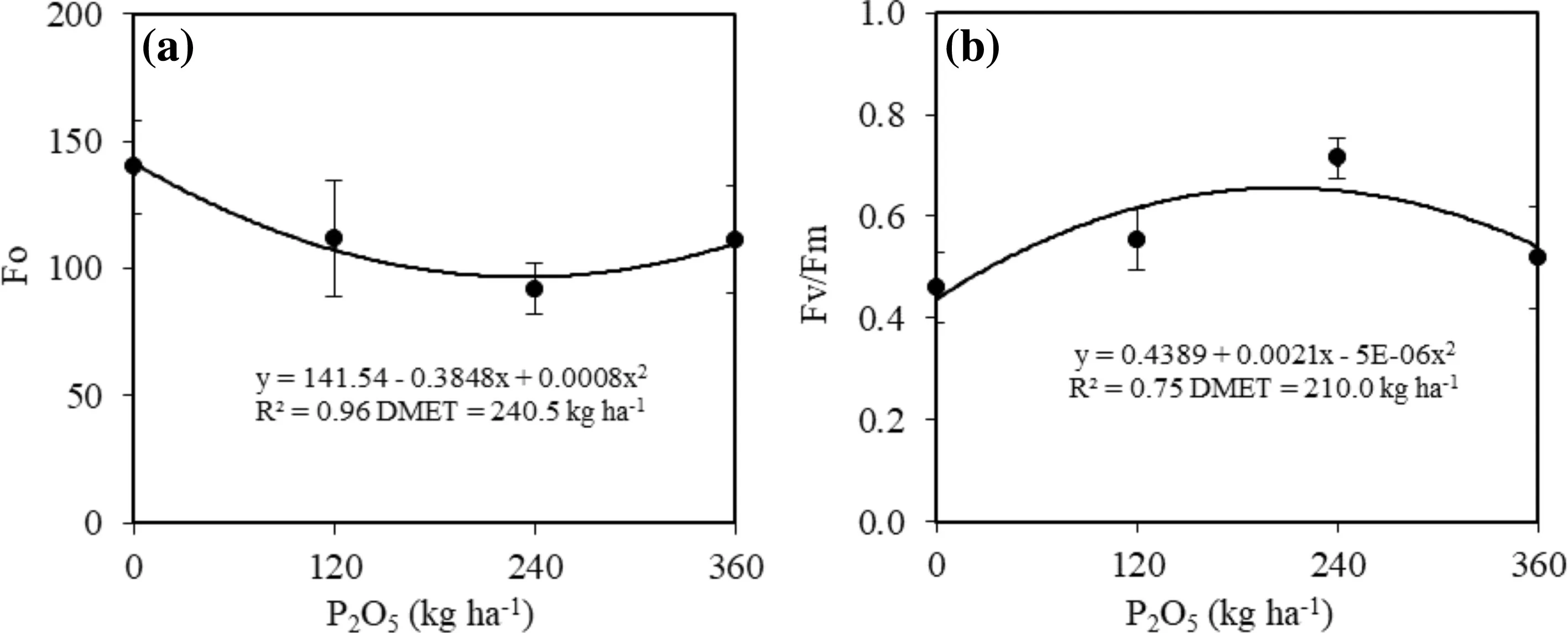
Fig.5 a initial fluorescence (F o ) and b maximum quantum yield (F v /F m ) of C. trichotoma seedlings in soil with different phosphorous dosages 24 months after planting
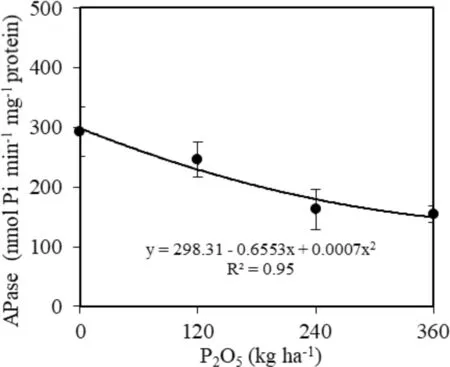
Fig.6 Activity of APase in C. trichotoma seedlings in soil with different P dosages 24 months after planting
Acid phosphatase enzyme (APase)
APase activity in the leaves decreased with the increase of P (Fig.6). Seedlings cultivated without the addition of P had higher enzyme activity (292.67 nmol Pi min?1mg?1protein), which increased 79.8% in relation to plants treated with 360 kg P2O5ha?1(Fig.6).
Phosphorous in plant tissue and in soil
Phosphorous application did not modify its concentration in seedling roots, resulting in a general average of 1.51 mg kg?1(Fig.7 a). Concentrations in the stems and branches increased linearly with the increase in P (Fig.7 b, c), without effects from the polymer. Seedlings raised soil with 360 kg P2O5ha?1showed increase P concentrations of 5.6 and 4.0 times in tissues of stems and branches, respectively, relative to the controls.
Concentrations of P in the leaves increased quadratically with the increase P dosage applied to the soil without influence of the polymer (Fig.7 d). Seedlings cultivated without phosphorus added 1.84 mg kg?1of dry matter of P in the leaves, on average 2.5 times lower than in leaves of seedlings grown in soil with an application of 360 kg P2O5ha?1(Fig.7 d). The levels of P extracted with Mehlich-1 in the upper 20 cm and 20–40 cm layers increased quadratically with increases in dosages of P2O5, without effect of the water-retaining polymer (Fig.8). The highest levels of P were in the upper 20 cm layer.
Discussion
The 100% survival of seedlings grown in soil with or without the water-retaining polymer, regardless of the phosphorous dosage, may possibly be associated with the quality of seedlings used in planting and the intensity and regularity of rainfall in the initial months after planting (Fig.1). This guaranteed the maintenance of soil moisture and replenishment of water to the seedlings, avoiding dehydration of theroot system (Barbosa et al. 2013). Results similar to the present study were obtained by Dranski et al. ( 2013) in Jatropha curcas L. seedlings in spring and summer plantations and by Barbosa et al. ( 2013) in 30 native tree species transplanted with and without water-retaining polymer.
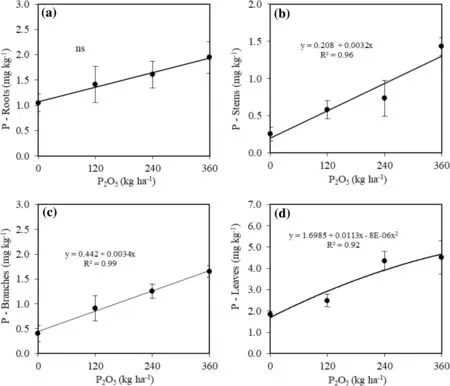
Fig.7 a Concentrations of P in roots, b stem, c branches, and d leaves of C. trichotoma seedlings in soil with different phosphorous dosages, 24 months after planting. ns F not significant to 5% probability
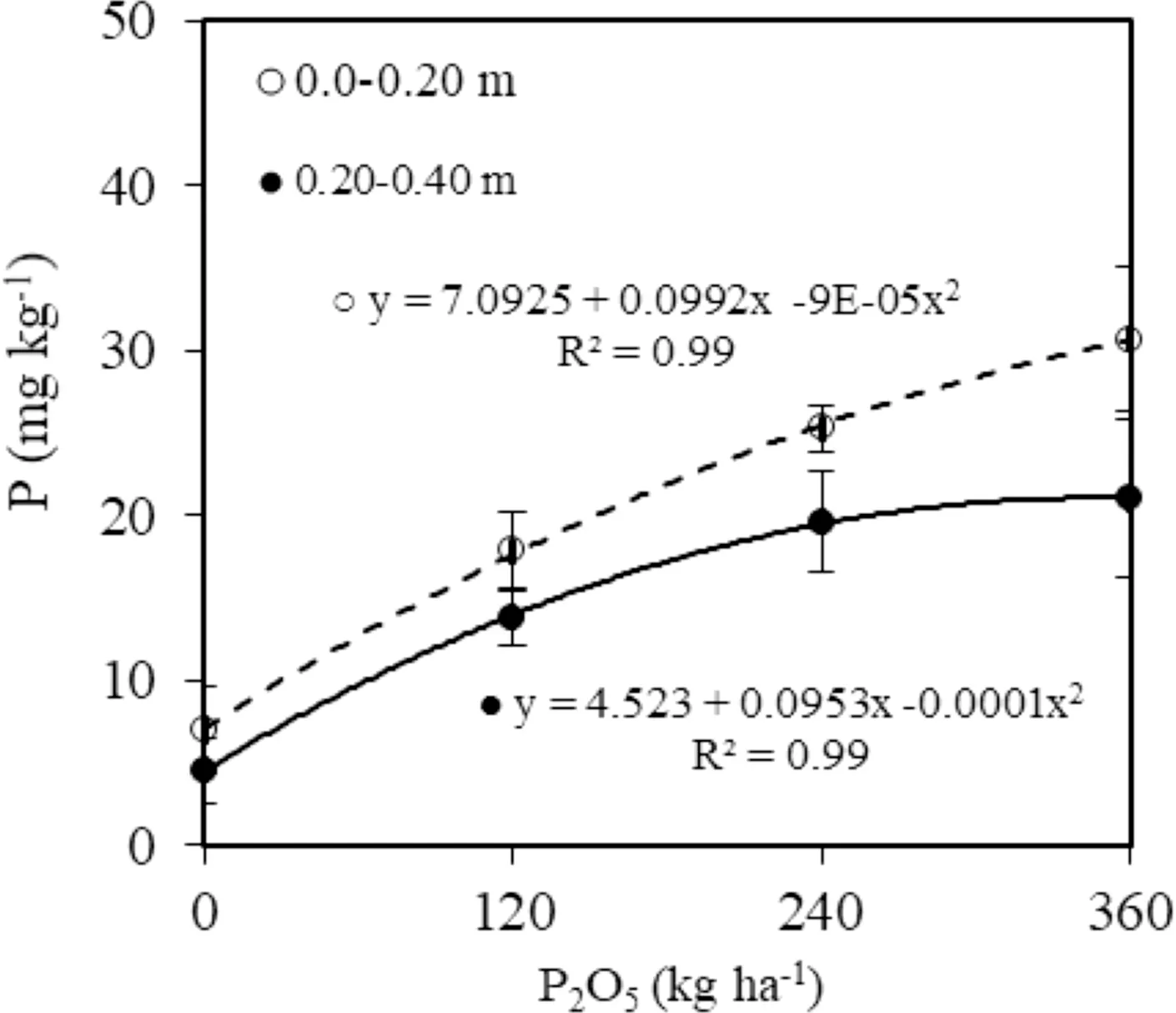
Fig.8 Phosphorous content in the upper 20 cm and 20–40 cm layers 24 months after planting
The increase in the values of morphological traits of C. trichotoma with increasing dosage (Fig.2) may be attributed to the higher availability of P in the soil (Fig.8), which enhances phosphate root uptake (Piccin et al. 2017a). Phosphorous was possibly absorbed preferentially in H2PO4–and HPO42?forms (Elanchezhian et al. 2015), and part transported to seedling organs, accumulating mainly in the leaves (Fig.7 d) because they are annual organs that undergo intense cell division and dry matter increase in the growing season.
Phosphorous in leaves stimulated energy metabolism, cell division and expansion (Noack et al. 2014), reflected in increased leaf area (Fig.2 c). Increases in LA are directly related to light absorption, CO2assimilation which, consequently, increases the photosynthetic rate (Zambrosi et al. 2012a, b; Crous et al. 2015). This provides greater growth (Zambrosi et al. 2011) and dry matter production (Veneklaas et al. 2012; Nielsen et al. 2015).
Morphological traits and absolute growth rate (AGR) decrease in plants grown with 360 kg P2O5ha?1compared to treatments with 240 kg P2O5ha?1(Figs. 2, 3). This is possibly because the seedlings may have absorbed levels of P above their metabolic requirements, resulting in the allocation of P in cell vacuoles (Zambrosi et al. 2011; Veneklaas et al. 2012; Noack et al. 2014). Previous studies with seedlings cultivated with increased P dosages showed similar effects (Stahl et al. 2013 ; Crous et al. 2015).
The higher levels of leaf pigment in plants grown in phosphate-fertilized soils may be attributed to increased P concentrations in tissue, which can accelerate energy metabolism, cell division (Marschner 2012) and stomatal conductance (Warren 2011), and consequently result in increased synthesis of photosynthetic pigments (Jiang et al. 2009). The higher levels of pigments indicate that C. trichotoma seedlings can show greater effi ciency in absorption and energy transfer to photosynthetic processes (Ferreira et al. 2018). This is because the increase in chlorophyll a and b and carotenoids levels in leaves promotes greater light capture and absorption in different regions of the spectrum in the early stages of photosynthesis. With this, there is a greater energy transfer by resonance in the antenna complexes to the reaction centers where the energy can be used for the photochemical reactions responsible for the production of biomass.
The lower initial fluorescence (Fo) values obtained with the maximum technical efficiency dosage (DMET) of 240.5 kg P2O5ha?1, demonstrates that, in this condition, the seedlings showed little damage in the reaction center of photosystem II (PSII) and have high transfer excitation energy from the light collecting system to the reaction center (Stirbet and Govindjee 2011; Schansker et al. 2014). The lower energy loss (Fig.5 a) was reflected in seedlings with higher growth rates (Fig.3) because, when energy dissipation increases in the form of fluorescence, there is a reduction in the formation of ATP and NADPH formed during the light reactions, and in the assimilation of carbon (Taiz and Zeiger 2013).
The higher Fv/Fmof PSII (0.66) found with the DMET of 210.0 kg P2O5ha?1(Fig.5 b) indicates that most of light energy is being directed to the photochemical stage of photosynthesis rather than being lost by chlorophyll a fluorescence (Baker 2008). In fact, in this study it was possible to observe higher growth and dry matter production (Figs. 2, 3) when the C. trichotoma seedlings were exposed to intermediate doses of P, corroborating the results of Fv/Fmof the PSII. Thus, values close to 0.66 can be considered good predictors of growth because, under these conditions, the seedlings had higher growth and dry matter production. Similar results were observed in studies carried out in southern Brazil with seedlings of native tree species, indicating that values between 0.55 and 0.70 can be considered good indicators of initial growth (Turchetto et al. 2016; Brucker Kelling et al. 2017; Zavistanovicz et al. 2017).
The reduction of the Fv/Fmin control seedlings may be related to the reduction of chlorophyll levels (Cambrollé et al. 2015). In addition, with the reduction of Fv/Fm, a smaller amount ofenergy absorbed by the plant through the antenna complex is used to transport electrons and produce dry matter, which reinforces the explanation for low dry matter production due to the low availability of P (Tiecher et al. 2016, 2017).
Higher APase activities in leaves of plants cultivated without addition of phosphorous (Fig.6) happened because, with the lower availability of P in the soil, lower absorption of forms of P is expected and, consequently, lower concentrations in the tissues (Tang et al. 2013). Thus, the induction of intracellular APase activity occurs in order to maintain the internal homeostasis of inorganic phosphate (Pi) to provide it from organic phosphorous compounds in the cytoplasm and vacuoles (Pandey et al. 2017). These results show that there is an intracellular mechanism of Pi utilization (Lazali and Drevon 2014) associated with high P remobilization, providing greater effi ciency in the use of P when seedlings are grown under nutrient-deficient conditions (Ferreira et al. 2018). Hinsinger et al. ( 2011) indicates that this is a common strategy of forest species for absorption and reallocation of P.
The higher concentrations of P in the roots, stems, branches and leaves of the plants grown in soil with 360 kg P2O5ha?1(Fig.7) can be attributed to the higher phosphorous availability (Ferreira et al. 2018). The concentrations of P in leaves (Fig.7 d) can be interpreted as high because they are above the ideal levels suggested for Eucalyptus, which range from 1.0 to 1.3 g kg?1(Melo et al. 2016).
The higher P levels in upper 20 cm layer occurred because the phosphate fertilizer was applied to the soil in pits without incorporation in order to avoid mechanical damage to the roots. Phosphorous is normally adsorbed in functional groups of soil reactive particles such as oxides, oxyhydroxides, and clay minerals, reducing their mobility in the soil profile, but also lower P availability may occur (Brunetto et al. 2015; Fink et al. 2016; Freitas et al. 2017). However, a portion of the applied phosphorous migrated in the soil profile down to the 40 cm layer because an increase of P levels as a result of higher dosages added was observed. This possibly occurred because it was a sandy with low P-adsorption capacity (Pizzeghello et al. 2011). This may be desirable because the roots grow in depth over time and can increase the effi ciency of use of the P applied.
Conclusions
With regular, suffi cient rainfall in the initial post-planting phase, the addition of a water-retaining polymer did not alter the survival, morphological, physiological and biochemical traits of C. trichotoma plants.
The increased availability of phosphorous provided better growth of C. trichotoma seedlings, and an application of 212 kg P2O5ha?1is recommended for the establishment of stands of this species. The application of P2O5stimulated the concentration of phosphorous in the tissues, preferentially in the leaves, increasing photosynthetic pigment levels and use. In addition, it promoted greater height growth, stem diameters and shoot and root dry matter production.
On the other hand, the highest photochemical energy losses in the photosystem II complex occurred in seedlings in soil without phosphate fertilization. In this condition, seedlings had higher APase (acid phosphatase enzyme) activity due to lower availability of P and, consequently, lower concentration of the mineral in tissues.
AcknowledgementsAppreciation is extended to the Council for Scientific and Technological Development (CNPq) and to the Coordination for the Improvement of Education Personnel (CAPES) for funding and grants awarded.
References
Aimi SC, Araujo MM, Tonetto TS, Tabaldi LA, Saldanha CW, Farias JG, de Oliveira GG (2017) Shading as a conditioning factor to forest species planting: a study with Apuleia leiocarpa. Bosque (Valdivia) 38:371–379. https ://doi.org/10.4067/S0717 -92002 01700 02000 14
Alvares CA, Stape JL, Sentelhas PC, de Moraes Gon?alves JL, Sparovek G (2013) K?ppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Antonelli PV, Brun EJ, dos Santos MAB, Sartor LR, Brun FGK (2015) Desenvolvimento de Cordia trichotoma em fun??o da aduba??o, em sistema silvipastoril no Sudoeste do Paraná-Brasil. Rev Ecol e Nutr Florest - ENFLO 3:59–70. https://doi.org/10.5902/23169 80X19 054
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annur ev.arpla nt.59.03260 7.09275 9
Barbosa TC, Rodrigues RR, de Couto HTZ (2013) Tamanhos de recipientes e o uso de hidrogel no estabelecimento de mudas de espécies florestais nativas. Hoehnea 40:537–556. https://doi.org/10.1590/S2236-89062 01300 03000 13
Bdmet-Inmet (2019) Banco de Dados Meteorológicos para Ensino e Pesquisa. Instituto Nacional de Meteorologia. Temperaturas máximas e mínimas e umidade relativa do ar anos 2013/2015. Disponível em: http://www.inmet.gov.br/porta l/. Acesso em: 12 Jan 2017
Berghetti áLP, Maristela MA, Tonetto TS, Aimi SC, Navroski MC, Turchetto F, Thairini CZ (2016) Growth of Cordia trichotoma seedlings in different sizes of recipients and doses of fertilizer. Afr J Agric Res 11:2450–2455. https://doi.org/10.5897/AJAR2 016.10883
Bernardi MR, Sperotto Junior M, Daniel O, Vitorino ACT (2012) Crescimento de mudas de Corymbia citriodora em fun??o do uso de hidrogel e aduba??o. CERNE 18:67–74. https://doi.org/10.1590/S0104-77602 01200 01000 09
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brunetto G, Nava G, Ambrosini VG, Comin JJ, Kaminski J (2015) The pear tree response to phosphorus and potassium fertilization. Rev Bras Frutic 37:507–516. https ://doi.org/10.1590/0100-2945-027/14
Cambrollé J, García JL, Figueroa ME, Cantos M (2015) Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 120:171–178. https ://doi.org/10.1016/j.chemo spher e.2014.06.044
Coelho Filho MA, Villa-Nova NA, Angelocci LR, Marin FR, Righi CA (2012) Método para estimativa do IAF de árvores isoladas ou de planta??es com dossel fechado. Rev Bras Eng Agrícola e Ambient 16:529–538. https ://doi.org/10.1590/S1415 -43662 01200 05000 09
Coradin L, Siminski A, Reis A (2011) Espécies nativas da flora brasileira de valor econ?mico atual ou potencial: plantas para o futuroRegi?o Sul. MMA, Brasília
CQFS-RS/SC (2004) Manual de Aduba??o e de Calagem para os estados do Rio Grande do Sul e Santa Catarina. 10th edn. Porto Alegre
Crous KY, ósvaldsson A, Ellsworth DS (2015) Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilisation stimulates stem growth. Plant Soil 391:293–305. https://doi.org/10.1007/s1110 4-015-2426-4
Dranski JAL, Pinto Junior AS, Campagnolo MA, Malavasi UC, Malavasi MM (2013) Sobrevivência e crescimento do pinh?omanso em fun??o do método de aplica??o e formula??es de hidrogel. Rev Bras Eng Agrícola e Ambient 17:537–542. https ://doi.org/10.1590/S1415-43662 01300 05000 11
Elanchezhian R, Krishnapriya V, Pandey R, Rao AS, Abrol YP (2015) Physiological and molecular approaches for improving phosphorus uptake effi ciency of crops. Curr Sci 108:1271–1279
Embrapa (2013) Sistema Brasileiro de Classifica??o de Solos. Empres Bras Pesqui Agropecuária 3 ed:353. ISBN 978-85-7035-198-2
Felippe D, Navroski MC, Sampietro JA, Frigotto T, Albuquerque JA, Mota CS, Pereira MO (2016) Efeito do hidrogel no crescimento de mudas de Eucalyptus benthamii submetidas a diferentes frequências de irriga??o. Floresta 46:10
Ferreira DF (2014) Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia 38:109–112. https://doi.org/10.1590/S1413-70542 01400 02000 01
Ferreira PAA, Tiecher T, Tiecher TL, Rangel WM, Soares CRFS, Deuner S, Tarouco CP, Giachini AJ, Nicoloso FT, Brunetto G, Coronas MV, Ceretta CA (2018) Effects of Rhizophagus clarus and P availability in the tolerance and physiological response of Mucuna cinereum to copper. Plant Physiol Biochem 122:46–56. https://doi.org/10.1016/j.plaph y.2017.11.00
Fink JR, Inda AV, Bavaresco J, Barrón V, Torrent J, Bayer C (2016) Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res 155:62–68. https://doi.org/10.1016/j.still.2015.07.017
Fonseca L, Roitman I, Jacobson TKB, Ogata RS, Solari RAF, Ribeiro RJC (2017) Viabilidade do Hidrogel na Recupera??o de Cerrado sensu stricto com Espécies Nativas. Floresta e Ambient. https://doi.org/10.1590/2179-8087.02271 6
Freitas ECS, de Paiva HN, Leite HG, de Oliveira Neto SN (2017) Effect of phosphate fertilization and base saturation of substrate on the seedlings growth and quality of Plathymenia foliolosa Benth. Rev árvore. https://doi.org/10.1590/1806-90882 01700 01000 11
Gon?alves JLM, Stape JL, Laclau JP, Bouillet JP, Ranger J (2008a) Assessing the effects ofearly silvicultural management on longterm site productivity of fast-growing eucalypt plantations: the Brazilian experience. South For 70:105–118. https://doi.org/10.2989/SOUTH.FOR.2008.70.2.6.534
Gon?alves JLM, Wichert MCP, Gava JL, Masetto AV, Arthur JC, Serrano MIP, Mello SLM (2008b) Soil fertility and growth of Eucalyptus grandis in Brazil under different residue management practices. South Hemisph For J 69:95–102. https://doi.org/10.2989/SHFJ.2007.69.2.4.289
Hidaka A, Kitayama K (2013) Relationship between photosynthetic phosphorus-use effi ciency and foliar phosphorus fractions in tropical tree species. Ecol Evol 3:4872–4880. https://doi.org/10.1002/ece3.861
Hinsinger P, Brauman A, Devau N, Gérard F, Jourdan C, Laclau J-P, Le Cadre E, Jaillard B, Plassard C (2011) Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil 348:29. https://doi.org/10.1007/s1110 4-011-0903-y
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Jiang H-X, Tang N, Zheng J-G, Li Y, Chen L-S (2009) Phosphorus alleviates aluminum-induced inhibition of growth and photosynthesis in Citrus grandis seedlings. Physiol Plant 137:298–311. https://doi.org/10.1111/j.1399-3054.2009.01288.x
Kelling MB, Araujo MM, León EB, Aimi SC, Turchetto F (2017) Regímenes de riego y dosis de polímero hidroretenedor sobre características morfológicas y fisiológicas de plantas de Cordia trichotoma. Bosque (Valdivia) 38:123–131
Lazali M, Drevon JJ (2014) The nodule conductance to O2 diff usion increases with phytase activity in N2-fixing Phaseolus vulgaris L. Plant Physiol Biochem 80:53–59. https://doi.org/10.1016/j.plaph y.2014.03.023
Leciejewski P (2009) The effect of hydrogel additives on the water retention curve of sandy soil from forest nursery in Julinek. J Water Land Dev 13:239–247. https://doi.org/10.2478/v1002 5-010-0031-8
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lopes MBS, Tavares TCO, Veloso DA, Silva NC, Fidelis RR (2017) Cowpea bean production under water stress using hydrogels. Pesqui Agropecu Trop 47:87–92. https://doi.org/10.1590/1983-40632 016v4 74339 8
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London. https ://doi.org/10.1016/b978-0-12-38490 5-2.x0001-5
Melo EASC, Gon?alves JLM, Rocha JHT, Hakamada RE, Bazani JH, Wenzel AVA, Arthur JC Jr, Borges JS, Malheiros R, de Lemos CCZ, Ferreira EVO, Ferraz AV (2016) Responses of clonal eucalypt plantations to N, P and K fertilizer application in different edaphoclimatic conditions. Forests 7:1–15. https://doi.org/10.3390/f7010 002
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Ngatia LW, Hsieh YP, Nemours D, Fu R, Taylor RW (2017) Potential phosphorus eutrophication mitigation strategy: biochar carbon composition, thermal stability and pH influence phosphorus sorption. Chemosphere 180:201–211. https://doi.org/10.1016/j.chemo spher e.2017.04.012
Nielsen UN, Prior S, Delroy B, Walker JKM, Ellsworth DS, Powell JR (2015) Response of belowground communities to short-term phosphorus addition in a phosphorus-limited woodland. Plant Soil 391:321–331. https://doi.org/10.1007/s1110 4-015-2432-6
Noack SR, McLaughlin MJ, Smernik RJ, McBeath TM, Armstrong RD (2014) Phosphorus speciation in mature wheat and canola plants as affected by phosphorus supply. Plant Soil 378:125–137. https://doi.org/10.1007/s1110 4-013-2015-3
Pandey BK, Mehra P, Verma L, Bhadouria J, Giri J (2017) OsHAD1, a haloacid dehalogenase-like apase, enhances phosphate accumulation. Plant Physiol 174:2316–2332. https ://doi.org/10.1104/pp.17.00571
Piccin R, Couto RR, Bellinaso RJS, Gatiboni LC, Conti L, Rodrigues LAT, Michelon LS, Kulmann MSS, Brunetto G (2017a) Phosphorus forms in leaves and their relationships with must composition and yield in grapevines. Pesqui Agropecuária Bras 52:319–327. https ://doi.org/10.1590/s0100-204x2 01700 05000 05
Piccin R, Kaminski J, Ceretta CA, Tiecher T, Gatiboni LC, Bellinaso RJS, Marchezan C, de Souza ROS, Brunetto G (2017b) Distribution and redistribution of phosphorus forms in grapevines. Sci Hortic (Amsterdam) 218:125–131. https://doi.org/10.1016/j.scien ta.2017.02.023
Pizzeghello D, Berti A, Nardi S, Morari F (2011) Phosphorus forms and P-sorption properties in three alkaline soils after longterm mineral and manure applications in north-eastern Italy. Agric Ecosyst Environ 141:58–66. https ://doi.org/10.1016/j.agee.2011.02.011
Pontes Filho RA, Gondim FA, Costa MCG (2018) Seedling growth of tree species under doses of hydrogel and two levels of luminosity. Rev árvore. https://doi.org/10.1590/1806-90882 01800 01000 12
Rossi E, Sartoretto LM (2014) Caracteriza??o de três espécies florestais de importancia econ?mica. Unoesc & Ciência 5:145–152
Schansker G, Tóth SZ, Holzwarth AR, Garab G (2014) Chlorophyll a fluorescence: beyond the limits of the QA model. Photosynth Res 120:43–58. https://doi.org/10.1007/s1112 0-013-9806-5
Souza TC, Magalh?es PC, Mauro de Castro E, Pereira de Albuquerque PE, Marabesi MA (2013) The influence of ABA on water relation, photosynthesis parameters, and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance. Acta Physiol Plant 35:515–527. https://doi.org/10.1007/s1173 8-012-1093-9
Stahl JD, Cameron MD, Haselbach J, Aust SD (2000) Biodegradation of superabsorbent polymers in soil. Environ Sci Pollut Res. https ://doi.org/10.1065/espr1 99912.014
Stahl J, Ernani PR, Gatiboni LC, Chaves DM, Neves CU (2013) Produ??o de massa seca e eficiência nutricional de clones de Eucalyptus dunnii e Eucalyptus benthamii em fun??o da adi??o de doses de fósforo ao solo. Ciência Florest 23:287–295. https://doi.org/10.5902/19805 09892 75
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B Biol 104:236–257. https://doi.org/10.1016/j.jphot obiol.2010.12.010
Tabaldi LA, Ruppenthal R, Cargnelutti D, Morsch VM, Pereira LB, Schetinger MRC (2007) Effects of metal elements on acid phosphatase activity in cucumber ( Cucumis sativus L.) seedlings. Environ Exp Bot 59:43–48. https ://doi.org/10.1016/j.envex pbot.2005.10.009
Taiz L, Zeiger E (2013) Plant physiology, 5th edn. Artmed, Porto Alegre
Tang H, Li X, Zu C, Zhang F, Shen J (2013) Spatial distribution and expression of intracellular and extracellular acid phosphatases of cluster roots at different developmental stages in white lupin. J Plant Physiol 170:1243–1250. https://doi.org/10.1016/j.jplph .2013.04.015
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais. Universidade Federal do Rio Grande do Sul, Porto Alegre
Tiecher TL, Tiecher T, Ceretta CA, Ferreira PAA, Nicoloso FT, Soriani HH, Tassinari A, Paranhos JT, De Conti L, Brunetto G (2016) Physiological and nutritional status of black oat ( Avena strigosa Schreb.) grown in soil with interaction of high doses of copper and zinc. Plant Physiol Biochem 106:253–263. https://doi.org/10.1016/j.plaph y.2016.05.015
Tiecher TL, Tiecher T, Ceretta CA, Ferreira PAA, Nicoloso FT, Soriani HH, De Conti L, Kulmann MSS, Schneider RO, Brunetto G (2017) Tolerance and translocation of heavy metals in young grapevine ( Vitis vinifera) grown in sandy acidic soil with interaction of high doses of copper and zinc. Sci Hortic (Amsterdam) 222:203–212. https://doi.org/10.1016/j.scien ta.2017.05.026
Turchetto F, Araujo MM, Tabaldi LA, Griebeler AM, Rorato DG, Aimi SC, Berghetti áLP, Gomes DR (2016) Can transplantation of forest seedlings be a strategy to enrich seedling production in plant nurseries? For Ecol Manag 375:96–104. https://doi.org/10.1016/j.forec o.2016.05.029
Veneklaas EJ, Lambers H, Bragg J, Finnegan P, Lovelock C, Plaxton W, Price C, Scheible W, Shane M, White P, Raven J (2012) Opportunities for improving phosphurus-use effi ciency in crop plants. New Phytol 195(2):306–320. https ://doi.org/10.111 1/j.1469-8137.2012.04190.x
von Tucher S, H?rndl D, Schmidhalter U (2018) Interaction of soil pH and phosphorus effi cacy: long-term effects of P fertilizer and lime applications on wheat, barley, and sugar beet. Ambio 47:41–49. https://doi.org/10.1007/s1328 0-017-0970-2
Wallace A, Wallace GA (1986) Effect of polymeric soil conditioners on emergence of tomato seedlings. Soil Sci. https ://doi.org/10.1097/00010 694-19860 5000-00004
Wallace A, Wallace GA, Abouzamzam AM (1986) Amelioration of sodic soils with polymers. Soil Sci. https://doi.org/10.1097/00010 694-19860 5000-00011
Warren CR (2011) How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol 31:727–739. https ://doi.org/10.1093/treep hys/tpr06 4
Wrege MS, Steinmetz S, Júnior Reisser C, Almeida IR (2012) Atlas climático da Regi?o Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul., 2 a Edi??o. Brasilia, DF
Wu PF, Ma XQ, Tigabu M, Huang Y, Zhou LL, Cai LP, Hu XL, Oden PC (2014) Comparative growth, dry matter accumulation and photosynthetic rate of seven species of Eucalypt in response to phosphorus supply. J For Res 25:377–383. https ://doi.org/10.1007/s1167 6-014-0465-y
Zambrosi CBF, Mattos D, Syvertsen JP (2011) Plant growth, leaf photosynthesis, and nutrient-use effi ciency of citrus rootstocks decrease with phosphite supply. J Plant Nutr Soil Sci 174:487–495. https://doi.org/10.1002/jpln.20100 0320
Zambrosi FCB, Mattos D Jr, Boaretto RM, Quaggio JA, Muraoka T, Syvertsen JP (2012a) Contribution of phosphorus (32P) absorption and remobilization for citrus growth. Plant Soil 355:353–362. https://doi.org/10.1007/s1110 4-011-1107-1
Zambrosi FCB, Mattos D Jr, Furlani PR, Quaggio JA, Boaretto RM (2012b) Eficiência de absor??o e utiliza??o de fósforo em portaenxertos cítricos. Rev Bras Ciência do Solo 36:485–496. https://doi.org/10.1590/S0100-06832 01200 02000 18
Zavistanovicz TC, Araujo MM, Aimi SC, Flores R, Berghetti áLP, Deponti G (2017) Morphophysiological responses of Ilex paraguariensis seedlings to different substrates and fertilizations. Rev Bras Eng Agrícola e Ambient 21:111–115. https ://doi.org/10.1590/1807-1929/agria mbi.v21n2 p111-115
 Journal of Forestry Research2021年2期
Journal of Forestry Research2021年2期
- Journal of Forestry Research的其它文章
- Stem taper functions for Betula platyphylla in the Daxing’an Mountains, northeast China
- Leaf phenotypic variation of endangered plant Tetracentron sinense Oliv. and influence of geographical and climatic factors
- Ecological variations of woody species along an altitudinal gradient in the Qinling Mountains of Central China: area-based versus mass-based expression of leaf traits
- Variations in stem radii of Larix principis-rupprechtii to environmental factors at two slope locations in the Liupan Mountains, northwest China
- A review of ecological mechanisms for management practices of protective forests
- Variation of basic density, calorific value and volumetric shrinkage within tree height and tree age of Ugandan grown Eucalyptus grandis wood
